3. Experimental Section
3.1. General Information
All melting points were taken on an Electrothermal IA 9100 series digital melting point apparatus (Shimadzu, Tokyo, Japan). Elemental analyses were performed on Vario EL (Elementar, Langenselbold, Germany). Microanalytical data were processed in the microanalytical center, Faculty of Science, Cairo University and National Research Centre. The IR spectra (KBr disc) were recorded using a Perkin-Elmer 1650 spectrometer (Waltham, MA, USA). NMR spectra were determined using JEOL 270 MHz and JEOL JMS-AX 500 MHz (JEOL, Tokyo, Japan) spectrometers with Me4Si as an internal standard. Mass spectra were recorded on an EI Ms-QP 1000 EX instrument (Shimadzu, Japan) at 70 eV. Biological evaluations were done by the antimicrobial unit, Department of Chemistry of Natural and Microbial Products, National Research Centre, Egypt. All starting materials and solvents were purchased from Sigma-Aldrich (Saint Louis, MO, USA).
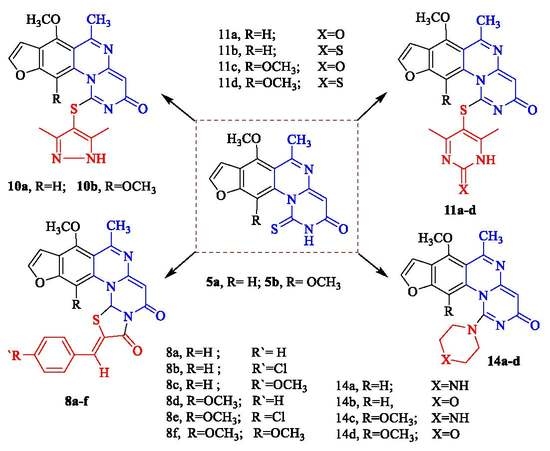
3.2. General Procedure for Synthesis of 6-((1-(6-Hydroxy-(4-methoxy or 4,7-dimethoxy)-benzofuran-5-yl)ethylidene)amino)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one (4a,b)
A mixture of visnaginone 2a (2.06 g, 0.01 mol) or khellinone 2b (2.36 g, 0.01 mol) with 6-amino-2-thioxo-2,3-dihydropyrimidin-4(1H)-one 3 (1.43 g, 0.01 mol) in dimethylformamide (40 mL) was refluxed for 4–6 h. The solid formed was filtered off, dried, and crystallized from the proper solvent to give 4a and 4b, respectively.
3.3. Synthesis of 6-((1-(6-Hydroxy-4-methoxybenzofuran-5-yl)ethylidene)amino)-2-thioxo-2,3-dihydro pyrimidin-4(1H)-one (4a)
The compound was obtained from the reaction of visnaginone (2a) with 6-amino-thiouracil (3) as yellow crystals, crystallized from methanol in 90% yield, m.p. 231 °C. IR (ν, cm−1) KBr: 3370 (brs, 2NH), 3030 (CH-aryl), 2960 (CH-aliph), 1685 (CO, amide), 1630 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.38 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 6.79 (d, 1H, J = 2.35 Hz, furan), 7.80 (d, 1H, J = 2.38 Hz, furan), 7.92 (s, 1H, phenyl), 7.94 (s, 1H, pyrimidine), 10.80, 11.19 (br, 2H, 2NH, D2O exchangeable), 16.11 (br, 1H, OH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 21.3 (1C, CH3), 61.7 (1C, OCH3), 93.1 (1C, CH, pyrimidine), 98.9 (1C, CH, phenyl), 100.5, 104.5, 108.9, 146.6, 146.9, 154.5, 160.9, 163.2, (8C, Ar-C), 164.1 (1C, C=N-ph), 168.1(1C, C=O), 173.5 (1C, C=S); MS (70 eV, %) m/z 331 (M+, 100%); Anal. Calc. (Found) for C15H13N3O4S (331.35): C, 54.37 (54.30); H, 3.95 (3.91); N, 12.68(12.60).
3.4. Synthesis of 6-((1-(6-Hydroxy-4,7-dimethoxybenzofuran-5-yl)ethylidene)amino)-2-thioxo-2,3-dihydro pyrimidin-4(1H)-one (4b)
The compound was obtained from the reaction of khellinone (2b) with 6-amino-thiouracil (3) as yellowish crystals, crystallized from dioxane in 84% yield, m.p. 211 °C. IR (ν, cm−1) KBr: 3371(brs, 2NH), 3032 (CH-aryl), 2965 (CH-aliph), 1682 (CO, amide), 1631 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.39 (s, 3H, CH3), 3.94 (s, 6H, 2OCH3), 6.80 (d, 1H, J = 2.34 Hz, furan), 7.83 (d, 1H, J = 2.37 Hz, furan), 7.95 (s, 1H, pyrimidine), 10.81,11.20 (br, 2H, 2NH, D2O exchangeable), 16.12 (br, 1H, OH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 21.4 (1C, CH3), 61.6 (2C, OCH3), 93.2 (1C, CH, pyrimidine), 100.5, 104.7, 109.9, 129.3, 146.2, 147.1, 153.8, 158.9, 164.7, (9C, Ar-C), 165.2 (1C, C=N-ph), 167.9 (1C, C=O), 173.8 (1C, C=S); MS (70 eV, %) m/z 361 (M+, 100%); Anal. Calc. (Found) for C16H15N3O5S (361.37): C, 53.18 (53.23); H, 4.18 (4.13); N, 11.63 (11.68).
3.5. General Procedure for Synthesis of (7-Methoxy or 7,11-dimethoxy)-6-methyl-1-thioxo-1,2-dihydro-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (5a,b)
Method A: A mix of visnaginone 2a (2.06 g, 0.01 mol) or khellinone 2b (2.36 g, 0.01 mol) with 6-amino-2-thiouracil (3) in DMF (40 mL) was refluxed for 14–16 h. The product precipitated was filtered off and washed with 100 mL water, dried and crystallized from the proper solvent to give 5a and 5b, respectively. Method B: A refluxing of 4a (3.31 g, 0.01 mol) or 4b (3.61 g, 0.01 mol) in DMF (40 mL) was refluxed for 8–10 h under control (TLC). The final precipitated was filtered off and washed with 50 mL ethanol, dried, and crystallized from the proper solvent to give 5a and 5b, respectively.
3.6. Synthesis of 7-Methoxy-6-methyl-1-thioxo-1,2-dihydro-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (5a)
The compound was obtained from the reaction of visnaginone (2a) with 6-aminothiouracil (3) or compound (4a) in dimethylformamide, as yellowish crystals, crystallized from benzene in 85% yield, m.p. 271 °C. IR (ν, cm−1) KBr: 3350 (brs, NH), 3028 (CH-aryl), 2955 (CH-aliph), 1680 (CO, amide), 1635 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.32 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 6.75 (d, 1H, J = 2.33 Hz, furan), 7.33 (s, 1H, pyrimidine), 7.80 (d, 1H, J = 2.36 Hz, furan), 8.29 (s, 1H, phenyl), 11.20 (br, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 24.5 (1C, CH3), 61.8 (1C, OCH3), 79.7 (1C, CH, pyrimidine), 99.1 (1C, CH, phenyl), 104.9, 108.9,116.6, 140.9, 146.6, 148.9, 160.1, 161.2, 164.1 (9C, Ar-C), 168.2 (1C, C=O), 173.6 (1C, C=S); MS (70 eV, %) m/z 313 (M+, 100%); Anal. Calc. (Found) for C15H11N3O3S (313.33): C, 57.50 (57.58); H, 3.54 (3.59); N, 13.41(13.50).
3.7. Synthesis of 7,11-Dimethoxy-6-methyl-1-thioxo-1,2-dihydro-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (5b)
The compound was obtained from the reaction of khellinone (2b) with 6-amino- thiouracil (3) or compound (4b) in dimethylformamide, as yellow crystals, crystallized from toluene in 82% yield, m.p. 251 °C. IR (ν, cm−1) KBr: 3352 (brs, NH), 3029 (CH-aryl), 2957 (CH-aliph), 1681 (CO, amide), 1633 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.35 (s, 3H, CH3), 3.95 (s, 6H, 2OCH3), 6.77 (d, 1H, J = 2.31 Hz, furan), 7.30 (s, 1H, pyrimidine), 7.82 (d, 1H, J = 2.37 Hz, furan), 11.25 (br, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 24.6 (1C, CH3), 61.9 (2C, 2OCH3), 80.2 (1C, CH, pyrimidine), 105.1, 109.2, 115.9, 125.8, 137.5, 142.8, 146.5, 149.2, 161.7, 164.8 (10C, Ar-C), 168.4 (1C, C=O), 173.8 (1C, C=S); MS (70 eV, %) m/z 343 (M+, 100%); Anal. Calc. (Found) for C16H13N3O4S (343.36): C, 55.97 (55.90); H, 3.82 (3.75); N, 12.24 (12.29).
3.8. General Procedure for Synthesis of 2-(((7-Methoxy or 7,11-dimethoxy)-6-methyl-3-oxo-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-1-yl)thio) Acetic Acid (6a,b)
A mixture from 5a (3.13 g, 0.01 mol) or 5b (3.43 g, 0.01 mol), chloroacetic acid (0.94 g, 0.01 mol) and (0.02 mol) of anhydrous sodium acetate was stirred under reflux in 40 mL of glacial acetic acid and 20 mL of acetic anhydride for 3–5 h. The reaction mixture was cooled and poured into cold water (100 mL). The deposited precipitate was filtered off, and crystallized from appropriate solvent to produce 6a and 6b, respectively.
3.9. Synthesis of 2-((7-Methoxy-6-methyl-3-oxo-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-1-yl)thio) Acetic Acid (6a)
The compound was obtained from the reaction of (5a) with chloroacetic acid, as brownish crystals, crystallized from hexane in 88% yield, m.p. 294 °C. IR (ν, cm−1) KBr: 3340 (brs, OH), 3025 (CH-aryl), 2950 (CH-aliph), 1745 (CO, acid), 1684 (CO, amide), 1631 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.30 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 4.27 (s, 2H, CH2), 6.77 (d, 1H, J = 2.32 Hz, furan), 7.35 (s, 1H, pyrimidine), 7.78 (d, 1H, J = 2.35 Hz, furan), 7.90 (s, 1H, phenyl), 13.70 (br, 1H, OH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 23.6 (1C, CH3), 32.8 (1C, CH2), 61.7 (1C, OCH3), 97.2 (1C, CH, phenyl), 100.6 (1C, CH, pyrimidine), 103.4, 105.3, 108.5, 143.7, 146.5, 152.2,158.4, 160.6, 163.9,164.8 (10C, Ar-C), 169.1, 172.8 (2C, 2C=O); MS (70 eV, %) m/z 371 (M+, 100%); Anal. Calc. (Found) for C17H13N3O5S (371.37): C, 54.98 (54.91); H, 3.53 (3.58); N, 11.32(11.40).
3.10. Synthesis of 2-((7,11-Dimethoxy-6-methyl-3-oxo-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-1-yl)thio) Acetic Acid (6b)
The compound was obtained from the reaction of (5b) with chloroacetic acid, as yellowish crystals, crystallized from ethanol in 86% yield, m.p. 283 °C. IR (ν, cm−1) KBr: 3345 (brs, OH), 3027 (CH-aryl), 2952 (CH-aliph), 1748 (CO, acid), 1681 (CO, amide), 1632 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.31 (s, 3H, CH3), 3.95 (s, 6H, 2OCH3), 4.25 (s, 2H, CH2), 6.79 (d, 1H, J = 2.35 Hz, furan), 7.38 (s, 1H, pyrimidine), 7.80 (d, 1H, J = 2.35 Hz, furan), 13.75 (br, 1H, OH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 23.4 (1C, CH3), 32.6 (1C, CH2), 61.3 (2C, 2OCH3), 100.1 (1C, CH, pyrimidine), 101.2, 105.4, 108.8, 124.1, 130.1, 146.2, 146.7, 150.5, 160.1, 164.4, 166.9 (11C, Ar-C), 169.5, 172.9 (2C, 2C=O); MS (70 eV, %) m/z 401 (M+, 100%); Anal. Calc. (Found) for C18H15N3O6S (401.39): C, 53.86 (53.80); H, 3.77 (3.71); N, 10.47 (10.42).
3.11. General Procedure for Synthesis of (9-Methoxy or 9,13-dimethoxy)-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo[2′,3′:2,3] pyrimido[1,6-a]quinazoline-3,5-dione (7a,b)
Method A: A mixture from 5a (3.13 g, 0.01 mol) or 5b (3.43 g, 0.01 mol), chloroacetic acid (0.94 g, 0.01 mol) and (0.02 mol) of anhydrous sodium acetate was stirred under reflux in 40 mL of glacial acetic acid and 20 mL of acetic anhydride on a water bath (60–70 °C) for 13–15 h (TLC). The reaction mixture was allowed to cool to room temperature and poured into water (100 mL). The solid precipitate was filtered off, and crystallized from appropriate solvent to produced 7a and 7b in good yields, respectively. Method B: A mixture of 6a (3.71 g, 0.01 mol) or 6b (4.01 g, 0.01 mol) in dimethylformamide (35 mL) was refluxed for 6–8 h with (TLC). The final product was filtered off, dried and crystallized from the proper solvent to give 7a and 7b, respectively.
3.12. Synthesis of 9-Methoxy-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo[2′,3′:2,3]pyrimido[1,6-a]quinazoline-3,5-dione (7a)
The compound was obtained from the reaction of (5a) with chloroacetic acid or compound (6a) in DMF, as white crystals, crystallized from dioxane in 82% yield, m.p. 338 °C. IR (ν, cm−1) KBr: 3035 (CH-aryl), 2962 (CH-aliph), 1688, 1680 (2CO, amide), 1635 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.37 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 4.21 (s, 2H, CH2), 5.57 (s, 1H, CH, thiazole), 6.78 (d, 1H, J = 2.34 Hz, furan), 7.38 (s, 1H, pyrimidine), 7.75 (d, 1H, J = 2.32 Hz, furan), 7.88 (s, 1H, phenyl); 13C-NMR (DMSO-d6) δ 23.3 (1C, CH3), 32.6 (1C, CH2), 61.8 (1C, OCH3), 76.1 (1C, CH, thiazole), 94.5 (1C, CH, phenyl), 98.8 (1C, CH, pyrimidine), 101.2, 105.5, 107.9, 142.2, 146.4, 153.5, 159.7, 160.4, 164.5 (9C, Ar-C), 166.4, 170.1 (2C, 2C=O); MS (70 eV, %) m/z 355 (M+, 90%); Anal. Calc. (Found) for C17H13N3O4S (355.37): C, 57.46 (57.40); H, 3.69 (3.62); N, 11.82 (11.88).
3.13. Synthesis of 9,13-Dimethoxy-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo[2′,3′:2,3]pyrimido[1,6-a]quinazoline-3,5-dione (7b)
The compound was obtained from the reaction of (5b) with chloroacetic acid or compound (6b) in DMF, as yellowish crystals, crystallized from methanol in 80% yield, m.p. 303 °C. IR (ν, cm−1) KBr: 3038 (CH-aryl), 2966 (CH-aliph), 1685, 1682 (2CO, amide), 1632 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.33 (s, 3H, CH3), 3.97 (s, 6H, 2OCH3), 4.24 (s, 2H, CH2), 5.62 (s, 1H, CH, thiazole), 6.74 (d, 1H, J = 2.33 Hz, furan), 7.39 (s, 1H, pyrimidine), 7.77 (d, 1H, J = 2.31 Hz, furan); 13C-NMR (DMSO-d6) δ 23.1 (1C, CH3), 32.3 (1C, CH2), 61.7 (2C, 2OCH3), 76.4 (1C, CH, thiazole), 97.5 (1C, CH, pyrimidine), 101.4, 105.6, 108.2, 123.6, 127.8, 146.3, 146.8, 152.9, 160.1, 164.7 (10C, Ar-C), 166.2, 170.5 (2C, 2C=O); MS (70 eV, %) m/z 385 (M+, 95%); Anal. Calc. (Found) for C18H15N3O5S (385.39): C, 56.10 (56.18); H, 3.92 (3.85); N, 10.90 (10.98).
3.14. General Procedure for Synthesis of 2-(Substituted-benzylidene)-9,(substituted)-methoxy-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo[2′,3′:2,3]pyrimido[1,6-a]quinazoline-3,5-dione (8a–f)
Method A: One pot synthesis: A mixture from 5a (3.13g, 0.01 mol) or 5b (3.43 g, 0.01 mol), chloroacetic acid (0.94 g, 0.01 mol), the appropriate aromatic aldehyde (10 mmol) and (0.02 mol) of anhydrous sodium acetate was stirred under reflux in 40 mL of glacial acetic acid and 20 mL of acetic anhydride for 24–26 h. The reaction mixture was cooled and poured into ice water. The deposited precipitate was filtered off, and crystallized from appropriate solvent to give (8a–f).
Method B: A mixture of compound 7a (3.55 g, 10 mmol) or 7b (3.85 g, 10 mmol) and the appropriate aromatic aldehyde (10 mmol) in dioxane (40 mL) containing a catalyst amount of piperidine (0.5 mL) was stirred and heated under reflux for 10–12 h (TLC control). The reaction mixture was cooled, the formed precipitate filtered off, dried and recrystallized from the appropriate solvent to afford (8a–f).
3.15. Synthesis of 2-Benzylidene-9-methoxy-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo[2′,3′:2,3] pyrimido[1,6-a]quinazoline-3,5-dione (8a)
The compound was obtained from the reaction of (5a) and benzaldehyde (1.06 g, 10 mmol) with chloroacetic acid or compound (7a) with benzaldehyde, as yellowish crystals, crystallized from dimethylformamide in 75% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3050 (CH-aryl), 2945 (CH-aliph), 1685, 1672 (2CO, amide), 1630 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.30 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 5.59 (s, 1H, CH, thiazole), 6.75 (d, 1H, J = 2.31 Hz, furan), 7.32 (s, 1H, pyrimidine), 7.40–7.67(m, 5H, phenyl), 7.72 (d, 1H, J = 2.30 Hz, furan), 7.85 (s, 1H, phenyl), 8.05 (s, 1H, CH); 13C-NMR (DMSO-d6) δ 23.1 (1C, CH3), 61.6 (1C, OCH3), 77.5 (1C, CH, thiazole), 90.8 (1C, CH, phenyl), 92.9 (1C, CH, pyrimidine), 100.7, 104.8, 105.6, (3C, benzofurane), 121.9 (1C, CH), 127.2, 128.1, 128.7, 133.9 (6C, phenyl), 137.5 (1C, thiazole), 140.3, 146.4, 153.8, 160.1, 160.4, 164.2 (6C, pyrimidobenzo furane), 165.6, 168.4 (2C, 2C=O); MS (70 eV, %) m/z 443 (M+, 88%); Anal. Calc. (Found) for C24H17N3O4S (443.48): C, 65.00 (65.10); H, 3.86 (3.80); N, 9.48 (9.41).
3.16. Synthesis of 2-(4-Chlorobenzylidene)-9-methoxy-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo[2′,3′:2,3]pyrimido[1,6-a]quinazoline-3,5-dione (8b)
The compound was obtained from the reaction of (5a) and 4-chlorobenzaldehyde (1.40 g, 10 mmol) with chloroacetic acid or compound (7a) with 4-chloro- benzaldehyde, as yellow crystals, crystallized from dioxane in 80% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3052 (CH-aryl), 2948 (CH-aliph), 1687, 1674 (2CO, amide), 1631 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.31 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 5.60 (s, 1H, CH, thiazole), 6.74 (d, 1H, J = 2.32 Hz, furan), 7.33 (s, 1H, pyrimidine), 7.45–7.50 (dd, 2H, J = 7.60, 7.64 Hz, 4-chlorophenyl), 7.55–7.60 (dd, 2H, J = 7.62, 7.66 Hz, 4-chloro phenyl),7.70 (d, 1H, J = 2.34 Hz, furan), 7.83 (s, 1H, phenyl), 8.08 (s, 1H, CH); 13C-NMR (DMSO-d6) δ 23.2 (1C, CH3), 61.4 (1C, OCH3), 77.8 (1C, CH, thiazole), 90.2 (1C, CH, phenyl), 92.1 (1C, CH, pyrimidine), 100.3, 104.9, 105.5, (3C, benzofurane), 122.1 (1C, CH), 128.4, 129.2, 132.5, 132.8 (6C, 4-chlorophenyl), 137.9 (1C, thiazole), 140.6, 146.3,153.5, 160.3, 160.7, 164.3 (6C, pyrimidobenzo furane), 165.8, 168.7 (2C, 2C=O); MS (70 eV, %) m/z 477 (M+, 90%); Anal. Calc. (Found) for C24H16ClN3O4S (477.92): C, 60.32 (60.39); H, 3.37 (3.44); N, 8.79 (8.85).
3.17. Synthesis of 9-Methoxy-2-(4-methoxybenzylidene)-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo[2′,3′:2,3]pyrimido[1,6-a]quinazoline-3,5-dione (8c)
The compound was obtained from the reaction of (5a) and 4-methoxy- benzaldehyde (1.36 g, 10 mmol) with chloroacetic acid or compound (7a) with 4-methoxybenzaldehyde, as brownish crystals, crystallized from ethanol in 78% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3054 (CH-aryl), 2944 (CH-aliph), 1683, 1671 (2CO, amide), 1636 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.29 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 4.10 (s, 3H, OCH3), 5.59 (s, 1H, CH, thiazole), 6.71 (d, 1H, J = 2.32 Hz, furan), 7.28 (s, 1H, pyrimidine), 7.48–7.53 (dd, 2H, J = 7.61, 7.65 Hz, 4-methoxyphenyl), 7.58–7.63 (dd, 2H, J = 7.63, 7.67 Hz, 4-methoxyphenyl), 7.69 (d, 1 H, J = 2.37 Hz, furan), 7.86 (s, 1 H, phenyl), 8.06 (s, 1H, CH); 13C-NMR (DMSO-d6) δ 23.4 (1C, CH3), 58.5 (1C, OCH3), 61.7 (1C, OCH3), 77.2 (1C, CH, thiazole), 90.5 (1C, CH, phenyl), 92.4 (1C, CH, pyrimidine), 100.4, 105.1, 105.5, (3C, benzofurane), 122.6 (1C, CH), 123.1, 127.7, 130.1, 155.5 (6C, 4-methoxyphenyl), 137.4 (1C, thiazole), 140.8, 146.2,153.6, 160.1, 160.4, 164.3 (6C, pyrimidobenzofurane), 165.1, 168.6 (2C, 2C=O); MS (70 eV, %) m/z 473 (M+, 87%); Anal. Calc. (Found) for C25H19N3O5S (473.50): C, 63.42 (63.49); H, 4.04 (4.12); N, 8.87 (8.95).
3.18. Synthesis of 2-Benzylidene-9,13-dimethoxy-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo [2′,3′:2,3]pyrimido[1,6-a]quinazoline-3,5-dione (8d)
The compound was obtained from the reaction of (5b) and benzaldehyde (1.06 g, 10 mmol) with chloroacetic acid or compound (7b) with benzaldehyde, as yellow crystals, crystallized from acetone in 79% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3060 (CH-aryl), 2950 (CH-aliph), 1688, 1678 (2CO, amide), 1638 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.33 (s, 3H, CH3), 3.97 (s, 6H, 2OCH3), 5.60 (s, 1H, CH, thiazole), 6.74 (d, 1H, J= 2.30 Hz, furan), 7.34 (s, 1H, pyrimidine), 7.45–7.65 (m, 5H, phenyl), 7.74 (d, 1H, J = 2.35 Hz, furan), 8.02 (s, 1H, CH); 13C-NMR (DMSO-d6) δ 23.5 (1C, CH3), 61.8 (2C, 2OCH3), 77.7 (1C, CH, thiazole), 91.7 (1C, CH, pyrimidine), 101.4, 105.3, 108.8, 120.7 (4C, benzo furane), 122.2 (1C, CH), 126.1 (1C, pyrimidine), 127.7, 128.3, 128.8, 134.6 (6C, phenyl), 137.7 (1C, thiazole), 146.2, 146.7, 151.9, 160.6, 164.8 (5C, pyrimidobenzofurane), 165.1, 168.3 (2C, 2C=O); MS (70 eV, %) m/z 473 (M+, 84%); Anal. Calc. (Found) for C25H19N3O5S (473.50): C, 63.42 (63.50); H, 4.04 (4.10); N, 8.87 (8.80).
3.19. Synthesis of 2-(4-Chlorobenzylidene)-9,13-dimethoxy-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo [2′,3′:2,3]pyrimido[1,6-a]quinazoline-3,5-dione (8e)
The compound was obtained from the reaction of (5b) and 4-chlorobenzaldehyde (1.40 g, 10 mmol) with chloroacetic acid or compound (7b) with 4-chlorobenzaldehyde, as yellowish crystals, crystallized from methanol in 88% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3055 (CH-aryl), 2945 (CH-aliph), 1686, 1674 (2CO, amide), 1634 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.30 (s, 3H, CH3), 3.98 (s, 6H, 2OCH3), 5.59 (s, 1H, CH, thiazole), 6.73 (d, 1H, J= 2.31 Hz, furan), 7.35 (s, 1H, pyrimidine), 7.47–7.52 (dd, 2H, J = 7.68, 7.62 Hz, 4-chlorophenyl), 7.57–7.62 (dd, 2H, J = 7.61, 7.65 Hz, 4-chloro phenyl), 7.75 (d, 1H, J = 2.36 Hz, furan), 8.04 (s, 1H, CH); 13C-NMR (DMSO-d6) δ 23.7 (1C, CH3), 61.9 (2C, 2OCH3), 77.6 (1C, CH, thiazole), 91.2 (1C, CH, pyrimidine), 100.5, 105.6, 108.4, 120.8 (4C, benzo furane), 122.4 (1C, CH), 126.3 (1C, pyrimidine), 128.2, 128.8, 131.5, 131.9 (6C, 4-chloro phenyl), 137.5 (1C, thiazole), 146.3, 146.9, 150.8, 160.4, 164.9 (5C, pyrimidobenzofurane), 165.3, 168.1 (2C, 2C=O); MS (70 eV, %) m/z 507 (M+, 98%); Anal. Calc. (Found) for C25H18ClN3O5S (507.94): C, 59.12 (59.22); H, 3.57 (3.50); N, 8.27 (8.35).
3.20. Synthesis of 9,13-Dimethoxy-2-(4-methoxybenzylidene)-8-methyl-2-hydro-5H,14aH-furo[3,2-g]thiazolo [2′,3′:2,3]pyrimido[1,6-a]quinazoline-3,5-dione (8f)
The compound was obtained from the reaction of (5b) and 4-methoxy- benzaldehyde (1.36 g, 10 mmol) with chloroacetic acid or compound (7b) with 4-methoxybenzaldehyde, as brownish crystals, crystallized from benzene in 73% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3052 (CH-aryl), 2944 (CH-aliph), 1685, 1672 (2CO, amide), 1630 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.28 (s, 3H, CH3), 3.95 (s, 6H, 2OCH3), 4.12 (s, 3H, OCH3), 5.55 (s, 1H, CH, thiazole), 6.71 (d, 1H, J= 2.32 Hz, furan), 7.37 (s, 1H, pyrimidine), 7.49–7.54 (dd, 2H, J = 7.65, 7.61 Hz, 4-methoxyphenyl), 7.58–7.63 (dd, 2H, J = 7.62, 7.66 Hz, 4-methoxyphenyl), 7.76 (d, 1H, J = 2.35 Hz, furan), 8.06 (s, 1H, CH); 13C-NMR (DMSO-d6) δ 23.4 (1C, CH3), 58.2 (1C, OCH3), 61.8 (2C, 2OCH3), 77.3 (1C, CH, thiazole), 91.5 (1C, CH, pyrimidine), 100.2, 105.5, 108.6, 120.5 (4C, benzofurane), 122.7 (1C, CH), 126.1 (1C, pyrimidine), 127.2, 128.4, 129.1, 152.2 (6C, 4-methoxyphenyl), 137.8 (1C, thiazole), 146.1, 146.7, 150.6, 160.5, 164.8 (5C, pyrimidobenzo furane), 165.7, 168.2 (2C, 2C=O); MS (70 eV, %) m/z 503 (M+, 91%); Anal. Calc. (Found) for C26H21N3O6S (503.53): C, 62.02 (62.10); H, 4.20 (4.28); N, 8.35 (8.27).
3.21. General Procedure for Synthesis of 3-(((7-Methoxy or 7,11-dimethoxy)-6-methyl-3-oxo-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-1-yl)thio)pentane-2,4-dione (9a,b)
To a warmed ethanolic potassium hydroxide solution (prepared by dissolving 10 mmol of KOH in 50 mL ethanol) was added each of 5a (3.13 g, 0.01 mol) or 5b (3.43 g, 0.01 mol), the heating was continued for 40 min and the mixture was allowed to cool to room temperature, and the proper 3-chloro-pentane-2,4-dione (3-chloroacetylacetone, 1.12 mL, 0.01 mol) was added. The mixture was stirred under reflux for 6–8 h (control TLC), and then cool to room temperature, poured into cold water (100 mL). The solid product precipitated was filtered off, washed with 100 mL water; the product was dried and crystallized from the suitable solvent to afford 9a and 9b in good yields, respectively.
3.22. Synthesis of 3-((7-Methoxy-6-methyl-3-oxo-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-1-yl)thio)pentane- 2,4-dione (9a)
The compound was obtained from the reaction of (5a) with 3-chloro-pentane-2,4-dione (3-chloroacetylacetone), as white crystals, crystallized from hexane in 92% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3035 (CH-aryl), 2945 (CH-aliph), 1725, 1721 (2CO, acetyl), 1682 (CO, amide), 1638 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.26 (s, 6H, 2COCH3), 2.34 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 4.08 (s, 1H, CH), 6.73 (d, 1H, J = 2.31 Hz, furan), 7.37 (s, 1H, pyrimidine), 7.62 (s, 1H, phenyl), 7.75 (d, 1H, J = 2.33 Hz, furan), 13C-NMR (DMSO-d6) δ 23.8(1C, CH3), 26.5 (2C, 2COCH3), 61.6 (1C, OCH3), 68.2 (1C, CH), 91.3 (1C, CH, phenyl), 96.5 (1C, CH, pyrimidine), 100.1, 105.3, 108.5, 142.6, 146.1, 153.5, 157.4, 160.3, 163.8, 165.7 (10C, Ar-C), 168.6, 184.5, 188.2 (3C, 3C=O); MS (70 eV, %) m/z 411 (M+, 95%); Anal. Calc. (Found) for C20H17N3O5S (411.43): C, 58.39 (58.32); H, 4.16 (4.10); N, 10.21 (10.16).
3.23. Synthesis of 3-((7,11-Dimethoxy-6-methyl-3-oxo-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-1-yl)thio)pentane-2,4-dione (9b)
The compound was obtained from the reaction of (5b) with 3-chloro-pentane-2,4-dione (3-chloroacetylacetone), as white crystals, crystallized from dioxane in 90% yield, m.p. 348 °C. IR (ν, cm−1) KBr: 3038 (CH-aryl), 2949 (CH-aliph), 1728, 1722 (2CO, acetyl), 1684 (CO, amide), 1636 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.28 (s, 6H, 2COCH3), 2.37 (s, 3H, CH3), 3.98 (s, 6H, 2OCH3), 4.15 (s, 1H, CH), 6.72 (d, 1H, J = 2.34 Hz, furan), 7.35 (s, 1H, pyrimidine), 7.79 (d, 1H, J = 2.37 Hz, furan), 13C-NMR (DMSO-d6) δ 23.4 (1C, CH3), 26.1 (2C, 2COCH3), 61.9 (2C, 2OCH3), 68.5 (1C, CH), 97.1 (1C, CH, pyrimidine), 100.2, 105.7, 108.9, 122.5, 130.2, 146.3, 146.8, 150.7, 159.6, 164.2, 166.4 (11C, Ar-C), 168.8, 185.1, 188.7 (3C, 3C=O); MS (70 eV, %) m/z 441 (M+, 90%); Anal. Calc. (Found) for C21H19N3O6S (441.46): C, 57.14 (57.20); H, 4.34 (4.39); N, 9.52 (9.60).
3.24. General Procedure for Synthesis of 1-((3,5-Dimethyl-1H-pyrazol-4-yl)thio)-(7-methoxy or 7,11-dimethoxy)-6-methyl-3H-furo[3, 2-g]pyrimido[1,6-a]quinazolin-3-one (10a,b)
A mixture of 9a (4.11 g, 0.01 mol) or 9b (4.41 g, 0.01 mol), and hydrazine hydrate (99–100%) in dioxane (30 mL) and ethanol (20 mL) was stirred under reflux for 13–15 h. The reaction mixture was allowed to cool to room temperature, poured into cold water (100 mL). The deposited precipitate was filtered off, dried, and crystallized from the proper solvent to give 10a and 10b in good yields, respectively.
3.25. Synthesis of 1-((3,5-Dimethyl-1H-pyrazol-4-yl)thio)-7-methoxy-6-methyl-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (10a)
The compound was obtained from the reaction of (9a) with hydrazine hydrate, as yellow crystals, crystallized from dimethylformamide in 95% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3375 (brs, NH), 3030 (CH-aryl), 2940 (CH-aliph), 1684 (CO, amide), 1633 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.28 (s, 3H, CH3), 2.38 (s, 3H, CH3), 2.40 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 6.75 (d, 1H, J = 2.32 Hz, furan), 7.32 (s, 1H, pyrimidine), 7.60 (s, 1H, phenyl), 7.72 (d, 1H, J = 2.37 Hz, furan), 11.50 (brs, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 20.1, 20.3, 23.2 (3C, 3CH3), 61.4 (1C, OCH3), 91.1 (1C, CH, phenyl), 96.3 (1C, CH, pyrimidine), 100.2, 105.1, 107.2, 108.4, 142.3, 144.5, 146.6, 154.1, 158.2, 160.5, 164.4, 166.3 (13C, Ar-C), 168.8 (1C, C=O); MS (70 eV, %) m/z 407 (M+, 100%); Anal. Calc. (Found) for C20H17N5O3S (407.45): C, 58.96 (58.88); H, 4.21 (4.15); N, 17.19 (17.10).
3.26. Synthesis of 1-((3,5-Dimethyl-1H-pyrazol-4-yl)thio)-7,11-dimethoxy-6-methyl-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (10b)
The compound was obtained from the reaction of (9b) with hydrazine hydrate, as yellowish crystals, crystallized from dioxane in 91% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3370 (brs, NH), 3032 (CH-aryl), 2943 (CH-aliph), 1682 (CO, amide), 1635 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.30 (s, 3H, CH3), 2.39 (s, 3H, CH3), 2.41 (s, 3H, CH3), 3.98 (s, 6H, 2OCH3), 6.77 (d, 1H, J = 2.36 Hz, furan), 7.38 (s, 1H, pyrimidine), 7.79 (d, 1H, J = 2.34 Hz, furan), 11.55 (brs, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 20.4, 20.6, 23.5 (3C, 3CH3), 61.9 (2C, 2OCH3), 96.7 (1C, CH, pyrimidine), 100.3, 105.6, 107.8, 108.5, 122.5, 130.4, 144.8, 146.1, 146.8, 151.4, 160.5, 164.2, 166.6 (14C, Ar-C), 168.9 (1C, C=O); MS (70 eV, %) m/z 437 (M+, 100%); Anal. Calc. (Found) for C21H17N5O4S (437.47): C, 57.66 (57.72); H, 4.38 (4.30); N, 16.01 (16.10).
3.27. General Procedure for Synthesis of 1-((4,6-Dimethyl-2-(oxo or thioxo)-1,2-dihydropyrimidin-5-yl)thio)-(7-methoxy or 7,11-dimethoxy)-6-methyl-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (11a–d)
A mixture of 9a (4.11 g, 0.01 mol) or 9b (4.41 g, 0.01 mol), and urea (0.60 g, 0.01 mol) or thiourea (0. 76 g, 0.01 mol) was stirred under reflux in dioxane (40 mL) in the presence of catalytic amount of piperidine (1 mL) for 16–18 h. The reaction mixture was allowed to cool to room temperature, poured into water (100 mL), the deposited precipitate was filtered off, washed with ethanol (40 mL), dried and crystallized from proper solvent to afford (11a–d).
3.28. Synthesis of 1-((4,6-Dimethyl-2-oxo-1,2-dihydropyrimidin-5-yl)thio)-7-methoxy-6-methyl-3H-furo[3,2- g]pyrimido[1,6-a]quinazolin-3-one (11a)
The compound was obtained from the reaction of (9a) with urea, as yellowish crystals, crystallized from dioxane in 86% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3380 (brs, NH), 3032 (CH-aryl), 2942 (CH-aliph), 1685, 1680 (2C=O, amide), 1635 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.11 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.37 (s, 3H, CH3), 3.94 (s, 3H, OCH3), 6.72 (d, 1H, J = 2.31 Hz, furan), 7.36 (s, 1H, pyrimidine), 7.63 (s, 1H, phenyl), 7.79 (d, 1H, J = 2.38 Hz, furan), 10.60 (brs, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 20.6, 20.9, 23.5 (3C, 3CH3), 61.8 (1C, OCH3), 85.4 (1C, pyrimidine), 91.5 (1C, CH, phenyl), 97.7 (1C, CH, pyrimidine), 100.4, 105.7, 108.2, 143.1, 146.1, 153.6, 158.5, 160.4, 163.8, 164.7, 165.2, 166.8 (12C, Ar-C), 169.2, 172.5 (2C, 2C=O); MS (70 eV, %) m/z 435 (M+, 92%); Anal. Calc. (Found) for C21H17N5O4S (435.46): C, 57.92 (57.85); H, 3.94 (3.99); N, 16.08 (16.15).
3.29. Synthesis of 1-((4,6-Dimethyl-2-thioxo-1,2-dihydropyrimidin-5-yl)thio)-7-methoxy-6-methyl-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (11b)
The compound was obtained from the reaction of (9a) with thiourea, as yellow crystals, crystallized from dimethylformamide in 82% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3385 (brs, NH), 3035 (CH-aryl), 2944 (CH-aliph), 1681 (C=O, amide), 1632 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.10 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.36 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 6.70 (d, 1H, J = 2.30 Hz, furan), 7.34 (s, 1H, pyrimidine), 7.61 (s, 1H, phenyl), 7.78 (d, 1H, J = 2.36 Hz, furan), 12.30 (brs, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 21.4, 21.8, 23.7 (3C, 3CH3), 61.7 (1C, OCH3), 84.6 (1C, pyrimidine), 91.8 (1C, CH, phenyl), 98.2 (1C, CH, pyrimidine), 100.2, 105.8, 108.1, 143.7, 146.3, 153.8, 158.9, 160.5, 163.9, 164.2, 165.8, 169.6 (12C, Ar-C), 170.1 (1C, C=O), 177.8 (1C, C=S); MS (70 eV, %) m/z 451 (M+, 90%); Anal. Calc. (Found) for C21H17N5O3S2 (451.52): C, 55.86 (55.78); H, 3.80 (3.88); N, 15.51 (15.60).
3.30. Synthesis of 1-((4,6-Dimethyl-2-oxo-1,2-dihydropyrimidin-5-yl)thio)-7,11-dimethoxy-6-methyl-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (11c)
The compound was obtained from the reaction of (9b) with urea, as yellow crystals, crystallized from methanol in 80% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3377 (brs, NH), 3039 (CH-aryl), 2937 (CH-aliph), 1687, 1683 (2C=O, amide), 1638 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.09 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.33 (s, 3H, CH3), 3.99 (s, 6H, 2OCH3), 6.75 (d, 1H, J = 2.32 Hz, furan), 7.38 (s, 1H, pyrimidine), 7.76 (d, 1H, J = 2.37 Hz, furan), 10.74 (brs, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 20.5, 20.7, 23.7 (3C, 3CH3), 61.9 (2C, 2OCH3), 87.1(1C, pyrimidine), 98.9 (1C, CH, pyrimidine), 100.2, 105.4, 108.8, 122.3, 130.5, 146.1, 146.9, 152.1, 158.2, 164.1, 164.5, 166.2, 168.5 (13C, Ar-C), 169.8, 171.4 (2C, 2C=O); MS (70 eV, %) m/z 465 (M+, 88%); Anal. Calc. (Found) for C22H19N5O5S (465.48): C, 56.77 (56.70); H, 4.11 (4.05); N, 15.05 (15.14).
3.31. Synthesis of 1-((4, 6-Dimethyl-2-thioxo-1,2-dihydropyrimidin-5-yl)thio)-7,11-dimethoxy-6-methyl-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (11d)
The compound was obtained from the reaction of (9b) with thiourea, as yellowish crystals, crystallized from dioxane in 80% yield, m.p. > 350 °C. IR (ν, cm−1) KBr: 3388 (brs, NH), 3034 (CH-aryl), 2945 (CH-aliph), 1683 (C=O, amide), 1630 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.13 (s, 3H, CH3), 2.25 (s, 3H, CH3), 2.32 (s, 3H, CH3), 4.01 (s, 6H, 2OCH3), 6.73 (d, 1H, J = 2.34 Hz, furan), 7.36 (s, 1H, pyrimidine),7.80 (d, 1H, J = 2.38 Hz, furan), 12.50 (brs, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 21.5, 21.9, 23.8 (3C, 3CH3), 62.01 (2C, 2OCH3), 86.1 (1C, pyrimidine), 99.3 (1C, CH, pyrimidine), 100.4, 105.2, 108.7, 122.7, 130.4, 146.3, 146.7, 151.9, 159.1, 164.3, 164.5, 166.9, 168.2 (13C, Ar-C), 169.8(1C, C=O), 178.5 (1C, C=S); MS (70 eV, %) m/z 481 (M+, 86%); Anal. Calc. (Found) for C22H19N5O4S2 (481.54): C, 54.87 (54.95); H, 3.98 (3.90); N, 14.54 (14.47).
3.32. General Procedure for Synthesis of (7-Methoxy or 7,11-dimethoxy)-6-methyl-1-(methylthio)-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (12a,b)
To a warmed ethanolic KOH solution (prepared by dissolving 10 mmol of KOH in 50 mL ethanol) was added each of 5a (3.13 g, 0.01 mol) or 5b (3.43 g, 0.01 mol), the heating was continued for 30 min and the mixture was allowed to cool to room temperature, and methyl iodide (0.62 mL, 0.01 mol) was added. The mixture was stirred under reflux for 5–7 h, then cool to room temperature, poured into cold water (100 mL). The solid product precipitated was filtered off, washed with water; the product was dried and crystallized from the proper solvent to produce 12a and 12b, respectively.
3.33. Synthesis of 7-Methoxy-6-methyl-1-(methylthio)-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (12a)
The compound was obtained from the reaction of (5a) with methyl iodide, as yellow crystals, crystallized from methanol in 90% yield, m.p. 329 °C. IR (ν, cm−1) KBr: 3035 (CH-aryl), 2962 (CH-aliph), 1685 (CO, amide), 1630 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.30 (s, 3H, CH3), 2.80 (s, 3H, SCH3), 3.90 (s, 3H, OCH3), 6.73 (d, 1H, J = 2.31 Hz, furan), 7.34 (s, 1H, pyrimidine), 7.64 (s, 1H, phenyl), 7.78 (d, 1H, J = 2.35 Hz, furan); 13C-NMR (DMSO-d6) δ 18.9 (1C, SCH3), 23.8 (1C, CH3), 61.4 (1C, OCH3), 92.7 (1C, CH, phenyl), 98.9 (1C, CH, pyrimidine), 100.3, 105.2, 108.5, 145.2, 146.4, 153.7, 158.3, 160.9, 164.6, 167.8 (10C, Ar-C), 169.1 (1C, C=O); MS (70 eV, %) m/z 327 (M+, 100%); Anal. Calc. (Found) for C16H13N3O3S (327.36): C, 58.71 (58.78); H, 4.00 (4.10); N, 12.84 (12.90).
3.34. Synthesis of 7,11-Dimethoxy-6-methyl-1-(methylthio)-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (12b)
The compound was obtained from the reaction of (5b) with methyl iodide, as yellowish crystals, crystallized from ethanol in 86% yield, m.p. 311 °C. IR (ν, cm−1) KBr: 3038 (CH-aryl), 2965 (CH-aliph), 1683 (CO, amide), 1633 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.35 (s, 3H, CH3), 2.88 (s, 3H, SCH3), 3.97 (s, 6H, 2OCH3), 6.78 (d, 1H, J=2.32 Hz, furan), 7.38 (s, 1H, pyrimidine), 7.80 (d, 1H, J = 2.34 Hz, furan); 13C-NMR (DMSO-d6) δ 19.2 (1C, SCH3), 23.6 (1C, CH3), 61.8 (2C, 2OCH3), 99.4 (1C, CH, pyrimidine), 100.1, 105.7, 108.2, 122.7, 130.5, 146.1, 146.8, 151.5, 159.7, 164.4, 168.2 (11C, Ar-C), 169.7 (1C, C=O); MS (70 eV, %) m/z 357 (M+, 100%); Anal. Calc. (Found) for C17H15N3O4S (357.38): C, 57.13 (57.22); H, 4.23 (4.28); N, 11.76 (11.66).
3.35. General Procedure for Synthesis of (7-Methoxy or 7,11-dimethoxy)-6-methyl-1-(methylsulfonyl)-3H- furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (13a,b)
A mixture of 12a (3.27 g, 0.01 mol) or 12b (3.57 g, 0.01 mol), and excess amount of hydrogen peroxide (5 mL) in acetic acid (40 mL) was heated gently with stirring for 12–14 h with (TLC). The reaction mixture was allowed to cool to 0 °C. The deposited precipitate was filtered off, and crystallized from the proper solvent to afford 13a and 13b, respectively.
3.36. Synthesis of 7-Methoxy-6-methyl-1-(methylsulfonyl)-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (13a)
The compound was obtained from the reaction of (12a) with hydrogen peroxide, as yellowish crystals, crystallized from benzene in 75% yield, m.p. 241 °C. IR (ν, cm−1) KBr: 3032 (CH-aryl), 2920 (CH-aliph), 1687 (CO, amide), 1632 (C=N), 1162, 1340 (SO2). 1H-NMR (DMSO-d6, ppm) δ 2.34 (s, 3H, CH3), 2.95 (s, 3H, SO2CH3), 3.92 (s, 3H, OCH3), 6.76 (d, 1H, J = 2.36 Hz, furan), 7.37 (s, 1H, pyrimidine), 7.61 (s, 1H, phenyl), 7.81 (d, 1H, J = 2.32 Hz, furan); 13C-NMR (DMSO-d6) δ 23.5 (1C, CH3), 35.6 (1C, SO2CH3), 61.3 (1C, OCH3), 93.5 (1C, CH, phenyl), 99.4 (1C, CH, pyrimidine), 100.2, 105.4, 108.7, 140.3, 145.5, 146.2, 153.9, 160.8, 164.4, 168.1 (10C, Ar-C), 169.5 (1C, C=O); MS (70 eV, %) m/z 359 (M+, 84%); Anal. Calc. (Found) for C16H13N3O5S (359.36): C, 53.48 (53.40); H, 3.65 (3.57); N, 11.69 (11.61).
3.37. Synthesis of 7,11-Dimethoxy-6-methyl-1-(methylsulfonyl)-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin- 3-one (13b)
The compound was obtained from the reaction of (12b) with hydrogen peroxide, as yellow crystals, crystallized from toluene in 75% yield, m.p. 221 °C. IR (ν, cm−1) KBr: 3029 (CH-aryl), 2918 (CH-aliph), 1684 (CO, amide), 1628 (C=N), 1160, 1342(SO2). 1H-NMR (DMSO-d6, ppm) δ 2.29 (s, 3H, CH3), 2.97 (s, 3H, SO2CH3), 4.01 (s, 6H, 2OCH3), 6.80 (d, 1H, J = 2.30 Hz, furan), 7.31 (s, 1H, pyrimidine), 7.75 (d, 1H, J = 2.33 Hz, furan); 13C-NMR (DMSO-d6) δ 23.3 (1C, CH3), 35.8 (1C, SO2CH3), 61.9 (2C, 2OCH3), 99.8 (1C, CH, pyrimidine), 100.4, 105.7, 108.9, 122.4, 130.1, 140.6, 146.3, 146.8, 151.5, 164.7, 168.5(11C, Ar-C), 169.5 (1C, C=O); MS (70 eV, %) m/z 389 (M+, 80%); Anal. Calc. (Found) for C17H15N3 O6S (389.38): C, 52.44 (52.50); H, 3.88 (3.80); N, 10.79 (10.71).
3.38. General Procedure for Synthesis of (7-Methoxy or 7,11-dimethoxy)-6-methyl-1-((piperazin-1-yl) or morpholino)-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (14a–d)
In a warm solution of 12a (3.27 g, 0.01 mol) or 12b (3.57 g, 0.01 mol), in methanol (50 mL) was added the freshly distilled 2nd amine, namely piperazine (0.95 mL, 0.01 mol) or morpholine (0.86 mL, 0.01 mol). The reaction mixture was stirred under reflux for 7–9 h, then allowed to cool to 0 °C for 14 h and the solid obtained was filtered, washed with water (100 mL) dried and recrystallized from appropriate solvent to produce (14a–d).
3.39. Synthesis of 7-Methoxy-6-methyl-1-(piperazin-1-yl)-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (14a)
The compound was obtained from the reaction of (12a) with piperazine, as pale yellow crystals, crystallized from dioxane in 79% yield, m.p. 202 °C. IR (ν, cm−1) KBr: 3390 (br, NH), 3044 (CH-aryl), 2940 (CH-aliph), 1687 (CO, amide), 1620 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.29 (s, 3H, CH3), 2.82–2.91 (m, 8H, piperazine), 3.92 (s, 3H, OCH3), 6.78 (d, 1H, J = 2.36 Hz, furan), 7.38 (s, 1H, pyrimidine), 7.66 (s, 1H, phenyl), 7.81 (d, 1H, J = 2.37 Hz, furan), 10.30 (brs, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 23.2 (1C, CH3), 48.3 (2C, 2CH2, piperazine), 52.5 (2C, 2CH2, piperazine), 61.7 (1C, OCH3), 91.8 (1C, CH, phenyl), 98.4 (1C, CH, pyrimidine), 100.1, 105.3, 108.2, 144.6, 146.1, 153.8, 154.9, 160.3, 164.4, 168.1(10C, Ar-C), 169.3 (1C, C=O); MS (70 eV, %) m/z 365 (M+, 83%); Anal. Calc. (Found) for C19H19N5O3 (365.39): C, 62.46 (62.55); H, 5.24 (5.18); N, 19.17 (19.24).
3.40. Synthesis of 7-Methoxy-6-methyl-1-morpholino-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (14b)
The compound was obtained from the reaction of (12a) with morpholine, as yellow crystals, crystallized from methanol in 75% yield, m.p. 171 °C. IR (ν, cm−1) KBr: 3040 (CH-aryl), 2936 (CH-aliph), 1685 (CO, amide), 1622 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.30 (s, 3H, CH3), 3.14–3.23 (m, 8H, morpholine), 3.90 (s, 3H, OCH3), 6.76 (d, 1H, J = 2.34 Hz, furan), 7.33 (s, 1H, pyrimidine), 7.64 (s, 1H, phenyl), 7.80 (d, 1H, J = 2.35 Hz, furan); 13C-NMR (DMSO-d6) δ 23.1 (1C, CH3), 51.5 (2C, 2CH2, morpholine), 55.8 (2C, 2CH2, morpholine), 61.9 (1C, OCH3), 91.5 (1C, CH, phenyl), 99.5 (1C, CH, pyrimidine), 100.7, 105.6, 108.4, 144.7, 146.3, 153.9, 155.2, 160.7, 164.8, 168.9 (10C, Ar-C), 169.8 (1C, C=O); MS (70 eV, %) m/z 366 (M+, 78%); Anal. Calc. (Found) for C19H18N4O4 (366.38): C, 62.29 (62.37); H, 4.95 (4.88); N, 15.29 (15.22).
3.41. Synthesis of 7,11-Dimethoxy-6-methyl-1-(piperazin-1-yl)-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (14c)
The compound was obtained from the reaction of (12b) with piperazine, as yellowish crystals, crystallized from ethanol in 74% yield, m.p. 184 °C. IR (ν, cm−1) KBr: 3395 (br, NH), 3048 (CH-aryl), 2944 (CH-aliph), 1689 (CO, amide), 1624 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.31 (s, 3H, CH3), 2.85–2.94 (m, 8H, piperazine), 3.97 (s, 6H, 2OCH3), 6.81 (d, 1H, J = 2.32 Hz, furan), 7.36 (s, 1H, pyrimidine), 7.84 (d, 1H, J = 2.30 Hz, furan), 10.35 (brs, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ 23.5 (1C, CH3), 48.6 (2C, 2CH2, piperazine), 52.8 (2C, 2CH2, piperazine), 61.8 (2C, 2OCH3), 99.6(1C, CH, pyrimidine), 100.5, 105.7, 108.9, 122.3, 130.2, 146.4, 146.5, 151.8, 154.1, 164.8, 168.5 (10C, Ar-C), 169.7(1C, C=O); MS (70 eV, %) m/z 395 (M+, 76%); Anal. Calc. (Found) for C20H21N5O4 (395.42): C, 60.75 (60.82); H, 5.35 (5.43); N, 17.71 (17.78).
3.42. Synthesis of 7,11-Dimethoxy-6-methyl-1-morpholino-3H-furo[3,2-g]pyrimido[1,6-a]quinazolin-3-one (14d)
The compound was obtained from the reaction of (12b) with morpholine, as yellowish crystals, crystallized from n-hexane in 72% yield, m.p. 156 °C. IR (ν, cm−1) KBr: 3039 (CH-aryl), 2934 (CH-aliph), 1683 (CO, amide), 1626 (C=N). 1H-NMR (DMSO-d6, ppm) δ 2.32 (s, 3H, CH3), 3.16–3.25 (m, 8H, morpholine), 3.97 (s, 6H, 2OCH3), 6.79 (d, 1H, J = 2.33 Hz, furan), 7.37 (s, 1H, pyrimidine), 7.82 (d, 1H, J = 2.37 Hz, furan); 13C-NMR (DMSO-d6) δ 23.7 (1C, CH3), 53.8 (2C, 2CH2, morpholine), 57.6 (2C, 2CH2, morpholine), 61.9 (2C, 2OCH3), 99.8 (1C, CH, pyrimidine), 100.4, 105.3, 108.7, 122.6, 130.1, 146.2, 146.8, 151.3, 154.2, 164.5, 168.6 (11C, Ar-C), 169.4 (1C, C=O); MS (70 eV, %) m/z 396 (M+, 74%); Anal. Calc. (Found) for C20H20N4O5 (396.40): C, 60.60 (60.68); H, 5.09 (5.16); N, 14.13 (14.20).
3.43. Biological Screening
The antimicrobial activity of the newly prepared compounds was tested in vitro against Gram-positive bacteria
Staphylococcus aureus (ATCC
®6538™),
Streptococcus pyogenes (ATCC
®19615™), Gram-negative bacteria
Escherichia coli (ATCC
®25922™),
Klebsiella pneumoniae (ATCC
® 10031™) and the fungi
Aspergillus niger (ATCC
® 16888™),
Alternaria alternate, Curvularia lunata and
Candida albicans (ATCC
®10231™). The newly prepared compounds were dissolved in dimethyl sulfoxide (DMSO) and tested for their antimicrobial activity by the agar disk diffusion technique. Cefotaxime sodium and nystatin [
34,
35] were used as the standard drugs for antibacterial and antifungal assays, respectively. A solution of 100 μg mL
−1 of the tested compound was practical and microplate-wells, 1 cm in diameter, were used. Zones of inhibition were measured with calipers or automated scanners and were paralleled with those of the standards. Cefotaxime sodium (0.15 μmol mL
−1) and nystatin (0.037 μmol mL
−1) were used as the standard drugs for antibacterial and antifungal activity, respectively. Compound-impregnated disks were placed on an agar plate containing a standard suspension of microorganisms. The plate was incubated for 24 h at 37 °C. For the assessment of the minimum inhibitory concentration (MIC) by the serial plate dilution way [
34,
35], 5 mg of each tested compound was dissolved in 1 mL of DMSO separately to prepare stock solutions. Serial dilutions were prepared from each stock solution. The plates were incubated at 37 °C for 24 h. MIC is defined as the lowest concentration (μmol mL
−1) of the tested compound that results in no visible growth on the plates. DMSO was used as the solvent control to ensure that the solvent had no effect on bacterial growth. The results are shown in
Table 1 and
Table 2.