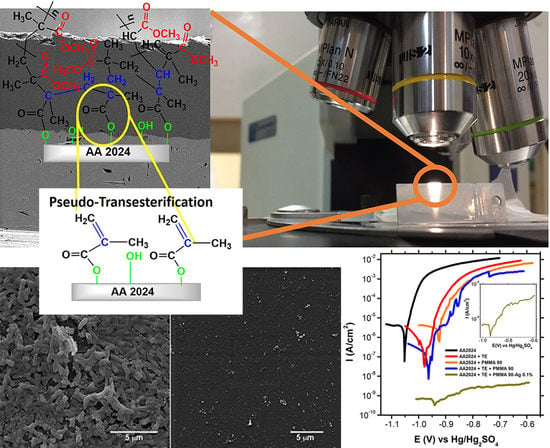
Surface Functionalization of an Aluminum Alloy to Generate an Antibiofilm Coating Based on Poly(Methyl Methacrylate) and Silver Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Functionalized Aluminum Alloy Surface
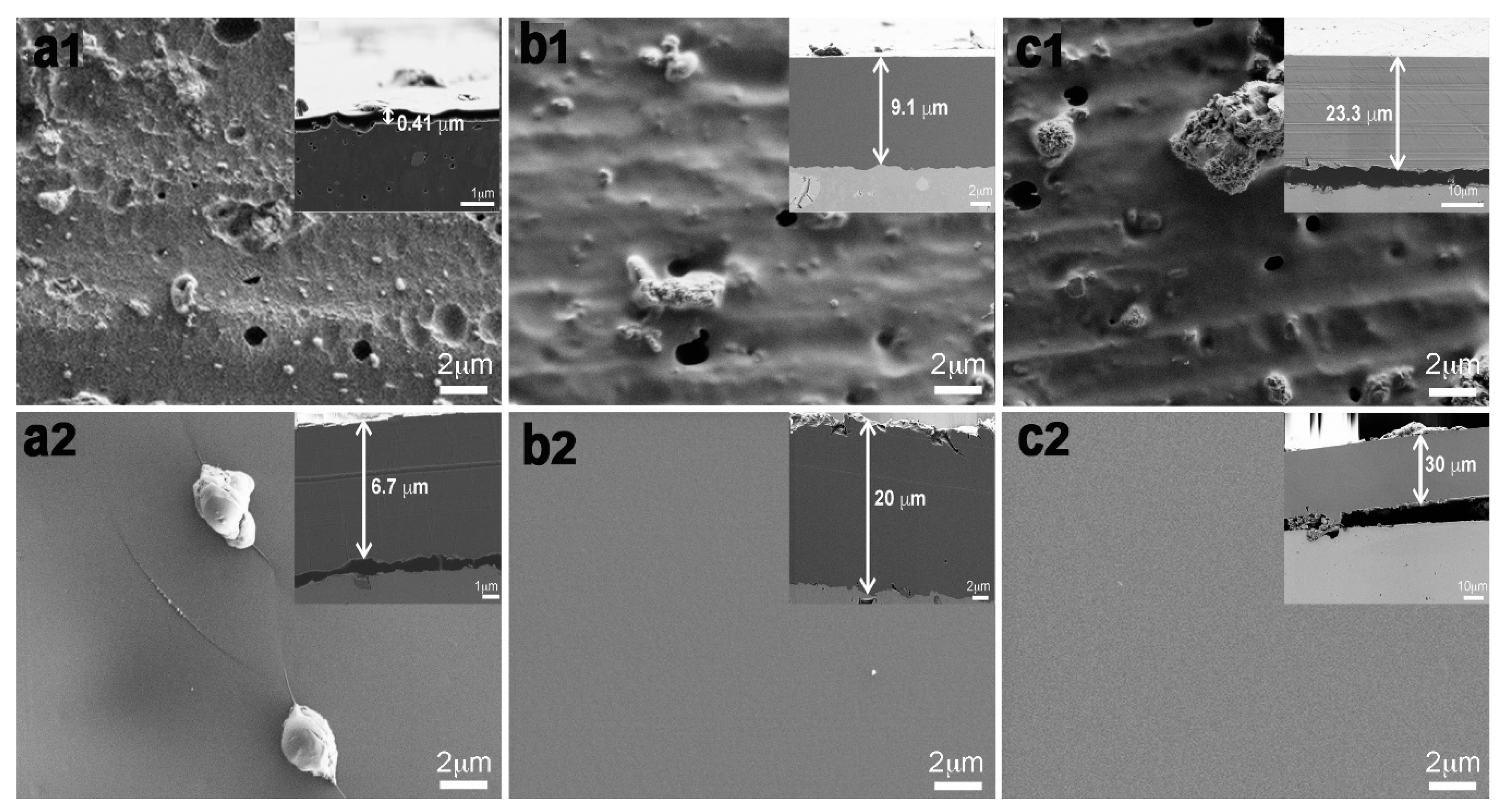
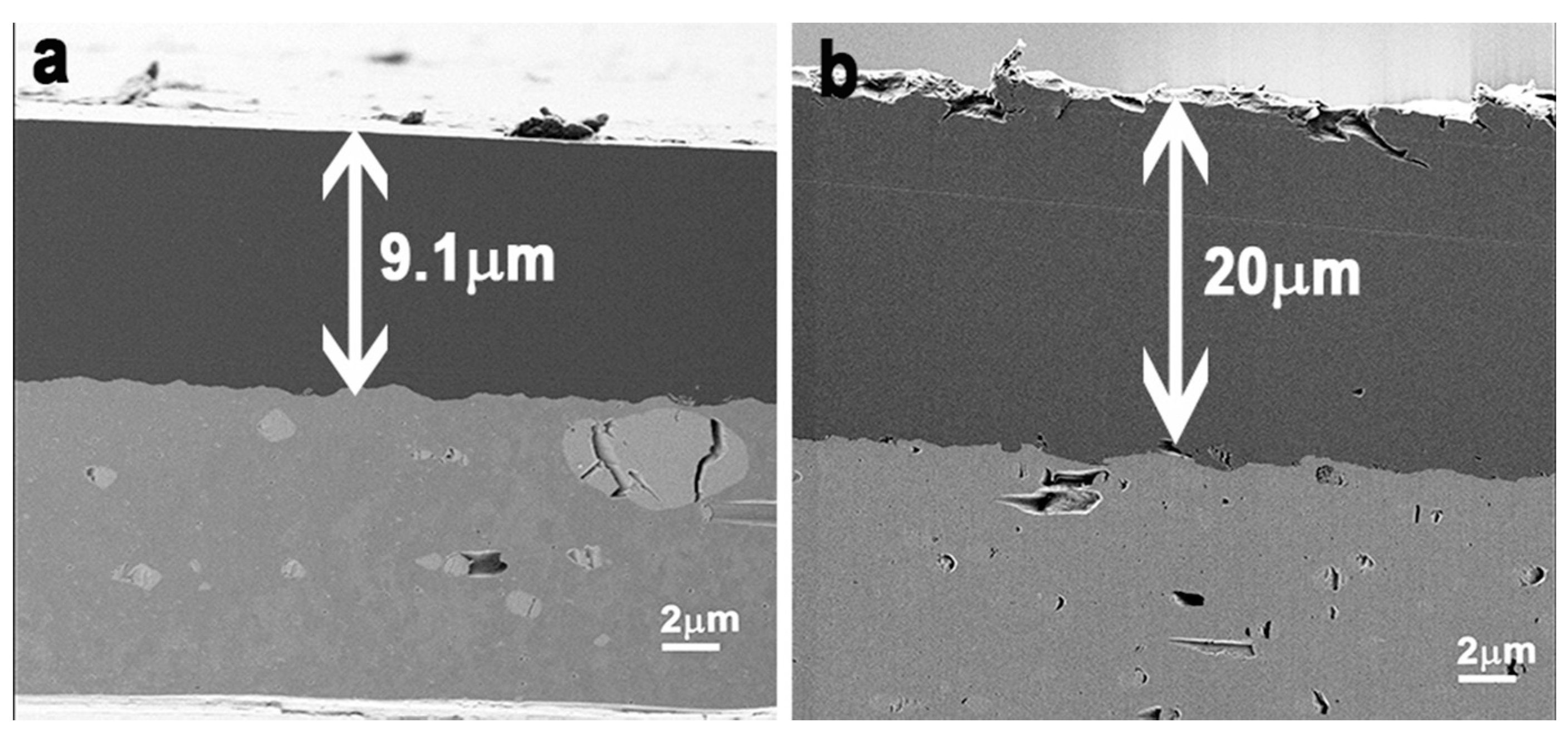
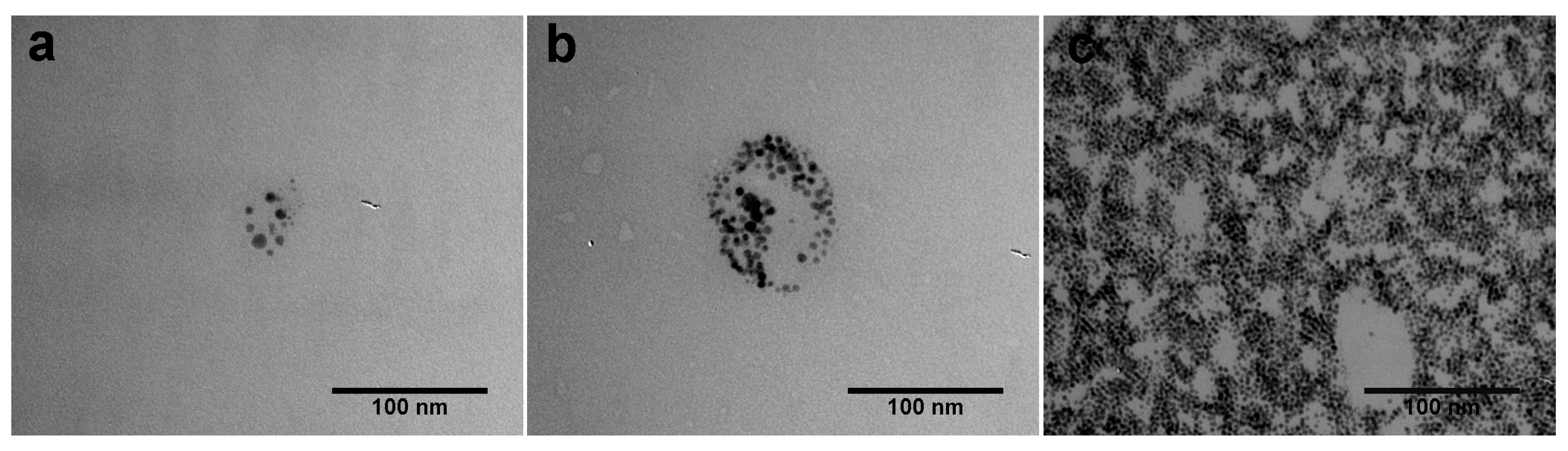
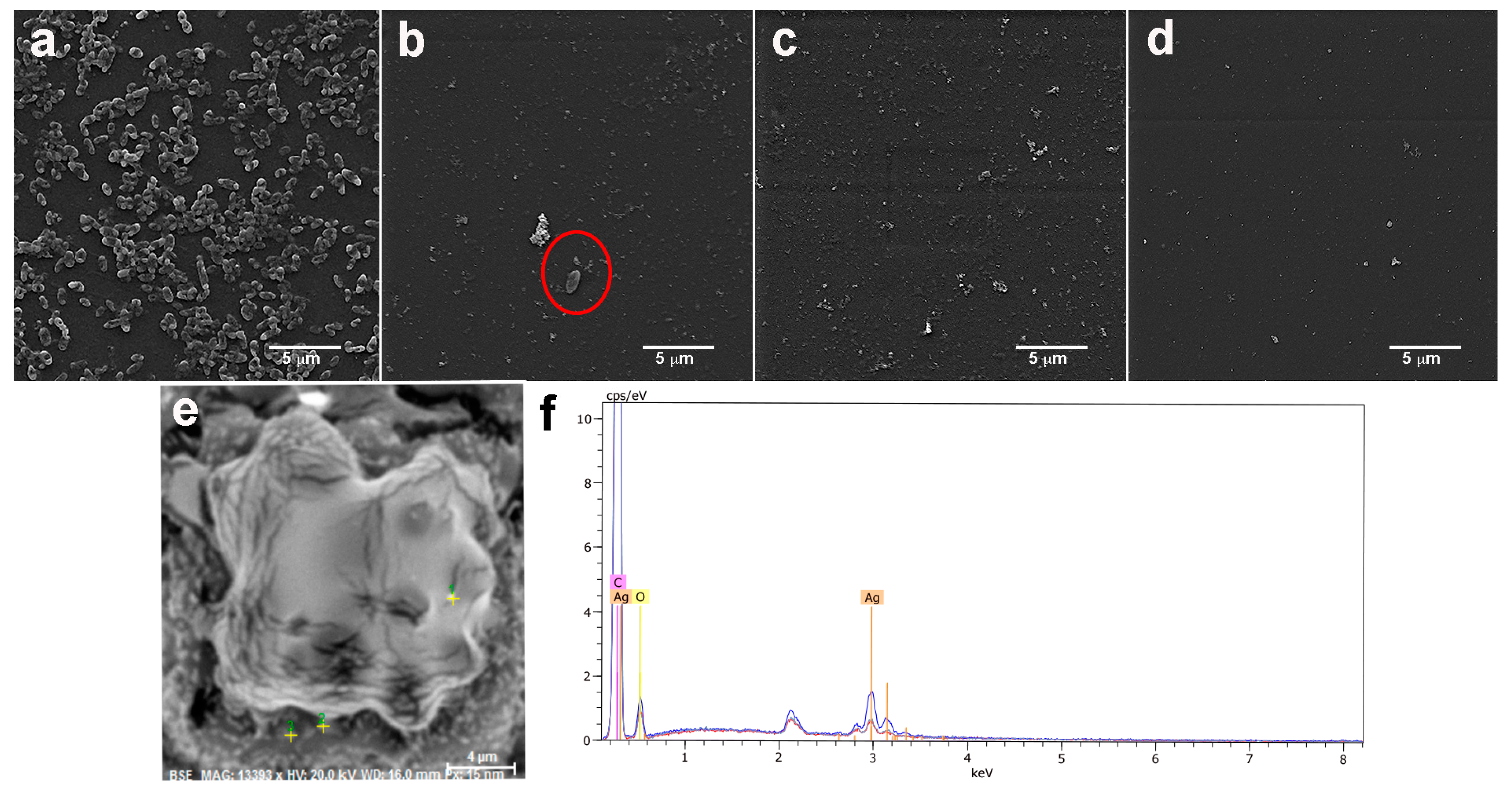
2.2. Morphological Characterization of the Coated Aluminum Alloy
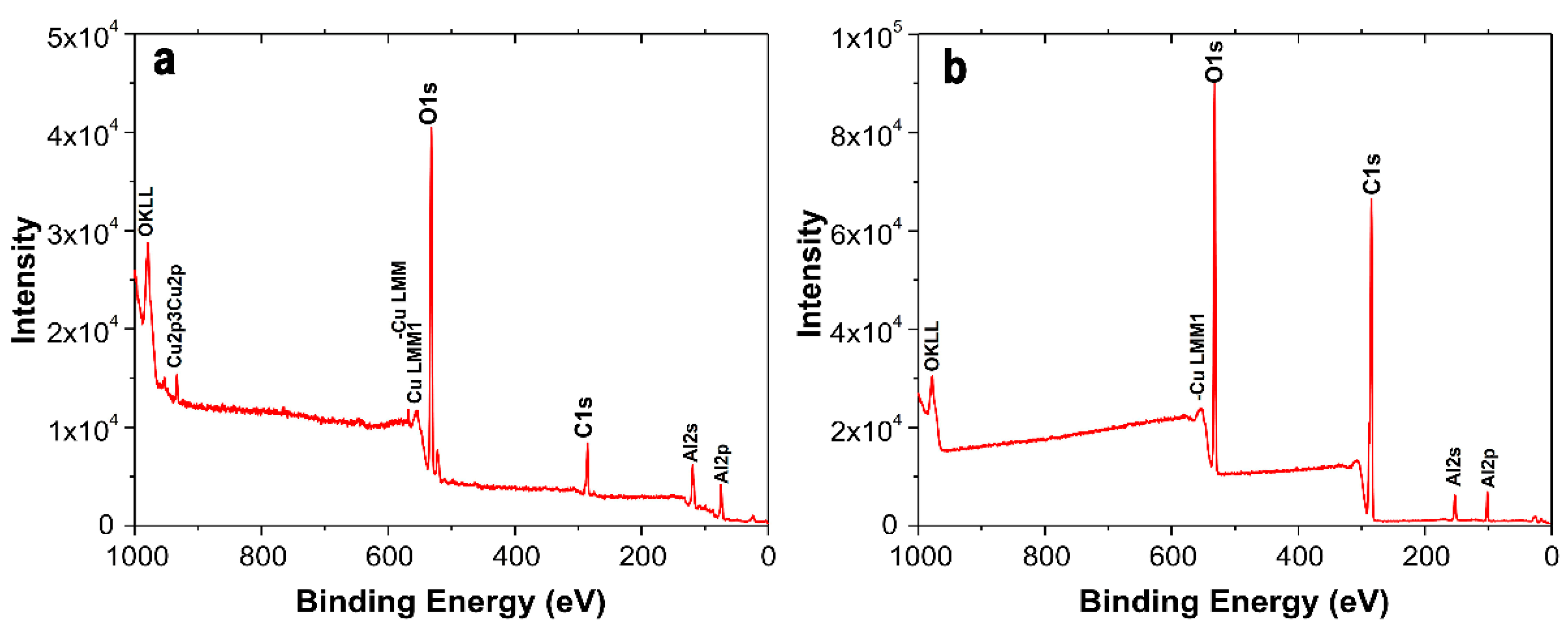
2.3. Characterization of the Coating Surfaces by XPS
2.4. Contact Angle Measurements
2.5. Antibacterial Properties of Coatings
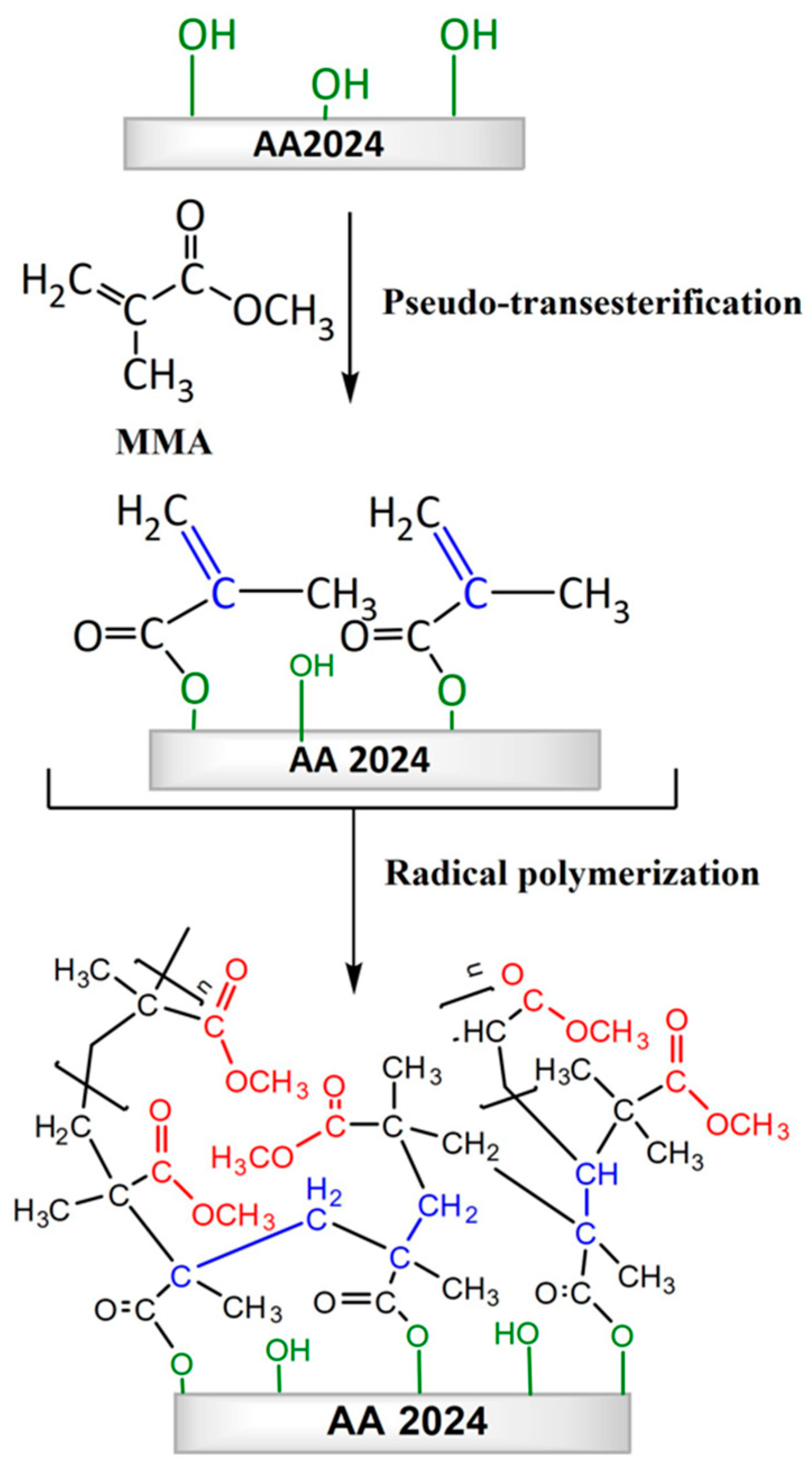
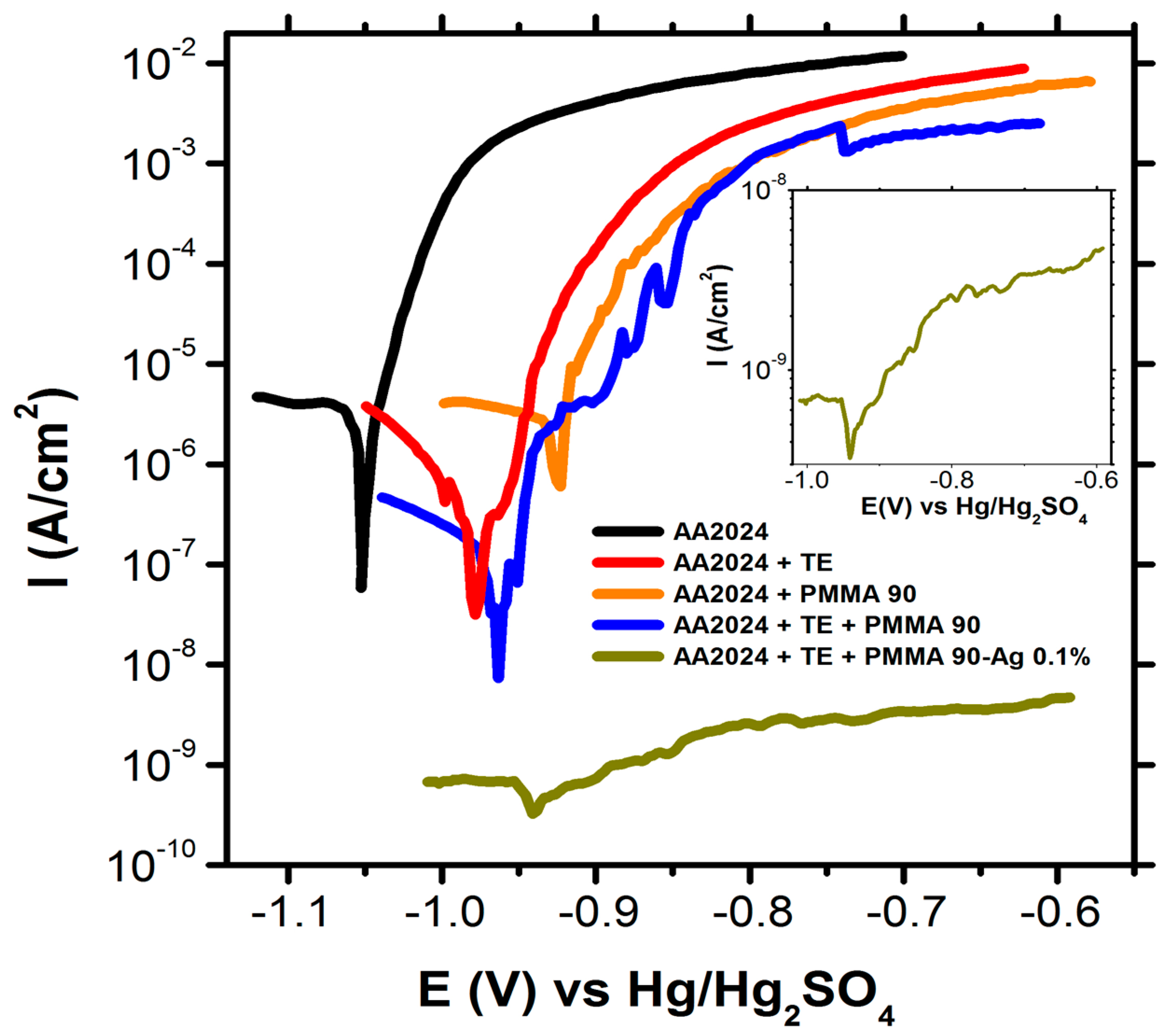
2.6. Effect of Pseudotransesterification on the Protective Capability of Coatings
3. Materials and Methods
3.1. Materials
3.2. Surface Treatment of the AA2024 Aluminum Alloy
3.3. Pseudotransesterification Process
3.4. Polymerization of Methyl Methacrylate on Aluminum Alloy
3.5. AgNPs Synthesis and PMMA-Ag Preparation
3.6. Surface Analysis
3.7. Antibiofilm Assay
3.8. Potentiodynamic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baldan, A. Adhesively-bonded joints and repairs in metallic alloys, polymers and composite materials: Adhesives, adhesion theories and surface pretreatment. J. Mater. Sci. 2004, 39, 1–49. [Google Scholar] [CrossRef]
- Hollaway, L.C.; Cadei, J. Progress in the technique of upgrading metallic structures with advanced polymer composites. Prog. Struct. Eng. Mater. 2002, 4, 131–148. [Google Scholar] [CrossRef]
- Islam, M.S.; Tong, L.; Falzon, P.J. Influence of metal surface preparation on its surface profile, contact angle, surface energy and adhesion with glass fibre prepreg. Int. J. Adhes. Adhes. 2014, 51, 32–41. [Google Scholar] [CrossRef]
- Forsgren, A.; Knudsen, O.Ø. Corrosion Control through Organic Coatings, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 137–147. [Google Scholar]
- Qi, J.T.; Hashimoto, T.; Walton, J.R.; Zhou, X.; Skeldon, P.; Thompson, G.E. Trivalent chromium conversion coating formation on aluminium. Surf. Coat. Technol. 2015, 280, 317–329. [Google Scholar] [CrossRef]
- Svoboda, J.; Kudlacek, J.; Kreibich, V.; Legutko, S. Alternative Methods of Chemical Pre-Treatment on Hot-Dip Galvanization Surface for Adhesion Organic Coatings. In Advances in Manufacturing, 1st ed.; Hamrol, A., Ciszak, O., Legutko, S., Jurczyk, M., Eds.; Springer: Gewerbestrasse, Switzerland, 2018; pp. 687–695. [Google Scholar]
- Iqbal, D.; Rechmann, J.; Sarfraz, A.; Altin, A.; Genchev, G.; Erbe, A. Synthesys of ultrathin Poly (methyl methacrylate) model coatings bound via organosilanes to zinc and investigation of their delamination kinetics. ACS Appl. Mat. Interfaces 2014, 6, 18112–18121. [Google Scholar] [CrossRef] [PubMed]
- Harb, S.V.; Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Siloxane–PMMA hybrid anti-corrosion coatings reinforced by lignin. Surf. Coat. Technol. 2015, 275, 9–16. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, T.X.; Cheng, F.; Cui, W.Z.; Chen, Y. Double-Layer Coating Films Prepared from Water-Borne Latexes of Acrylate-Vinylidene Chloride Copolymers: Investigating Their Heavy-Duty Anticorrosive Properties. Ind. Eng. Chem. Res. 2014, 53, 4534–4543. [Google Scholar] [CrossRef]
- Hidetoshi, Y. Stabilization of the polymer-metal interface. Prog. Org. Coat. 1996, 28, 9–15. [Google Scholar]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Raj, R.M.; Raj, V. Fabrication of superhydrophobic coatings for combating bacterial colonization on Al with relevance to marine and medical applications. J. Coat. Technol. Res. 2018, 15, 51–64. [Google Scholar]
- Wang, Z.; Elimelech, M.; Lin, S. Environmental applications of interfacial materials with special wettability. Environ. Sci. Technol. 2016, 50, 2132–2150. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly (methyl methacrylate) [PMMA]. Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Fang, J.; Wang, C.; Li, Y.; Zhao, Z.; Mei, L. Comparison of bacterial adhesion to dental materials of polyethylene terephthalate (PET) and polymethyl methacrylate (PMMA) using atomic force microscopy and scanning electron microscopy. Scanning 2016, 38, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Lin, W.T.; Tang, T.T. The use of antimicrobial-impregnated PMMA to manage periprosthetic infections: Controversial issues and the latest developments. Int. J. Artif. Organs 2012, 35, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.A.; Zapata, P.A.; Vejar, N.D.; Azócar, M.I.; Gulppi, M.A.; Zhou, X.; Thompson, G.; Páez, M.A. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater. Sci. Eng. C 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.; Acuña, D.; Riveros, A.L.; Kogan, M.J.; Azocar, M.I.; Páez, M.; Leal, M.; Urzúa, M.; Cerda, E. Porous Nanogold/Polyurethane Scaffolds with Improved Antibiofilm, Mechanical, and Thermal Properties and with Reduced Effects on Cell Viability: A Suitable Material for Soft Tissue Applications. ACS Appl. Mater. Interfaces 2018, 10, 13361–13372. [Google Scholar] [CrossRef] [PubMed]
- Lyutakov, O.; Goncharova, I.; Rimpelova, S.; Kolarova, K.; Svanda, J.; Svorcik, V. Silver release and antimicrobial properties of PMMA films doped with silver ions, nano-particles and complexes. Mater. Sci. Eng. C 2015, 49, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Petrochenko, P.E.; Zheng, J.; Casey, B.J.; Bayati, M.R.; Narayan, R.J.; Goering, P.L. Nanosilver-PMMA composite coating optimized to provide robust antibacterial efficacy while minimizing human bone marrow stromal cell toxicity. Toxicol. In Vitro 2017, 44, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Borse, S.; Temgire, M.; Khan, A.; Joshi, S. Photochemically assisted one-pot synthesis of PMMA embedded silver nanoparticles: Antibacterial efficacy and water treatment. RSC Adv. 2016, 6, 56674–56683. [Google Scholar] [CrossRef]
- Azioune, A.; Marcozzi, M.; Revello, V.; Pireaux, J.J. Deposition of polysiloxane-like nanofilms onto an aluminium alloy by plasma polymerized hexamethyldisiloxane: Characterization by XPS and contact angle measurements. Surf. Interface Anal. 2007, 39, 615–623. [Google Scholar] [CrossRef]
- Davies, M.C.; Lynn, R.A.P.; Hearn, J.; Paul, A.J.; Vickerman, J.C.; Watts, J.F. Surface Chemical Characterization Using XPS and TOF-SIMS of Latex Particles Prepared by the Emulsion Copolymerization of Functional Monomers with Methyl Methacrylate and 4-Winylpyridine. Langmuir 1995, 11, 4313–4322. [Google Scholar] [CrossRef]
- Watts, J.F.; Leadley, S.R.; Castle, J.E.; Biomfield, C.J. Adsorption of PMMA on Oxidized Al and Si Substrates: An Investigation by High-Resolution X-ray Photoelectron Spectroscopy. Langmuir 2000, 16, 2292–2300. [Google Scholar] [CrossRef]
- Ozge, O.; Eda, A.A.; Vasif, H.; Nesrin, H. Surface characterization and radical decay studies of oxygen plasma-treated PMMA films. Surf. Interface Anal. 2013, 45, 844–853. [Google Scholar] [CrossRef]
- Amor, B.S.; Baud, G.; Jacquet, M.; Nansé, G.; Fioux, P.; Nardin, M. XPS characterization of plasma-treated and alumina-coated PMMA. Appl. Surf. Sci. 2000, 153, 172–183. [Google Scholar] [CrossRef]
- Beamson, G. Conformation and orientation effects in the X-ray photoelectron spectra of organic polymers. J. Electron. Spectrosc. Relat. Phenom. 2001, 121, 163–181. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.; Liu, P.; Gao, Q.; Sun, Y.; Huang, C. Surface modification of hydrophobic PMMA intraocular lens by the immobilization of hydroxyethyl methacrylate for improving application in ophthalmology. Plasma Chem. Plasma Process. 2011, 31, 811–825. [Google Scholar] [CrossRef]
- DeQueiroz, G.A.; Day, D.F. Antimicrobial activity and effectiveness of a combination of sodium hypochlorite and hydrogen peroxide in killing and removing Pseudomonas aeruginosa biofilms from surfaces. J. Appl. Microbiol. 2007, 103, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Bruzaud, J.; Tarrade, J.; Coudreuse, A.; Canette, A.; Herry, J.M.; de Givenchy, E.T.; Darmanin, T.; Guittard, F.; Guilbaud, M.; Bellon-Fontaine, M.N. Flagella but not type IV pili are involved in the initial adhesion of Pseudomonas aeruginosa PAO1 to hydrophobic or superhydrophobic surfaces. Colloids Surf. B 2015, 131, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-Layer Assembled Nanocontainers for Self-Healing. Corrosion Protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Gonzalez, E.; Pavez, J.; Azocar, I.; Zagal, J.H.; Zhou, X.; Melo, F.; Thompson, G.E.; Páez, M.A. A silanol-based nanocomposite coating for protection of AA-2024 aluminum alloy. Electrochim. Acta 2011, 56, 7586–7595. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Efrima, S. Silver nanoparticles capped by long-chain unsaturated carboxylates. J. Phys. Chem. B 1999, 103, 7238–7246. [Google Scholar] [CrossRef]
Sample Availability: Samples of the PMMA coating and treated aluminum alloy are available from the authors. |
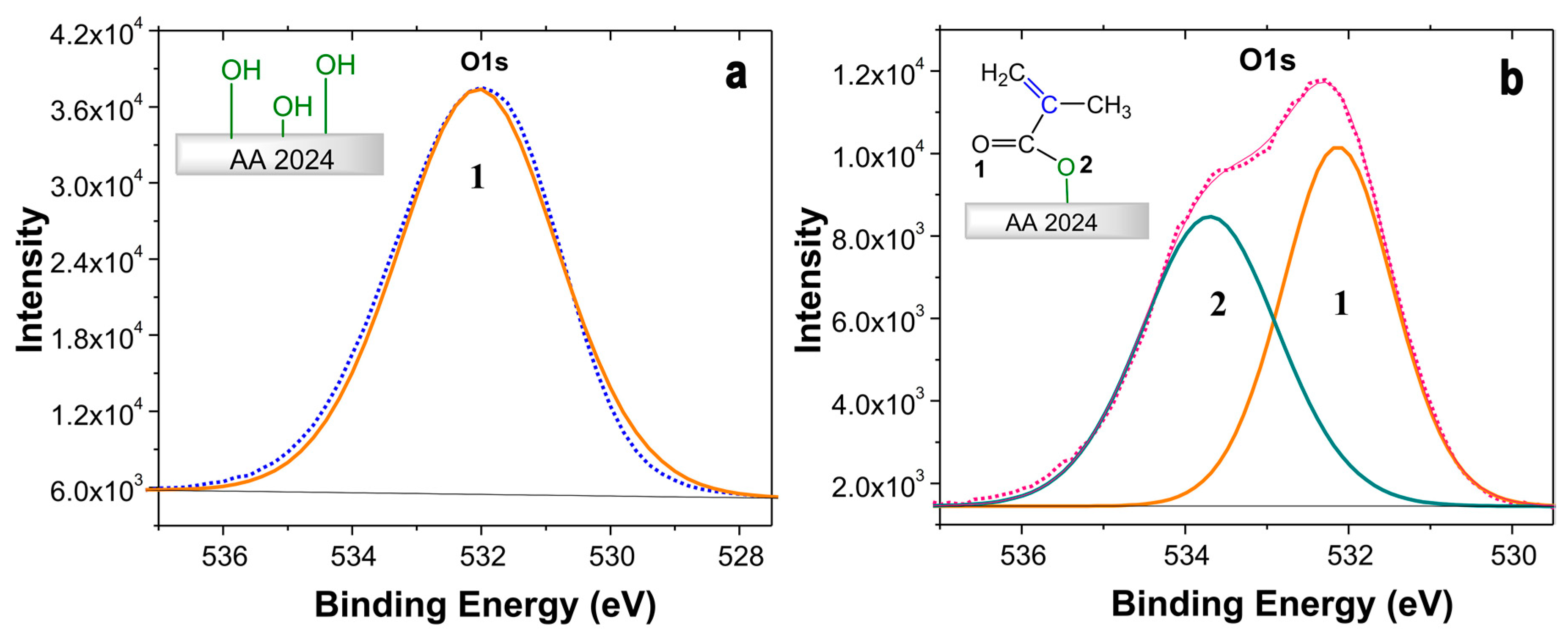
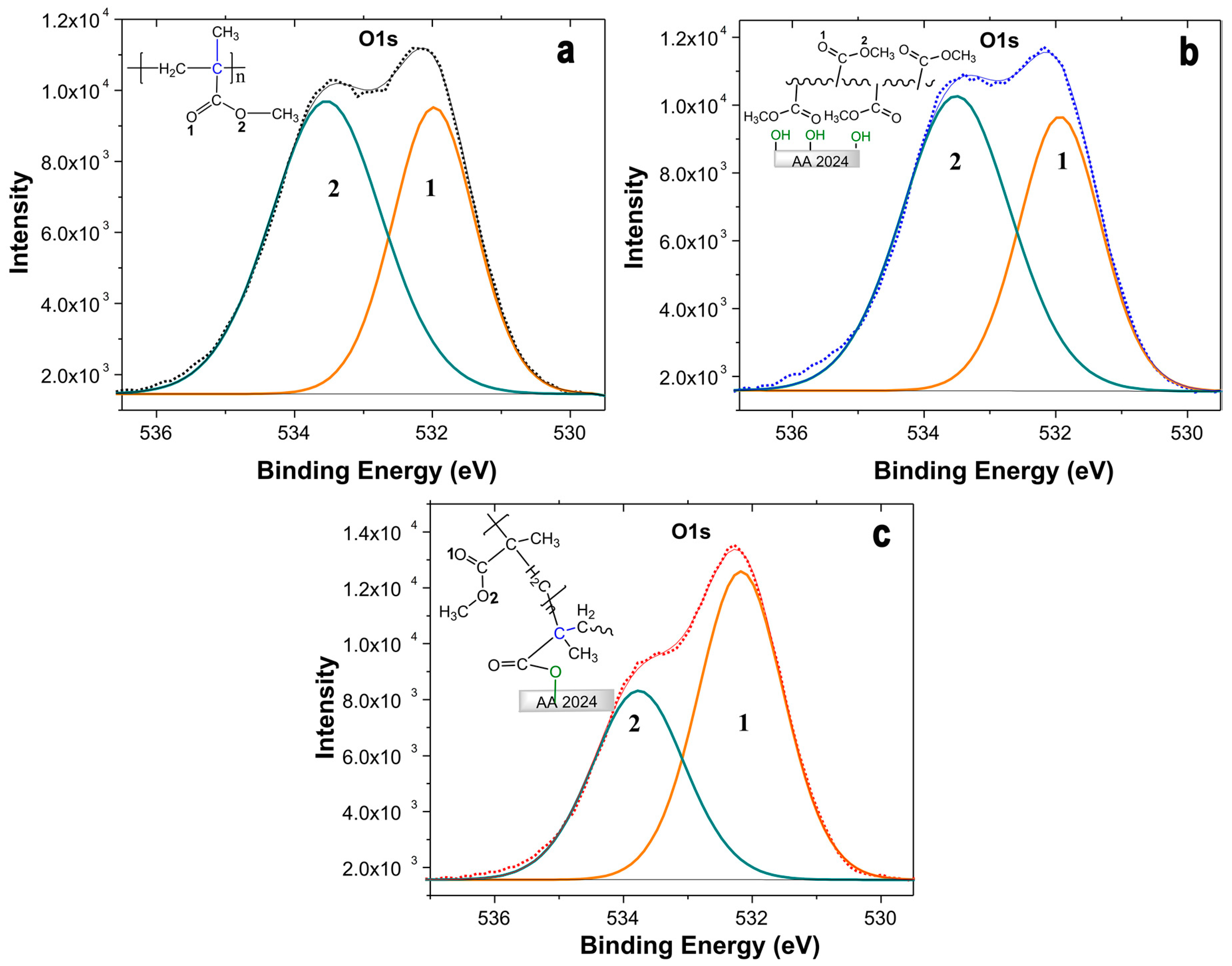


| Sample | O1s (1) O=C (%) | O1s (2) O-C (%) |
|---|---|---|
| PMMA | 45 | 56 |
| AA-PMMA | 42 | 58 |
| AA-TE | 57 | 43 |
| AA-TE-PMMA | 59 | 41 |
| Sample | OCP (V) | I (A/cm2) | IE (%) |
|---|---|---|---|
| AA | −1.06 ± 0.01 | 4.4 × 10−7 | n.d |
| AA + TE | −0.97 ± 0.01 | 4.3 × 10−8 | 90.0 |
| AA + PMMA | −0.93 ± 0.01 | 5.9 × 10−7 | n.d |
| AA + TE + PMMA | −0.95 ± 0.01 | 7.1 × 10−9 | 98.0 |
| AA2024 + TE + PMMA-Ag 0.1 wt% | −0.94 ± 0.02 | 4.1 × 10−10 | 99.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, L.; Tamayo, L.; Gulppi, M.; Rabagliati, F.; Flores, M.; Urzúa, M.; Azócar, M.; Zagal, J.H.; Encinas, M.V.; Zhou, X.; et al. Surface Functionalization of an Aluminum Alloy to Generate an Antibiofilm Coating Based on Poly(Methyl Methacrylate) and Silver Nanoparticles. Molecules 2018, 23, 2747. https://doi.org/10.3390/molecules23112747
Muñoz L, Tamayo L, Gulppi M, Rabagliati F, Flores M, Urzúa M, Azócar M, Zagal JH, Encinas MV, Zhou X, et al. Surface Functionalization of an Aluminum Alloy to Generate an Antibiofilm Coating Based on Poly(Methyl Methacrylate) and Silver Nanoparticles. Molecules. 2018; 23(11):2747. https://doi.org/10.3390/molecules23112747
Chicago/Turabian StyleMuñoz, Lisa, Laura Tamayo, Miguel Gulppi, Franco Rabagliati, Marcos Flores, Marcela Urzúa, Manuel Azócar, Jose H. Zagal, María V. Encinas, Xiaorong Zhou, and et al. 2018. "Surface Functionalization of an Aluminum Alloy to Generate an Antibiofilm Coating Based on Poly(Methyl Methacrylate) and Silver Nanoparticles" Molecules 23, no. 11: 2747. https://doi.org/10.3390/molecules23112747
APA StyleMuñoz, L., Tamayo, L., Gulppi, M., Rabagliati, F., Flores, M., Urzúa, M., Azócar, M., Zagal, J. H., Encinas, M. V., Zhou, X., Thompson, G., & Páez, M. (2018). Surface Functionalization of an Aluminum Alloy to Generate an Antibiofilm Coating Based on Poly(Methyl Methacrylate) and Silver Nanoparticles. Molecules, 23(11), 2747. https://doi.org/10.3390/molecules23112747