Limonene-Based Epoxy: Anhydride Thermoset Reaction Study
Abstract
:1. Introduction
2. Experimental Part
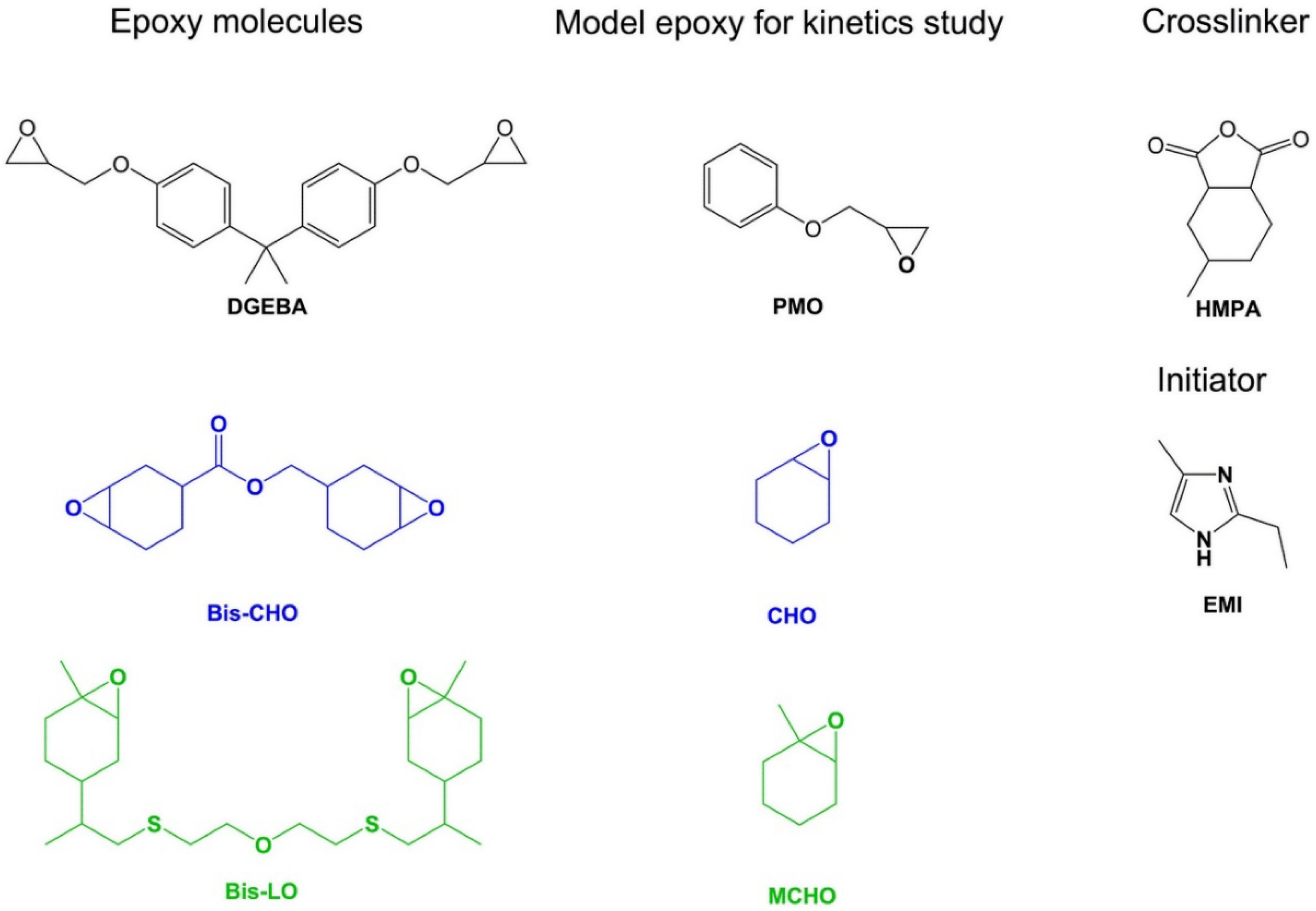
2.1. Materials
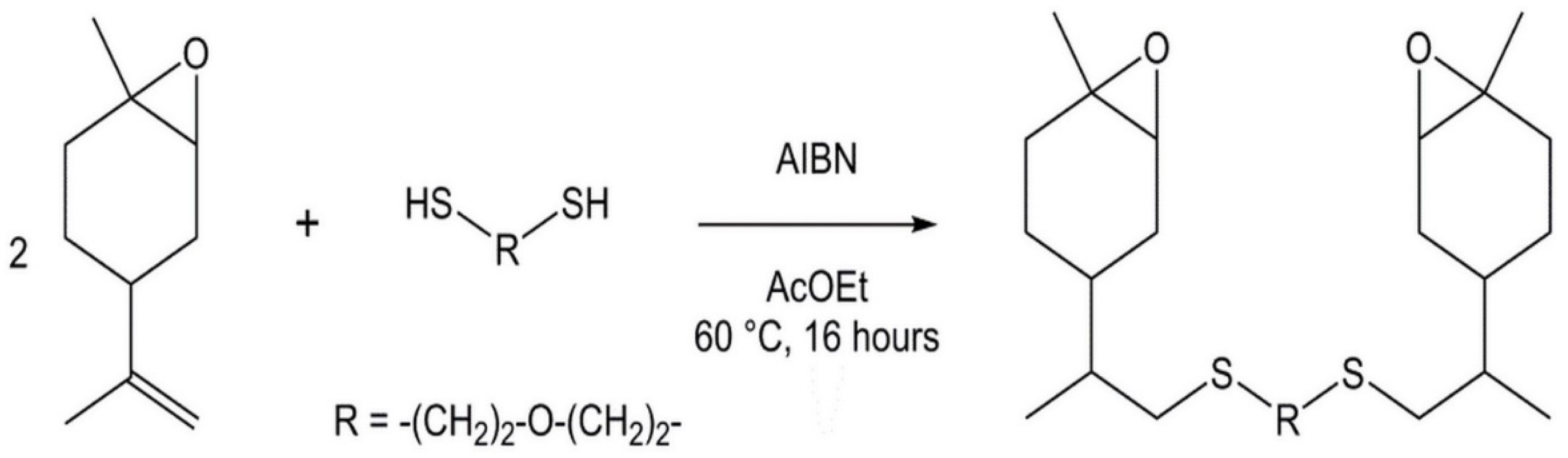
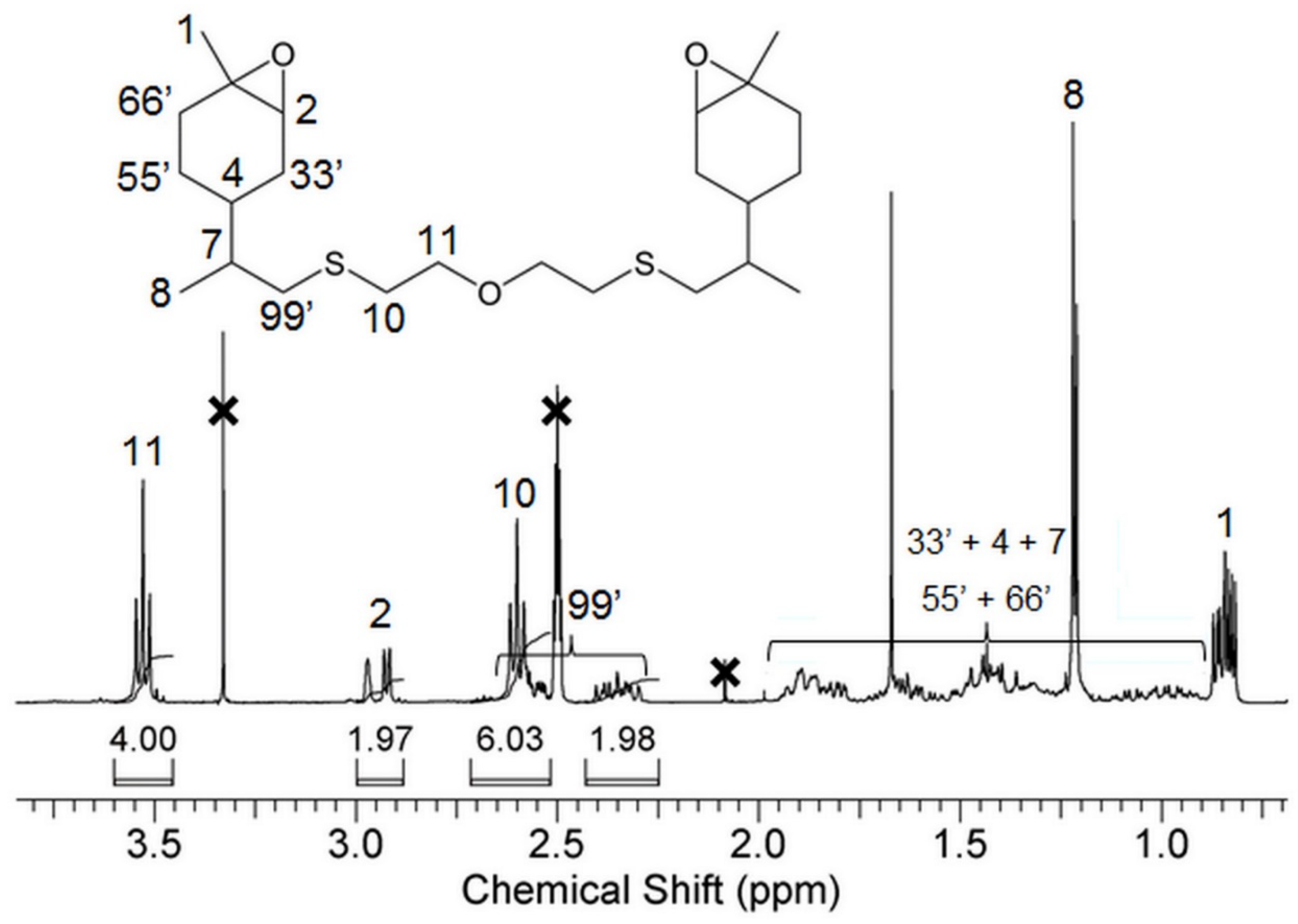
2.2. Synthesis of Bis-LO
2.3. Thermoset Curing
2.4. Characterization Techniques
3. Results and Discussion
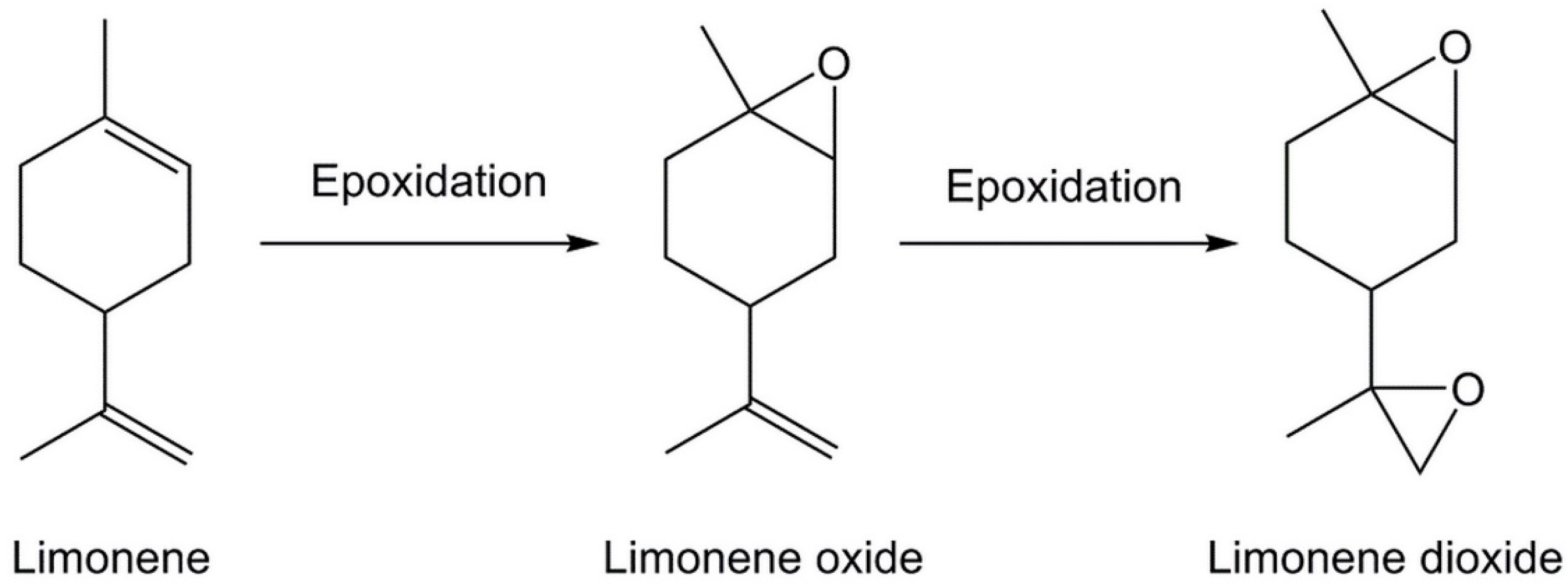
3.1. Synthesis of Bis-Limonene Oxide (Bis-LO)
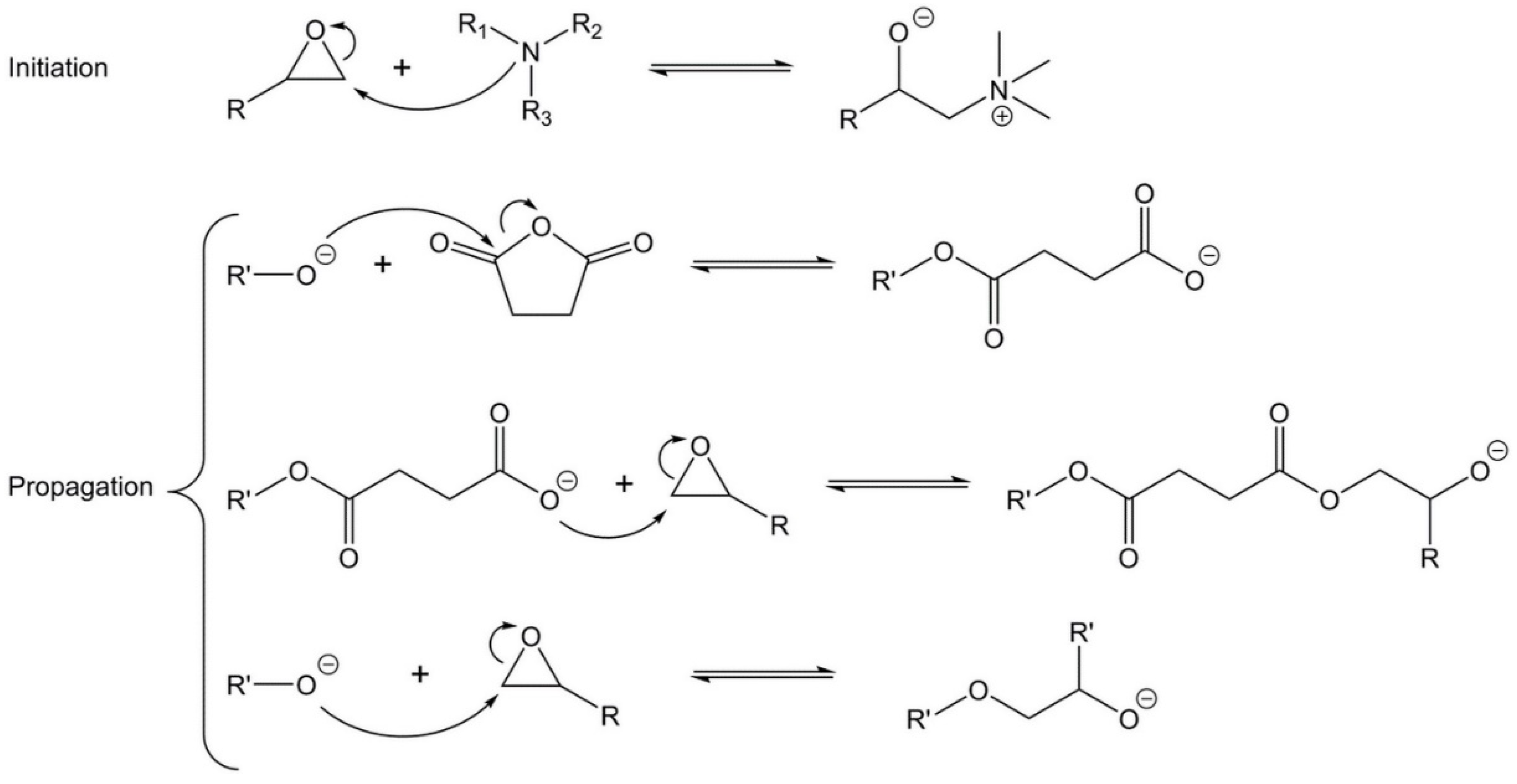
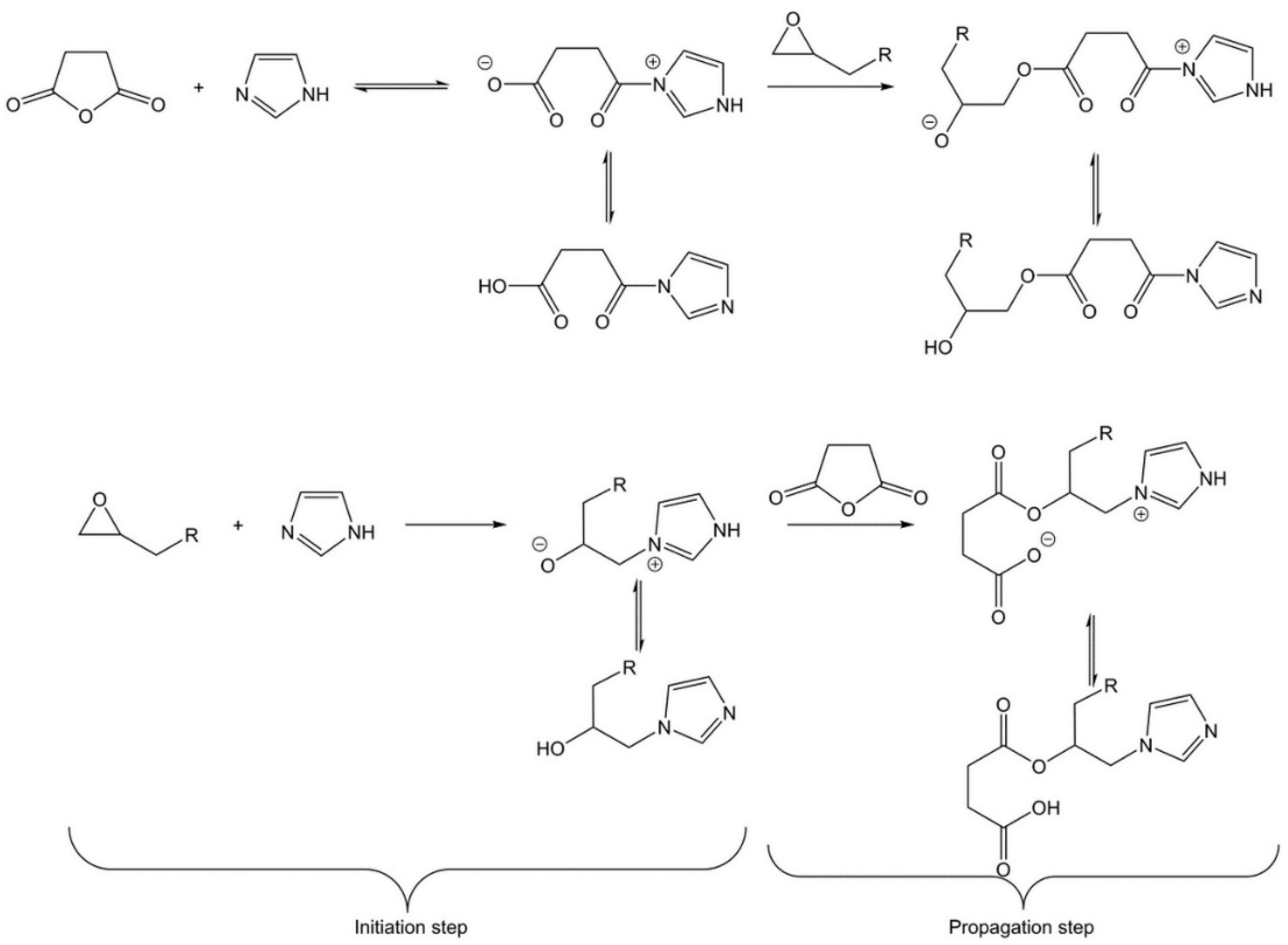
3.2. Kinetics Study on Model Molecules
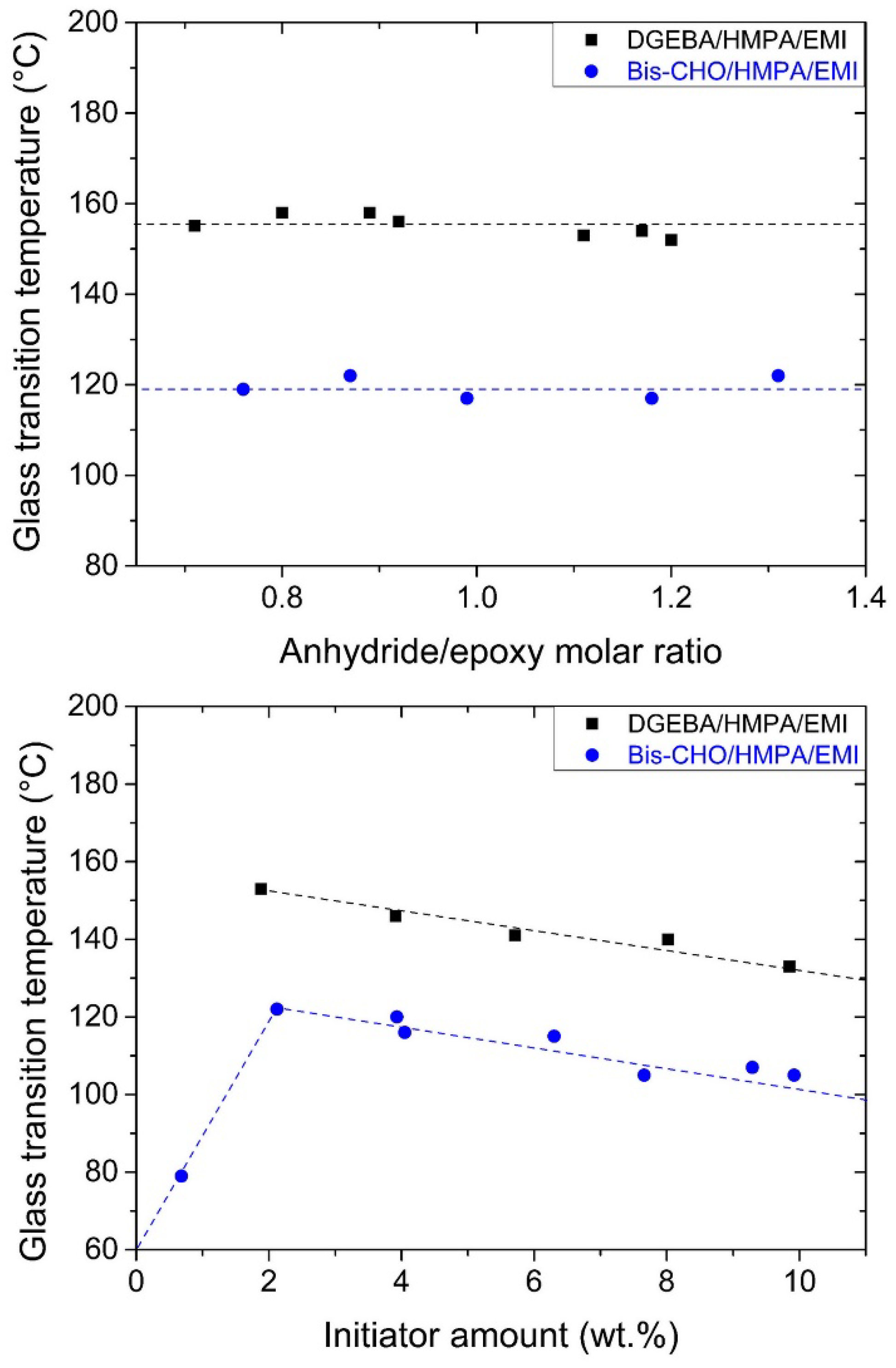
3.3. Influence of Stoichiometry and Initiator Amount on Tg
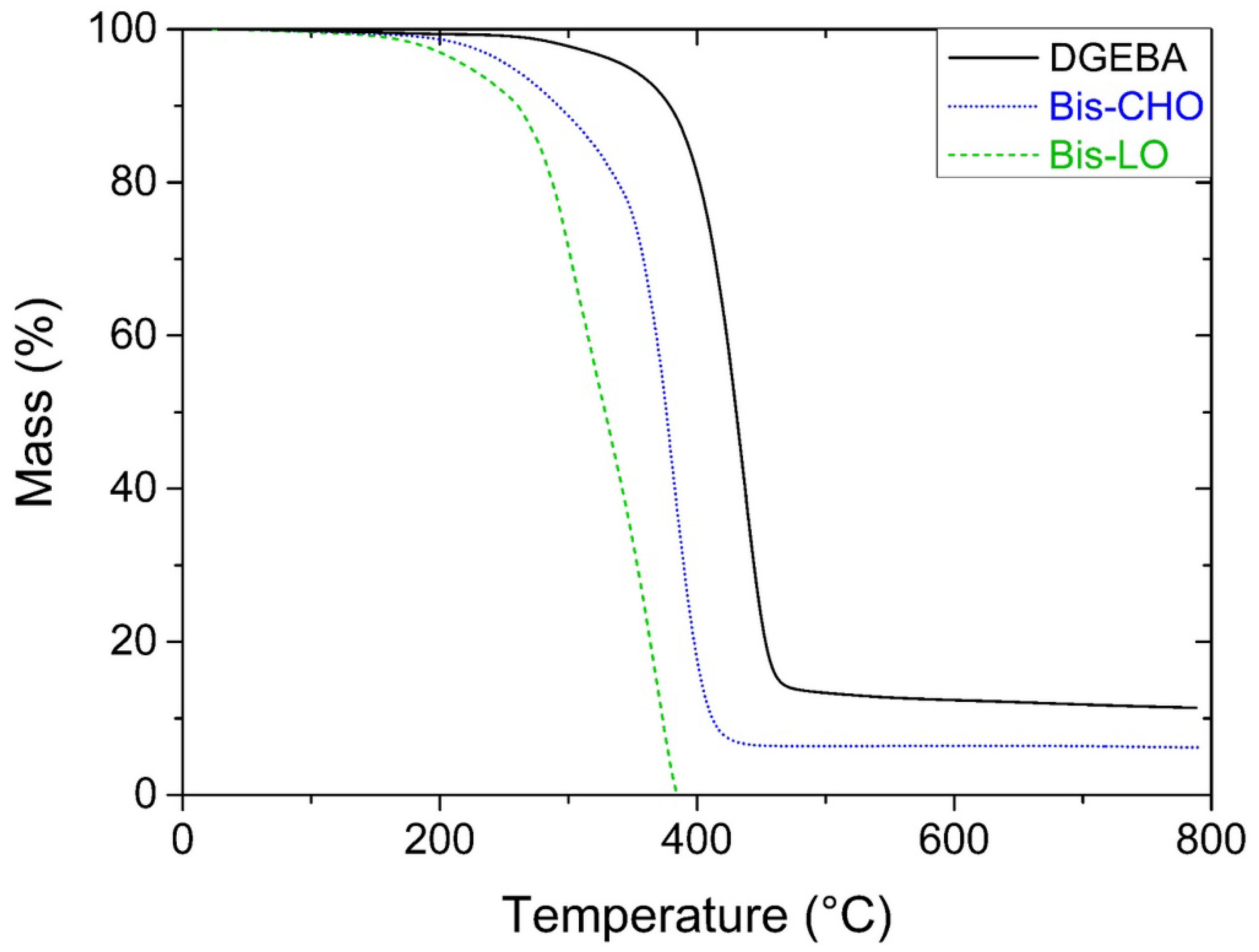
3.4. Thermal Degradation Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Pascault, J.-P.; Williams, R.J.J. Epoxy Polymers: New Materials and Innovations; Wiley-VCH Verlag GmbH Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-based aromatic epoxy monomers for thermoset materials. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Baroncini, E.A.; Kumar Yadav, S.; Palmese, G.R.; Stanzione, J.F. III. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Fache, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New vanillin-derived diepoxy monomers for the synthesis of biobased thermosets. Eur. Polym. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Fache, M.; Viola, A.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased epoxy thermosets from vanillin-derived oligomers. Eur. Polym. J. 2015, 68, 526–535. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. J. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevina, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhang, P.; Wolcott, M.; Zhang, J. Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym. Int. 2014, 63, 760–765. [Google Scholar] [CrossRef]
- Maiorana, A.; Reano, A.F.; Centore, R.; Grimaldi, M.; Balaguer, P.; Allais, F.; Gross, R.A. Structure property relationships of biobased n-alkyl bisferulate epoxy resins. Green Chem. 2016, 18, 4961–4973. [Google Scholar] [CrossRef]
- Chrysanthos, M.; Galy, J.; Pascault, J.-P. Influence of the Bio-Based Epoxy Prepolymer Structure on Network Properties. Macromol. Mater. Eng. 2013, 298, 1209–1219. [Google Scholar]
- Chrysanthos, M.; Galy, J.; Pascault, J.-P. Preparation and properties of bio-based epoxy networks derived from isosorbide diglycidyl ether. Polymer 2011, 52, 3611–3620. [Google Scholar] [CrossRef]
- Nouailhas, H.; Aouf, C.; Guerneve, C.L.; Caillol, S.; Boutevin, B.; Fulcrand, H. Synthesis and properties of biobased epoxy resins. part 1: Glycidylation of flavonoids by epichlorohydrin. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2261–2270. [Google Scholar] [CrossRef]
- Jaillet, F.; Darroman, E.; Ratsimihety, A.; Auvergne, R.; Boutevin, B.; Caillol, S. New biobased epoxy materials from cardanol. Eur. J. Lipid Sci. Technol. 2014, 116, 63–73. [Google Scholar] [CrossRef]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Biermann, U.; Friedt, W.; Siegmund Lang, S.; Wilfried Lühs, W.; Guido Machmüller, G.; Metzger, J.O.; Klaas, M.R.G.; Schäfer, H.J.; Schneider, M.P. New syntheses with oils and fats as renewable raw materials for the chemical industry. Angew. Chem. Int. Ed. 2000, 39, 2206–2224. [Google Scholar] [CrossRef]
- Zahradnik, L.; Tynova, E.; Kalouskova, H. Stable epoxy resins made from renewable nontraditional resources-economically and environmentally acceptable solution. Koroze Ochr. Mater. 2005, 49, 83–86. [Google Scholar]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Guenthner, A.J.; Koontz, T.A.; Storch, P.J.; Reamsc, J.T.; Groshens, T.J. Sustainable hydrophobic thermosetting resins and polycarbonates from turpentine. Green Chem. 2016, 18, 2416–2423. [Google Scholar] [CrossRef]
- Wiemann, L.O.; Faltl, C.; Sieber, V. Lipase-mediated epoxidation of the cyclic monoterpene limonene to limonene oxide and limonene dioxide. Z. Naturforsch. B J. Chem. Sci. 2012, 67, 1056–1060. [Google Scholar] [CrossRef]
- Morinaga, H.; Sakamoto, M. Synthesis of multi-functional epoxides derived from limonene oxide and its application to the network polymers. Tetrahedron Lett. 2017, 58, 2438–2440. [Google Scholar] [CrossRef]
- Yang, S.; Chen, J.S.; Körner, H.; Breiner, T.; Ober, C.K. Reworkable Epoxies: Thermosets with Thermally Cleavable Groups for Controlled Network Breakdown. Chem. Mater. 1998, 10, 1475–1482. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Xiong, L.; Zhao, L. Phosphorus-containing liquid cycloaliphatic epoxy resins for reworkable environment-friendly electronic packaging materials. Polymer 2010, 51, 4776–4783. [Google Scholar] [CrossRef]
- Wang, R.; Schuman, T.P. Vegetable oil-derived epoxy monomers and polymer blends: A comparative study with review. Exp. Polym. Lett. 2013, 7, 272–292. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, C.; Zhang, J.; Cheng, J. The influence of tertiary amine accelerators on the curing behaviors of epoxy/anhydride systems. Thermochim. Acta 2014, 577, 11–16. [Google Scholar] [CrossRef]
- Naumann, S.; Schmidt, F.G.; Speiser, M.; Bohl, M.; Epple, S.; Bonten, C.; Buchmeiser, M.R. Anionic Ring-Opening Homo- and Copolymerization of Lactams by Latent, Protected N-Heterocyclic Carbenes for the Preparation of PA 12 and PA 6/12. Macromolecules 2013, 46, 8426–8433. [Google Scholar] [CrossRef]
- Altmann, H.J.; Naumann, S.; Buchmeiser, M.R. Protected N-heterocyclic carbenes as latent organocatalysts for the low-temperature curing of anhydride-hardened epoxy resins. Eur. Polym. J. 2017, 95, 766–774. [Google Scholar] [CrossRef]
- Amirova, L.R.; Burilov, A.R.; Amirova, L.M.; Bauer, I.; Habicher, W.D. Kinetics and mechanistic investigation of epoxy-anhydride compositions cured with quaternary phosphonium salts as accelerators. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1088–1097. [Google Scholar] [CrossRef]
- Pinazo, J.M.; Domine, M.E.; Parvulescu, V.; Petru, F. Sustainability metrics for succinic acid production: A comparison between biomass-based and petrochemical routes. Catal. Today 2015, 239, 17–24. [Google Scholar] [CrossRef]
- Lin, Z.; Ierapetritou, M.; Nikolakis, V. Phthalic anhydride production from hemicellulose solutions: Technoeconomic analysis and life cycle assessment. AIChE J. 2015, 61, 3708–3718. [Google Scholar] [CrossRef]
- Park, W.H.; Lee, J.K.; Kwon, K.J. Cure behavior of an epoxy-anhydride-imidazole system. Polym. J. 1996, 28, 407–411. [Google Scholar] [CrossRef]
- Matejka, L.; Lovy, J.; Pokorny, S.; Bouchal, K.; Dusek, K. Curing epoxy resins with anhydrides. Model reactions and reaction mechanism. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 2873–2885. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chiu, W.-Y.; Lin, K.-F. Kinetics study of imidazole-cured epoxy-phenol resins. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3233–3242. [Google Scholar] [CrossRef]
- Antoon, M.K.; Koenig, J.L. Crosslinking mechanism of an anhydride-cured epoxy resin as studied by Fourier transform infrared spectroscopy. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 549–570. [Google Scholar] [CrossRef]
- Matejka, L.; Pokorny, S.; Dusek, K. Acid curing of epoxy resins. A comparison between the polymerization of diepoxide-diacid and monoepoxide-cyclic anhydride systems. Makromol. Chem. 1985, 186, 2025–2036. [Google Scholar] [CrossRef]
- Steinmann, B. Investigations on the curing of epoxy resins with hexahydrophthalic anhydride. J. Appl. Polym. Sci. 1989, 37, 1753–1776. [Google Scholar] [CrossRef]
- Steinmann, B. Investigations on the curing of epoxides with phthalic anhydride. J. Appl. Polym. Sci. 1990, 39, 2005–2026. [Google Scholar] [CrossRef]
- Paramarta, A.; Webster, D.C. Curing kinetics of bio-based epoxy-anhydride thermosets with zinc catalyst. J. Therm. Anal. Calorim. 2017, 130, 2133–2144. [Google Scholar] [CrossRef]
- Kuncho, C.N.; Schmidt, D.F.; Reynaud, E. Effects of Catalyst Content, Anhydride Blending, and Nanofiller Addition on Anhydride-Cured Epoxidized Linseed Oil-Based Thermosets. Ind. Eng. Chem. Res. 2017, 56, 2658–2666. [Google Scholar] [CrossRef]
- Woo, E.M.; Seferis, J.C. Cure kinetics of epoxy/anhydride thermosetting matrix systems. J. Appl. Polym. Sci. 1990, 40, 1237–1256. [Google Scholar] [CrossRef]
- Zou, Q.; Ba, L.; Tan, X.; Tu, M.; Cheng, J.; Zhang, J. Tunable shape memory properties of rigid-flexible epoxy networks. J. Mater. Sci. 2016, 51, 10596–10607. [Google Scholar] [CrossRef]
- Kretzschmar, K.; Hoffmann, K.W. Reaction enthalpies during the curing of epoxy resins with anhydrides. Thermochim. Acta 1985, 94, 105–112. [Google Scholar] [CrossRef]
- Simon, P. Isoconversional methods-fundamentals, meaning and application. J. Therm. Anal. Calorim. 2004, 76, 123–132. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Harsch, M.; Karger-Kocsis, J.; Holst, M. Influence of fillers and additives on the cure kinetics of an epoxy/anhydride resin. Eur. Polym. J. 2007, 43, 1168–1178. [Google Scholar] [CrossRef]
- Santiago, D.; Fernandez-Francos, X.; Ramis, X.; Salla, J.M.; Sangermano, M. Comparative curing kinetics and thermal-mechanical properties of DGEBA thermosets cured with a hyperbranched poly(ethyleneimine) and an aliphatic triamine. Thermochim. Acta 2011, 526, 9–21. [Google Scholar] [CrossRef]
- Leukel, J.; Burchard, W.; Krüger, R.P.; Much, H.; Schulz, G. Mechanism of the anionic copolymerization of anhydride-cured epoxies–analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Macromol. Rapid Commun. 1996, 17, 359–366. [Google Scholar] [CrossRef]
Sample Availability: not available. |
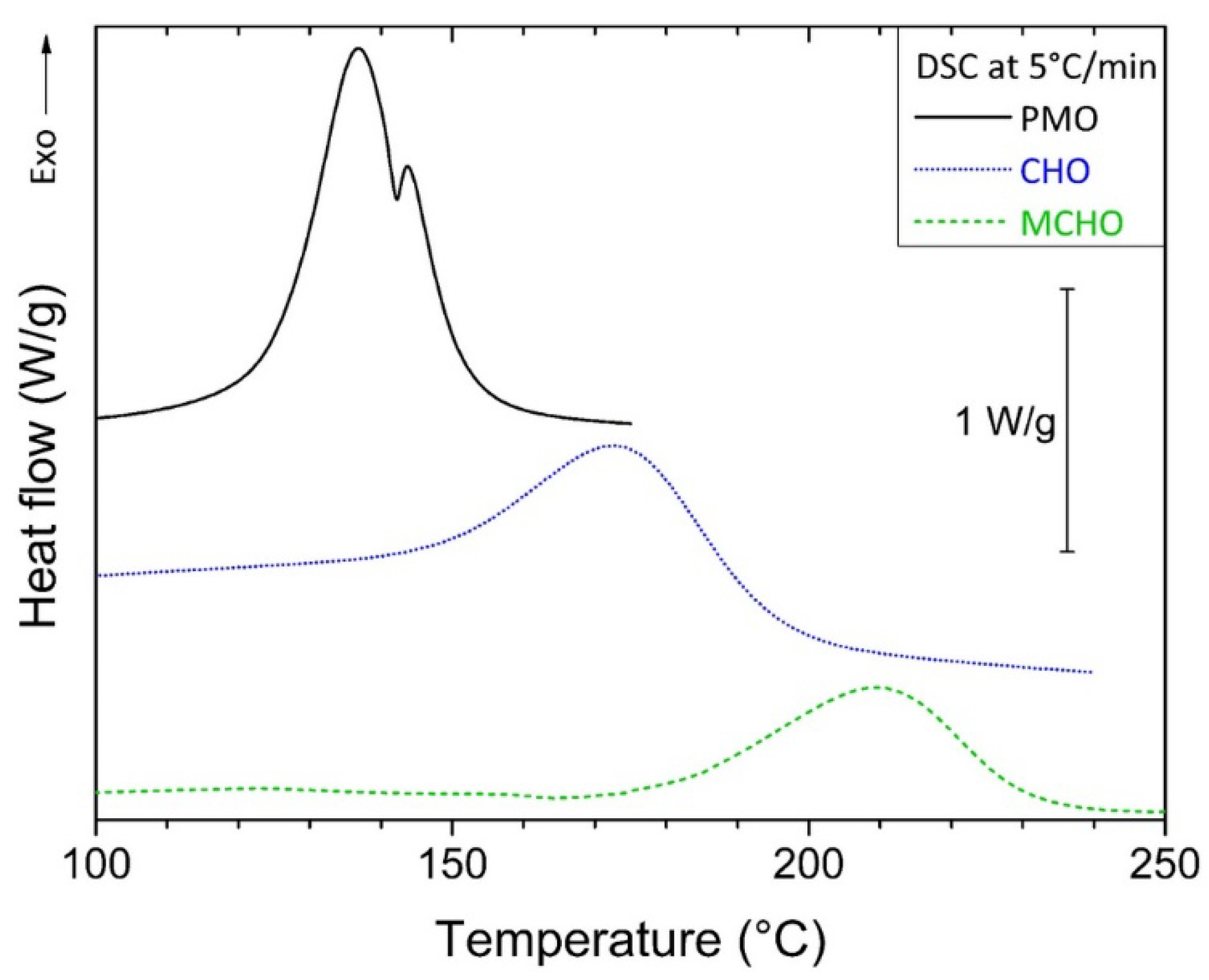
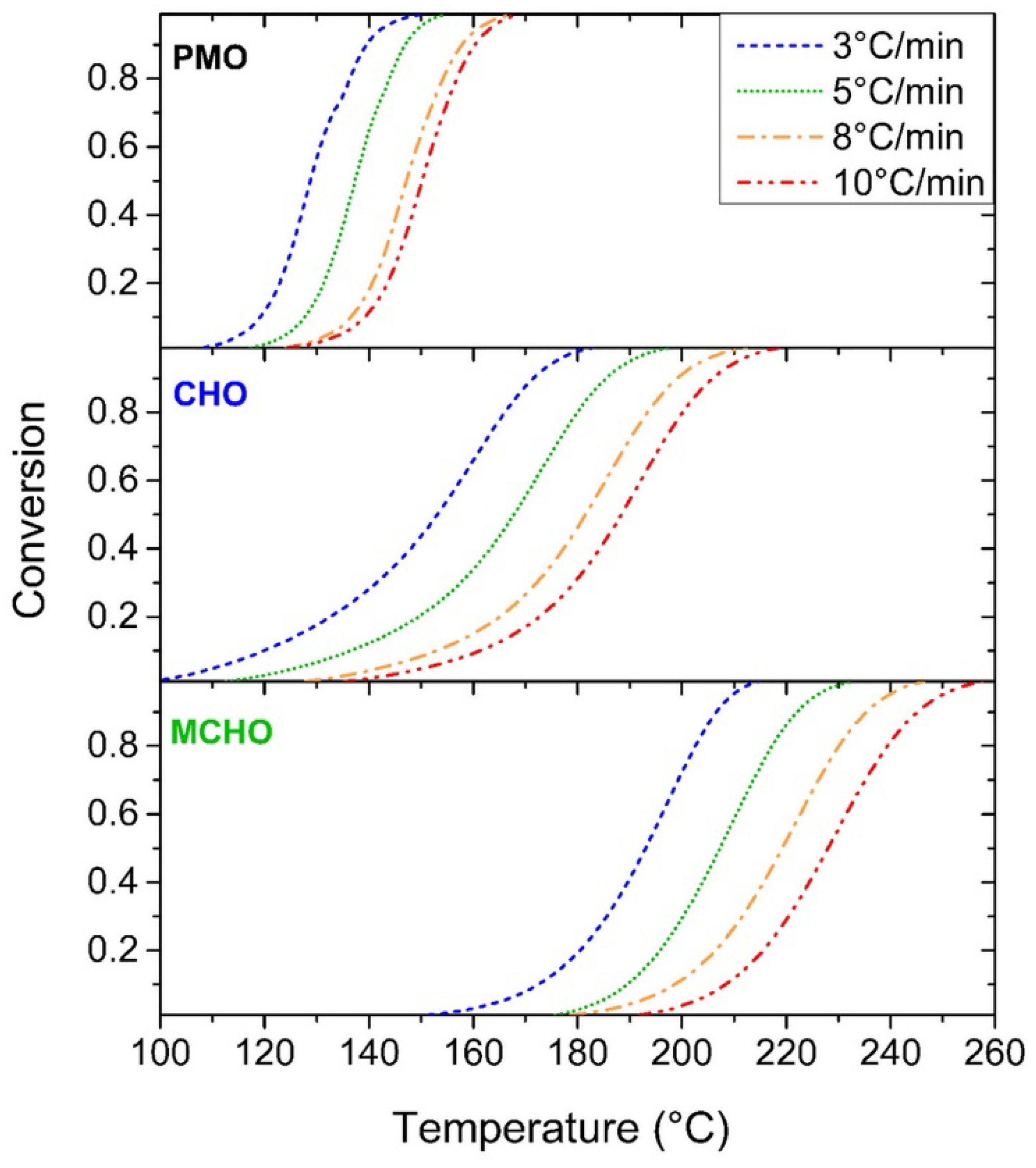
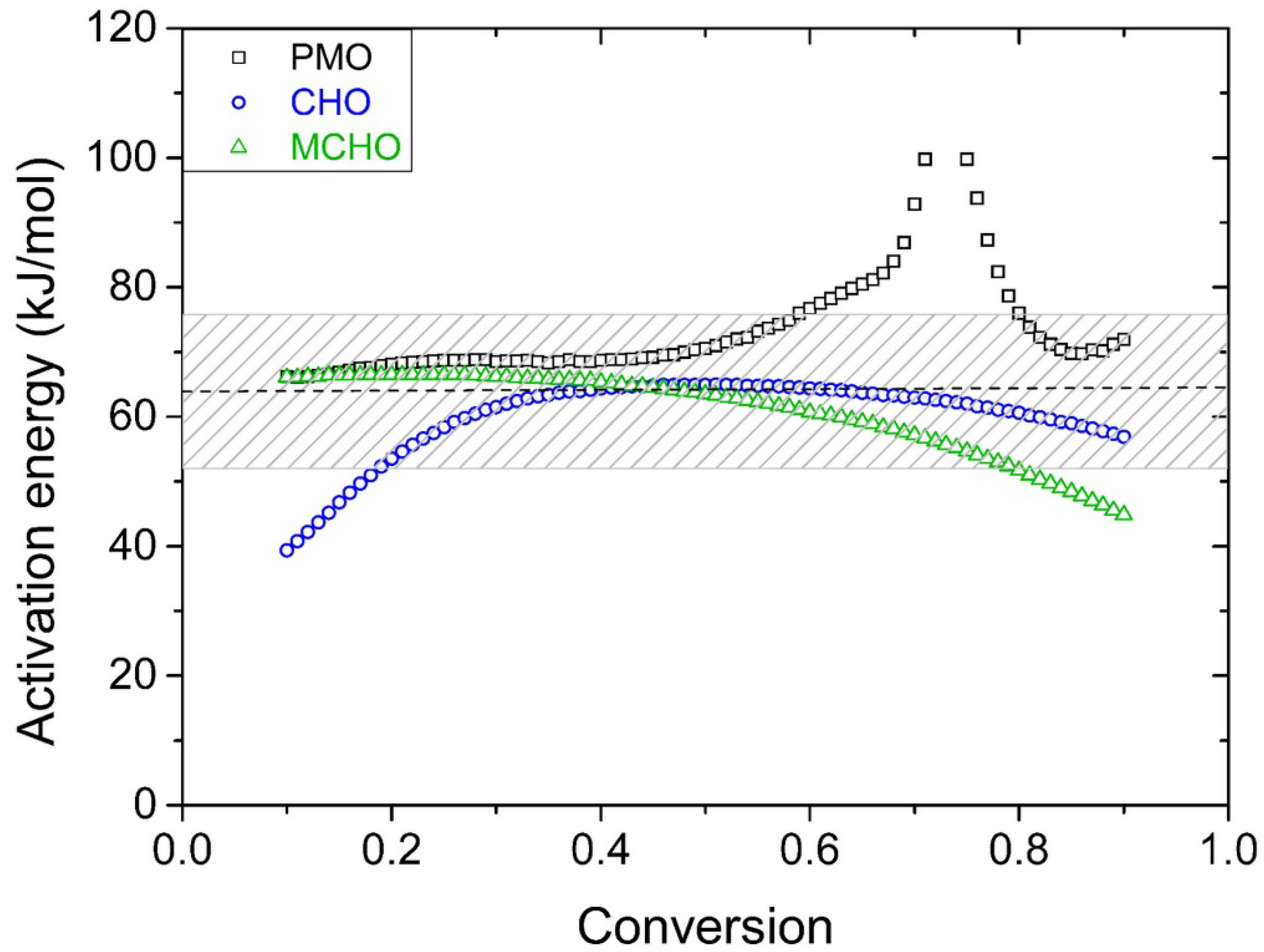
| β (°C/min) | ΔHTOTAL (J/g) | Peak Onset (°C) | ||||
|---|---|---|---|---|---|---|
| PMO | CHO | MCHO | PMO | CHO | MCHO | |
| 3 | 287.9 | 312.8 | 214.4 | 118 | 137 | 170 |
| 5 | 284.9 | 320.8 | 170.0 | 126 | 149 | 174 |
| 8 | 281.9 | 327.1 | 185.3 | 136 | 160 | 193 |
| 10 | 259.5 | 313.1 | 158.1 | 137 | 166 | 201 |
| Averages | 278.6 | 318.5 | 182.0 | |||
| Deviations | 12.9 | 6.8 | 24.3 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couture, G.; Granado, L.; Fanget, F.; Boutevin, B.; Caillol, S. Limonene-Based Epoxy: Anhydride Thermoset Reaction Study. Molecules 2018, 23, 2739. https://doi.org/10.3390/molecules23112739
Couture G, Granado L, Fanget F, Boutevin B, Caillol S. Limonene-Based Epoxy: Anhydride Thermoset Reaction Study. Molecules. 2018; 23(11):2739. https://doi.org/10.3390/molecules23112739
Chicago/Turabian StyleCouture, Guillaume, Lérys Granado, Florent Fanget, Bernard Boutevin, and Sylvain Caillol. 2018. "Limonene-Based Epoxy: Anhydride Thermoset Reaction Study" Molecules 23, no. 11: 2739. https://doi.org/10.3390/molecules23112739
APA StyleCouture, G., Granado, L., Fanget, F., Boutevin, B., & Caillol, S. (2018). Limonene-Based Epoxy: Anhydride Thermoset Reaction Study. Molecules, 23(11), 2739. https://doi.org/10.3390/molecules23112739