Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Artichoke Ethanolic Extract
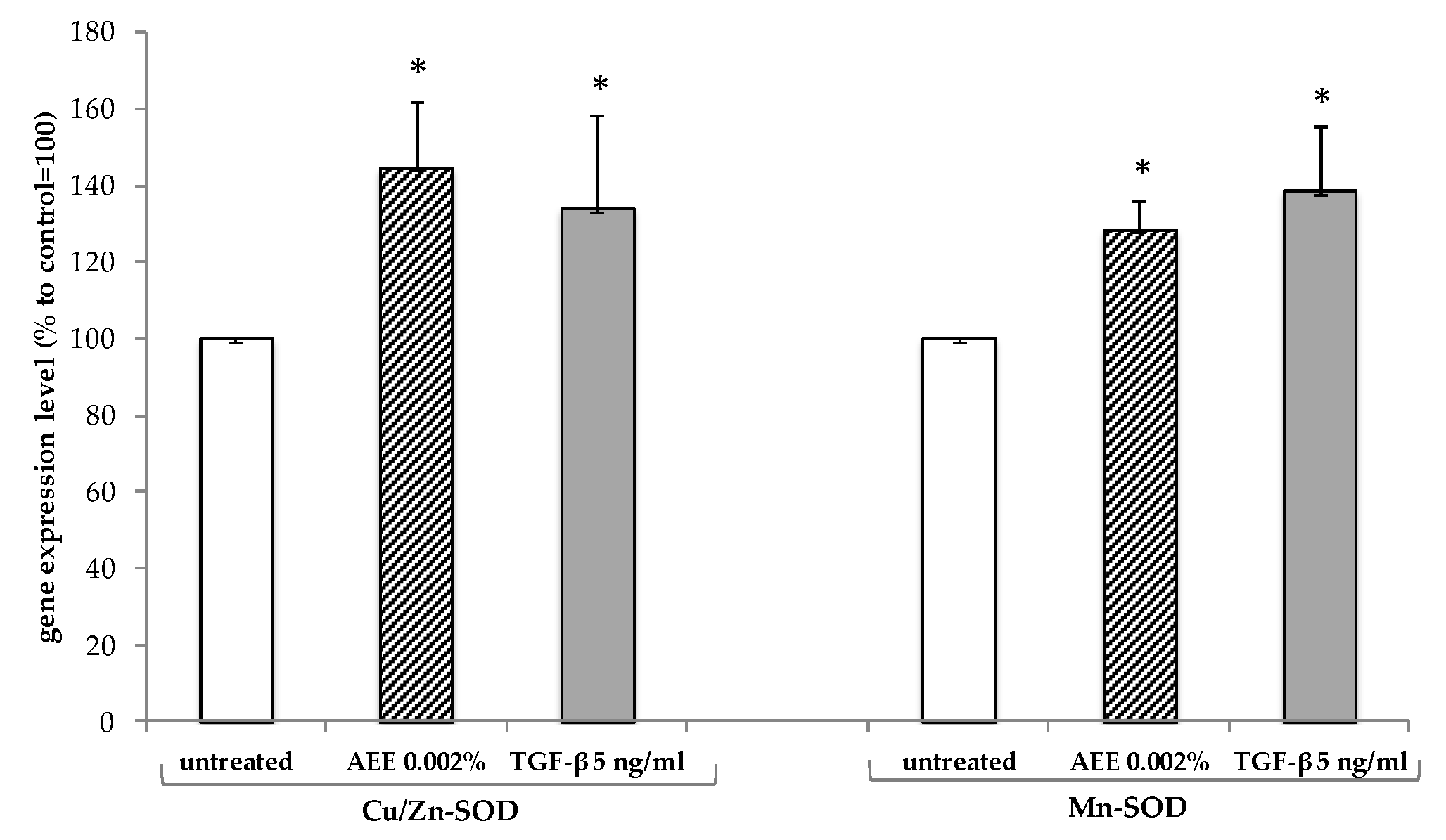
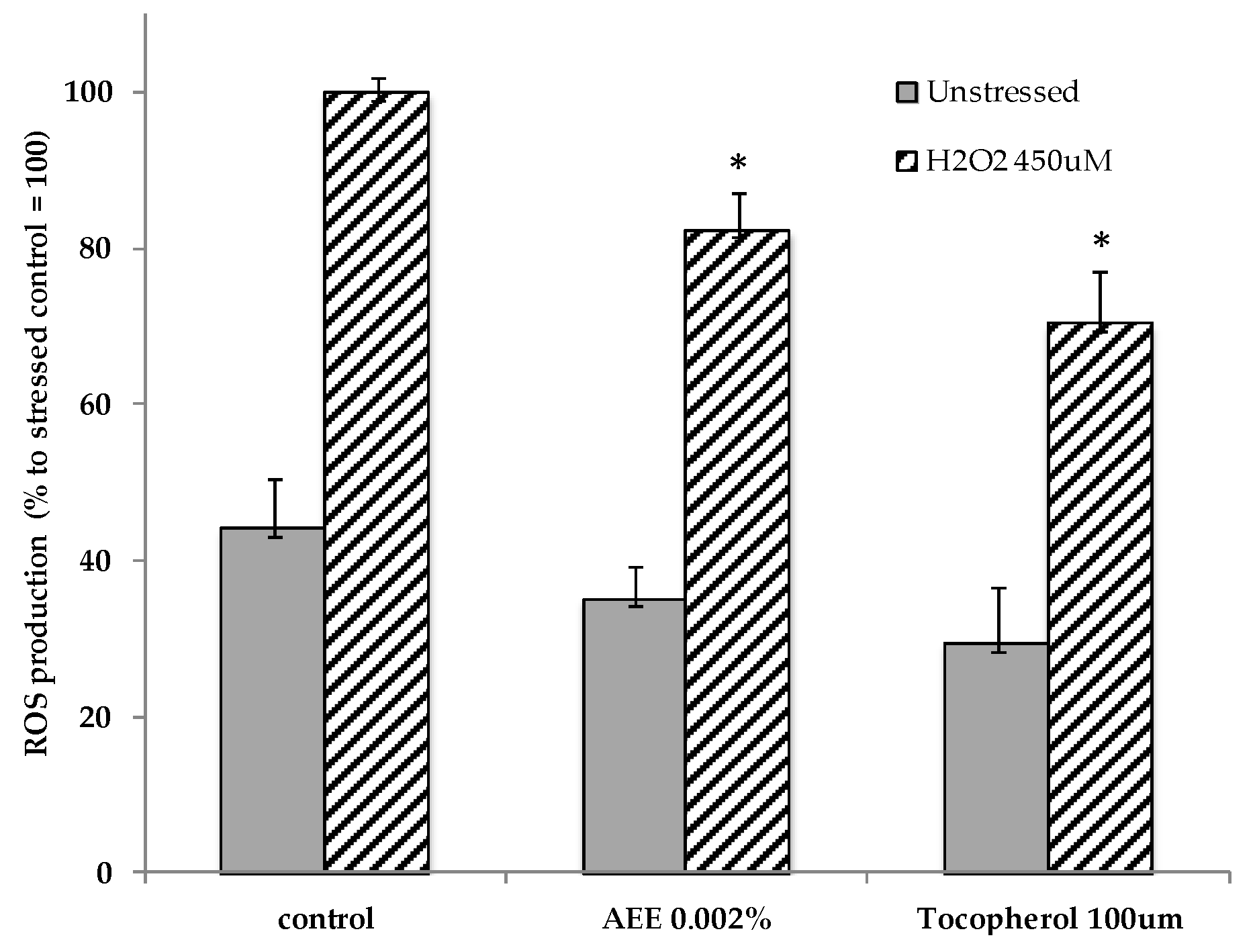
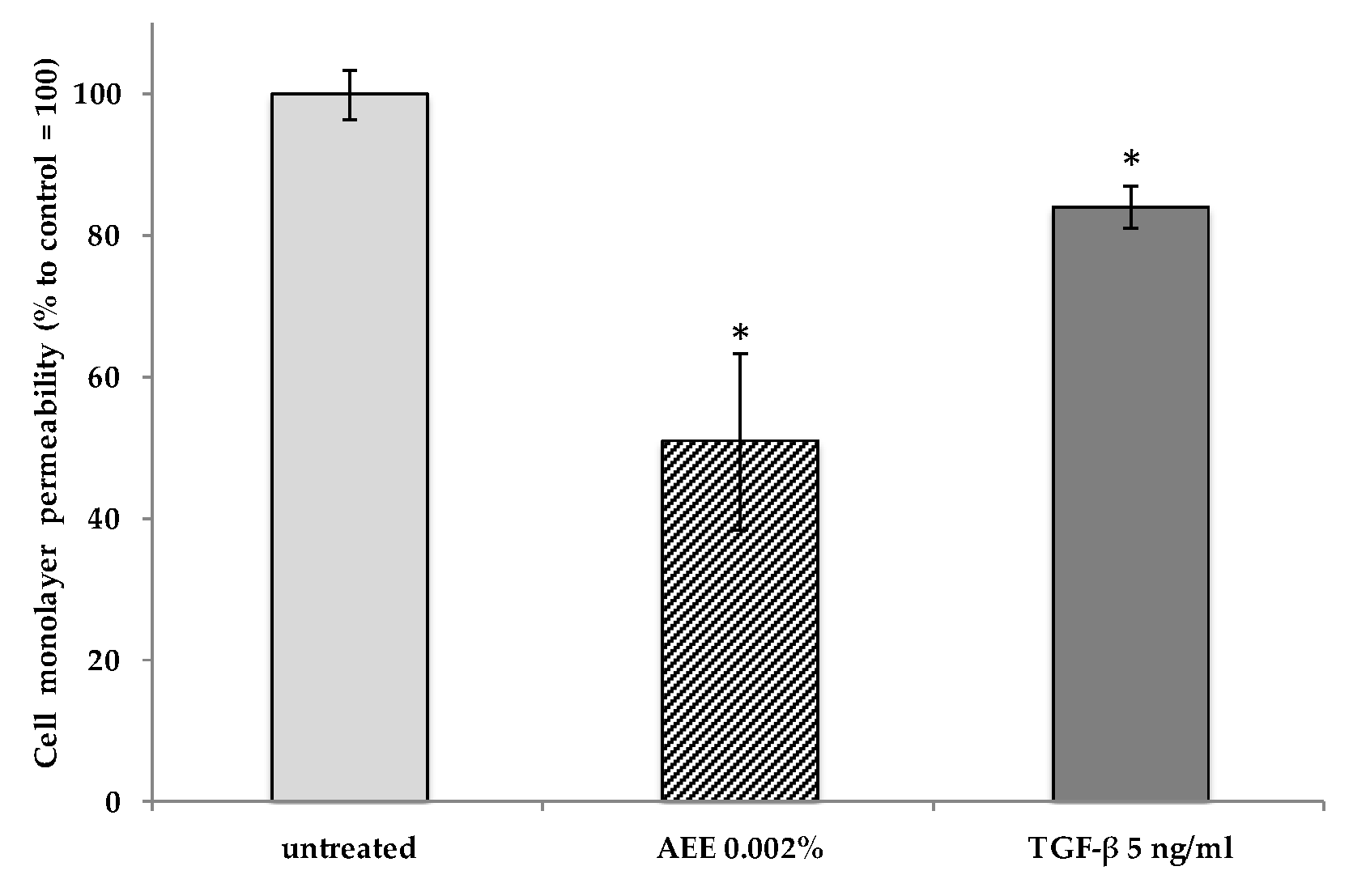
2.2. Artichoke Extract Activity in Endothelial Cells
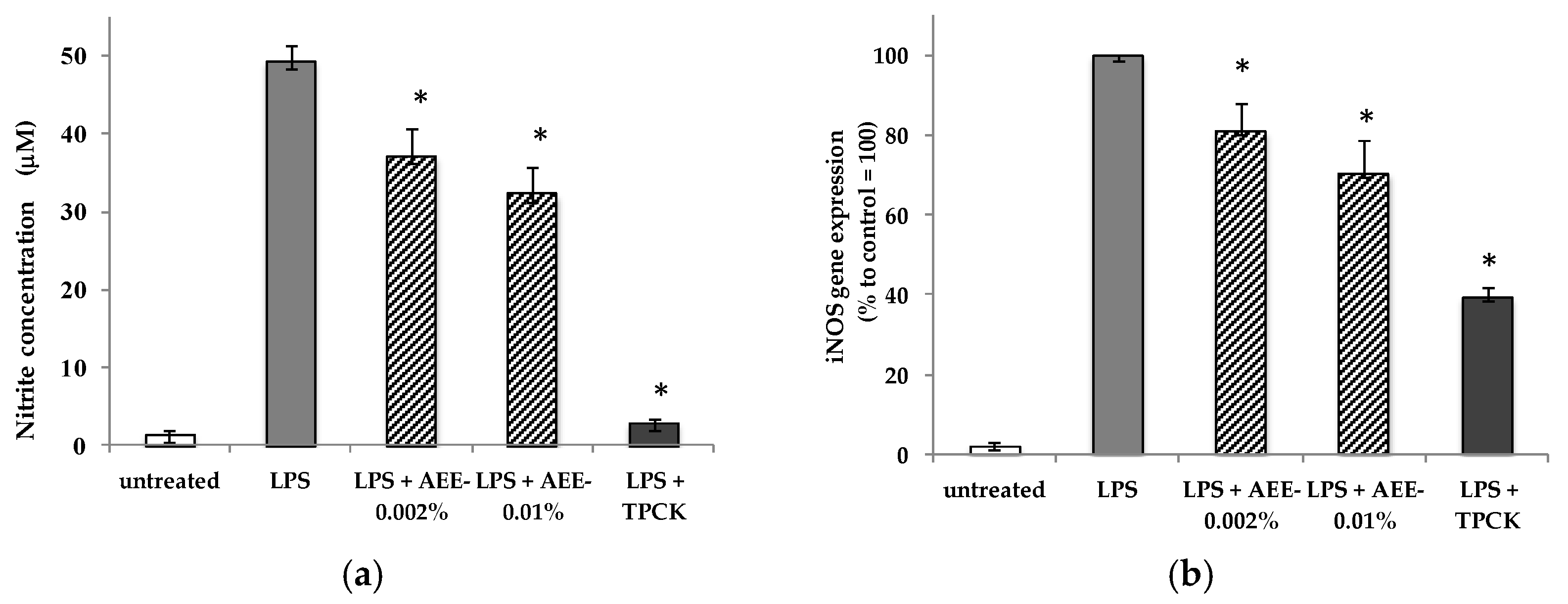
2.3. Artichoke Extract on Nitric Oxide Production in Macrophages
2.4. Artichoke Extract Activity in Lymphatic Endothelial Cells
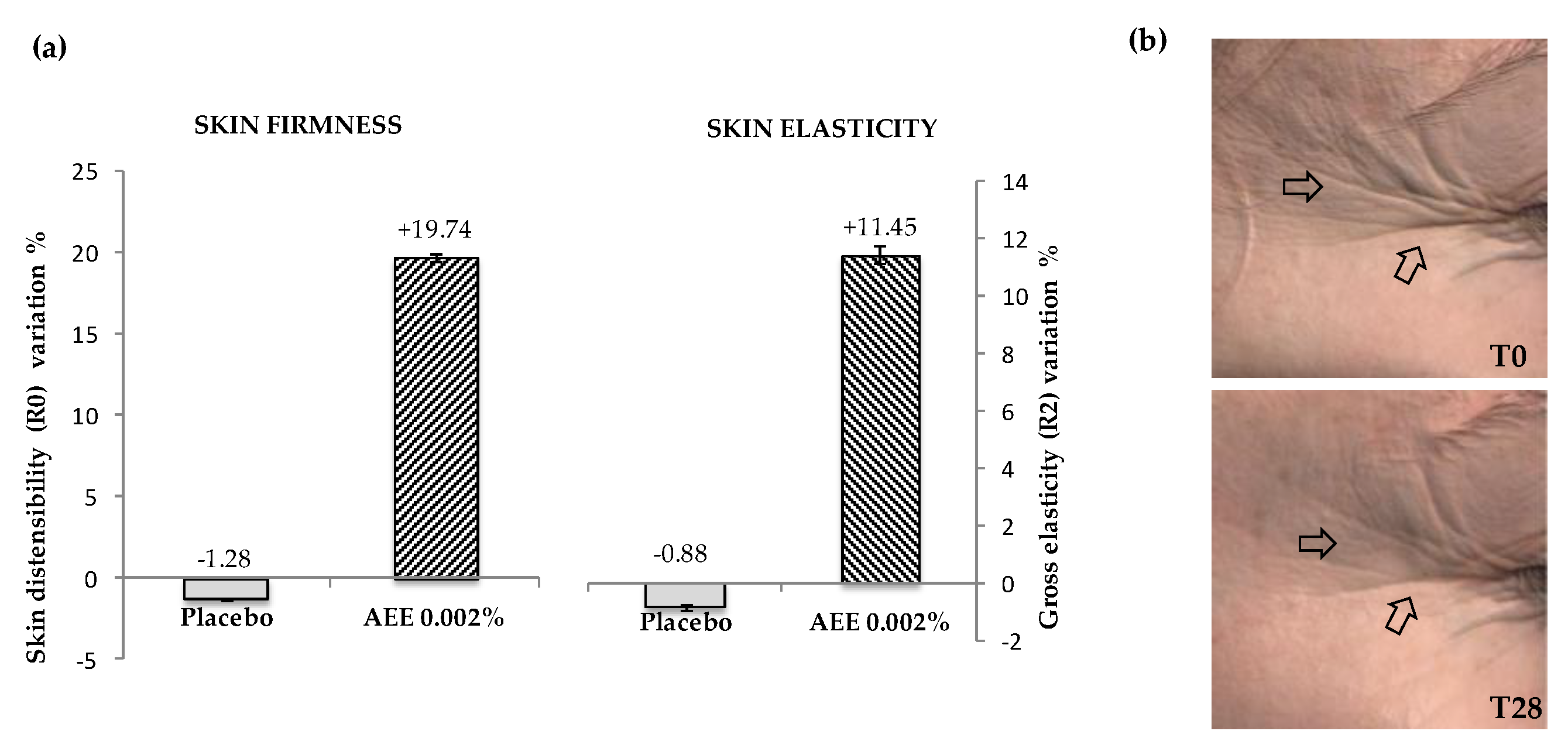
2.5. Artichoke Extract Activity in Clinical Tests
3. Materials and Methods
3.1. Reagents
3.2. Raw Materials
3.3. Preparation of Artichoke Polyphenol Extract
3.4. HPLC-DAD Analysis
3.5. Cell Cultures
3.6. Reactive Oxygen Species Determination
3.7. Gene Expression Analysis in HDLEC and HUVEC
3.8. Nitric Oxide Assay and iNOS Expression in Macrophages
3.9. Permeability Assay
3.10. Clinical Tests
3.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- FAO Food, Trade Statistics. Available online: http://www.fao.org/faostat (accessed on 31 July 2018).
- Marzi, V.; Lattanzio, V.; Vanadia, S. Il Carciofo Pianta Medicinale; Liantonio, Palo del Colle: Bari, Italy, 1975. [Google Scholar]
- Gil-Izquierdo, A.; Gil, M.I.; Conesa, M.A.; Ferreres, F. The effect of storage temperatures on vitamin C and phenolics content of artichoke (Cynara scolymus L.) heads. Innov. Food Sci. Emerg. Technol. 2001, 2, 199–202. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Cantini, C.; Cimato, A.; Heimler, D. Characterization of Violetto di Toscana, a typical Italian variety of artichoke (Cynara scolymus L.). Food Chem. 2006, 95, 221–225. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Mineral profile in globe artichoke as affected by genotype, head part and environment. J. Sci. Food Agric. 2011, 91, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauro, R.; Mauromicale, G. Variation of phenolic content in globe artichoke in relation to biological, technical and environmental factors. Ital. J. Agron./Riv. Agron. 2009, 4, 181–189. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar]
- Schutz, K.; Persike, M.; Carle, R.; Schieber, A. Characterization and quantification of anthocyanins in selected artichoke (Cynara scolymus L.) cultivars by HPLC-DADESI-MSn. Anal. Bioanal. Chem. 2006, 384, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R. Anticholestatic activity of flavonoids from artichoke (Cynara scolymus L.) and of their metabolites. Med. Sci. Monit. 2001, 7, 316–320. [Google Scholar] [PubMed]
- Coon, J.S.T.; Ernst, E. Herbs for serum cholesterol reduction: A systematic view. J. Fam. Pract. 2003, 52, 468–478. [Google Scholar]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Pizzi, F.; Minervini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: Influence of in vitro gastro-intestinal digestion. J. Funct. Foods 2014, 10, 456–464. [Google Scholar] [CrossRef]
- Coinu, R.; Carta, S.; Urgeghe, P.P.; Mulinacci, N.; Pinelli, P.; Franconi, F.; Romani, A. Dose-effect study on the antioxidant properties of leaves and outer bracts of extracts obtained from Violetto di Toscana artichoke. Food Chem. 2007, 101, 524–531. [Google Scholar] [CrossRef]
- Nazni, P.; Poongodi Vijayakumar, P.; Alagianambi, P.; Amirthaveni, M. Hypoglycemic and hypolipidemic effect of Cynara Scolymus among selected type 2 diabetic individuals. Pak. J. Nutr. 2006, 5, 147–151. [Google Scholar]
- Saénz Rodriguez, T.; García Giménez, D.; de la Puerta Vázquez, R. Choleretic activity and biliary elimination of lipids and bile acids induced by an artichoke leaf extract in rats. Phytomedicine 2002, 9, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molina, D.; Navarro-Martìnez, M.D.; Melgarejo, F.R.; Hiner, A.N.P.; Chazarra, S.; Neptuno –Lopez, J.R. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Sergio, L.; De Paola, A.; Linsalata, V.; Cardinali, A.; Vanadia, S. The use of artichoke peroxidase to remove phenols from olive mill waste water. Fresenius Environ. Bull. 2010, 19, 3028–3036. [Google Scholar]
- Fabbri, A.; Serranti, S.; Bonifazi, G. Biochemical methane potential (BMP) of artichoke waste: The inoculum effect. Waste Manag. Res. 2014, 32, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Llorach, R.; Espin, J.C.; Tomas-Barberan, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Effect of solvent type and extraction conditions on the recovery of Phenolic compounds from artichoke waste. Chem. Eng. Trans. 2014, 39, 463–468. [Google Scholar]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Reuse potential of artichoke (Cynara scolymus L.) waste for the recovery of phenolic compounds and bioenergy. J. Clean. Prod. 2016, 111, 279–284. [Google Scholar] [CrossRef]
- Barbulova, A.; Colucci, G.; Apone, F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Bonta, M.; Daina, L.; Muţiu, G. The process of ageing reflected by histological changes in the skin. Rom. J. Morphol. Embryol. 2013, 54, 797–804. [Google Scholar] [PubMed]
- Ryan, T. The ageing of the blood supply and the lymphatic drainage of the skin. Micron 2004, 35, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.; Tharakan, B. Vascular hyperpermeability and aging. Aging Dis. 2014, 5, 114–125. [Google Scholar] [PubMed]
- Rivelli, D.P.; Filho, C.A.H.; Almeida, R.L.; Ropke, C.D.; Sawada, T.C.H.; Barros, S.B.M. Chlorogenic acid UVA-UVB photostability. Photochem. Photobiol. 2010, 86, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.S.M.; Jiao, D.; Chan, B.C.L.; Hon, K.L.; Leung, P.C.; Lau, C.B.S.; Wong, E.C.W.; Cheng, L.; Chan, C.K.M.; Lam, C.W.K.; et al. Anti-inflammatory activities of pentaherbs formula, berberine, gallic acid and chlorogenic acid in atopic dermatitis-like skin inflammation. Molecules 2016, 21, 519. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Abdel Motaal, A.; Ezzat, S.M.; Tadros, M.G.; El-Askary, H.I. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm. Biol. 2016, 54, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knodler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic compositionof globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.G.; Palade, G.E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995, 108, 2369–2379. [Google Scholar] [PubMed]
- Chen, S.; Apostolova, M.D.; Cherian, M.G.; Chakrabarti, S. Interaction of endothelin-1 with vasoactive factors in mediating glucose-induced increased permeability in endothelial cells. Lab. Investig. 2000, 80, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Northcott, J.M.; Czubryt, M.P.; Wigle, J.T. Vascular senescence and ageing: A role for the MEOX proteins in promoting endothelial dysfunction. Can. J. Physiol. Pharmacol. 2017, 95, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.; Tucker, G.; Brameld, J. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br. J. Nutr. 2010, 103, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Mao, Z.; Zheng, Y.L.; Han, B.P.; Chen, L.T.; Li, J.; Li, F. Elevation of inducible nitric oxide synthase and cyclooxygenase-2 expression in the mouse brain after chronic nonylphenol exposure. Int. J. Mol. Sci. 2008, 9, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Han, S.C.; Kang, G.J.; Koo, D.H.; Koh, Y.S.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; Kang, H.K.; Yoo, E.S. Diphlorethohydroxycarmalol inhibits interleukin-6 production by regulating NF-κB, STAT5 and SOCS1 in lipopolysaccharide-stimulated RAW264.7 cells. Mar. Drugs 2015, 13, 2141–2157. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007. [Google Scholar] [CrossRef]
- Petrova, T.V.; Koh, G.Y. Organ-specific lymphatic vasculature: From development to pathophysiology. J. Exp. Med. 2018, 215, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, C.; Ridley, A.J. Endothelial cell-cell adhesion and signaling. Exp. Cell Res. 2017, 358, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Tsukita, S.; Miyachi, Y. Tight junction-associated proteins (occludin, ZO-1, claudin-1, claudin-4) in squamous cell carcinoma and Bowen’s disease. Br. J. Dermatol. 2004, 151, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.G. Hyaluronan in the lymphatics: The key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking. Matrix Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef]
- Kenney, W.L. Edward, F. Adolph distinguished lecture: Skin-deep insights into vascular aging. J. Appl. Physiol. 2017, 123, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Phenolic Compounds | Artichoke Extract | |
|---|---|---|
| Fresh (mg/100 g FW) | Lyophilized (mg/100 g powder) | |
| 1-O-caffeoylquinic acid | 3.4 ± 0.3 | 0.8 ± 0.1 |
| 3-O-caffeoylquinic acid | 2.1 ± 0.2 | 0.5 ± 0.1 |
| Chlorogenic acid | 236.2± 18.2 | 57.3 ± 1.4 |
| 1,4-O-dicaffeoylquinic acid | 5.0 ±0.4 | 1.3 ± 0.1 |
| 4,5-O-dicaffeoylquinic acid | 8.2 ± 0.7 | 2.0 ± 0.1 |
| 3,5-O-dicaffeoylquinic acid | 160.1 ± 13.1 | 39.8 ± 1.0 |
| 1,5-O-dicaffeoylquinic acid | 201.3 ± 17.9 | 50.1 ± 1.2 |
| 3,4-O-dicaffeoylquinic acid | 21.7 ± 1.9 | 5.4 ± 0.1 |
| Apigenin-7-O-glucoside | 19.9 ± 1.8 | 4.8 ± 0.4 |
| Total phenolics | 657.8 ± 56.3 | 162.0 ± 3.9 |
| Analyzed Gene | AEE 0.002% | AEE 0.01% | TGF-β 2.5 ng/mL |
|---|---|---|---|
| VEGF | 11.1 ± 6.1 * | 31.4 ± 7.4 * | 47.1 ± 11.2 * |
| ET-1 | 33.5 ± 14.3 * | 43.9 ± 9.4 * | 26.1 ± 7.4 * |
| eNOS | 22.2 ± 4.7 * | 59.4 ± 2.3 * | 10.6 ± 2.1 * |
| Analyzed Gene | AEE 0.002% | TGF-β 5 ng/mL |
|---|---|---|
| Cla-5 | 39.7 ± 6.8 * | 42.7 ± 6.5 * |
| ZO-1 | 46.9 ± 12.1 * | 42.5 ± 7.4 * |
| LYVE | 44.7 ± 7.7 * | 28.1 ± 2.3 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Antuono, I.; Carola, A.; Sena, L.M.; Linsalata, V.; Cardinali, A.; Logrieco, A.F.; Colucci, M.G.; Apone, F. Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality. Molecules 2018, 23, 2729. https://doi.org/10.3390/molecules23112729
D’Antuono I, Carola A, Sena LM, Linsalata V, Cardinali A, Logrieco AF, Colucci MG, Apone F. Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality. Molecules. 2018; 23(11):2729. https://doi.org/10.3390/molecules23112729
Chicago/Turabian StyleD’Antuono, Isabella, Antonietta Carola, Luigi M. Sena, Vito Linsalata, Angela Cardinali, Antonio F. Logrieco, Maria Gabriella Colucci, and Fabio Apone. 2018. "Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality" Molecules 23, no. 11: 2729. https://doi.org/10.3390/molecules23112729
APA StyleD’Antuono, I., Carola, A., Sena, L. M., Linsalata, V., Cardinali, A., Logrieco, A. F., Colucci, M. G., & Apone, F. (2018). Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality. Molecules, 23(11), 2729. https://doi.org/10.3390/molecules23112729







