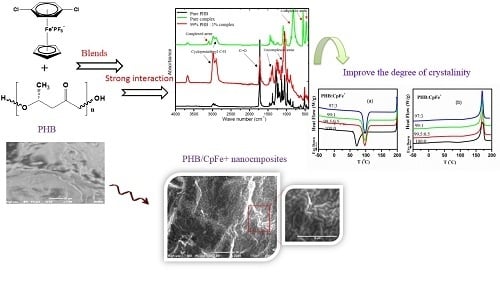
Cationic Cyclopentadienyliron Complex as a Novel and Successful Nucleating Agent on the Crystallization Behavior of the Biodegradable PHB Polymer
Abstract
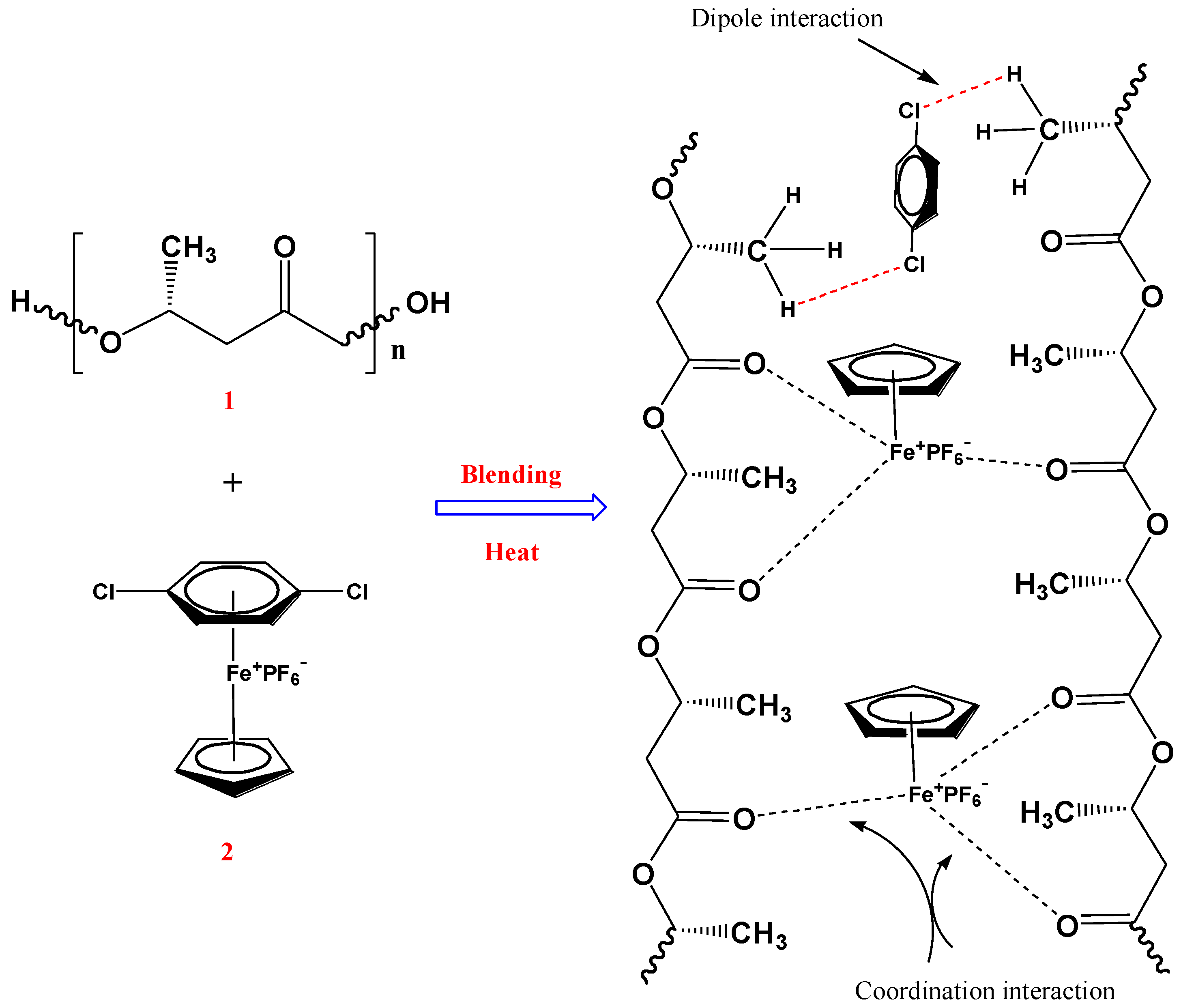
1. Introduction
2. Results and Discussion
2.1. Polarized Optical Microscopy
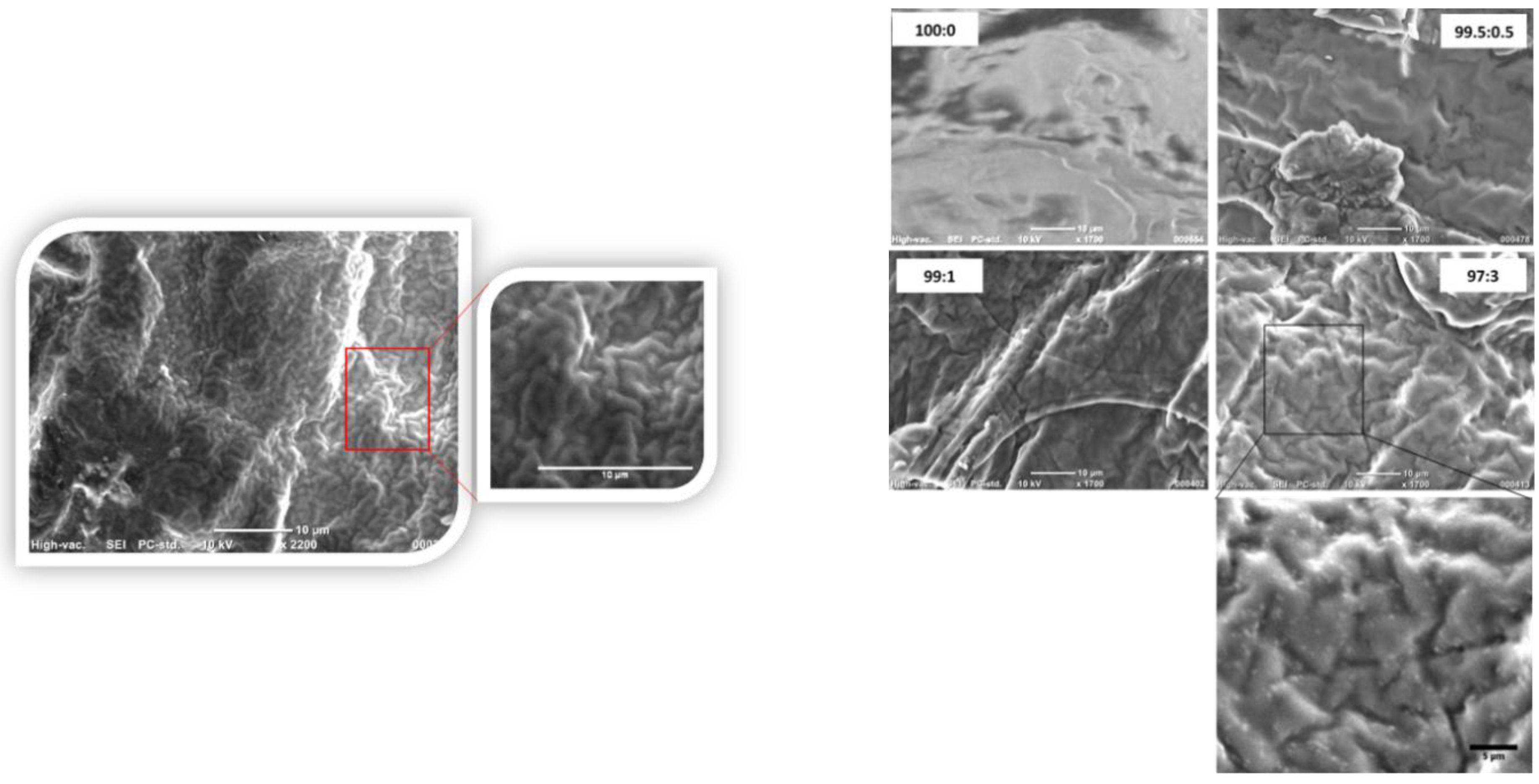
2.2. Scanning Electron Microscope (SEM)
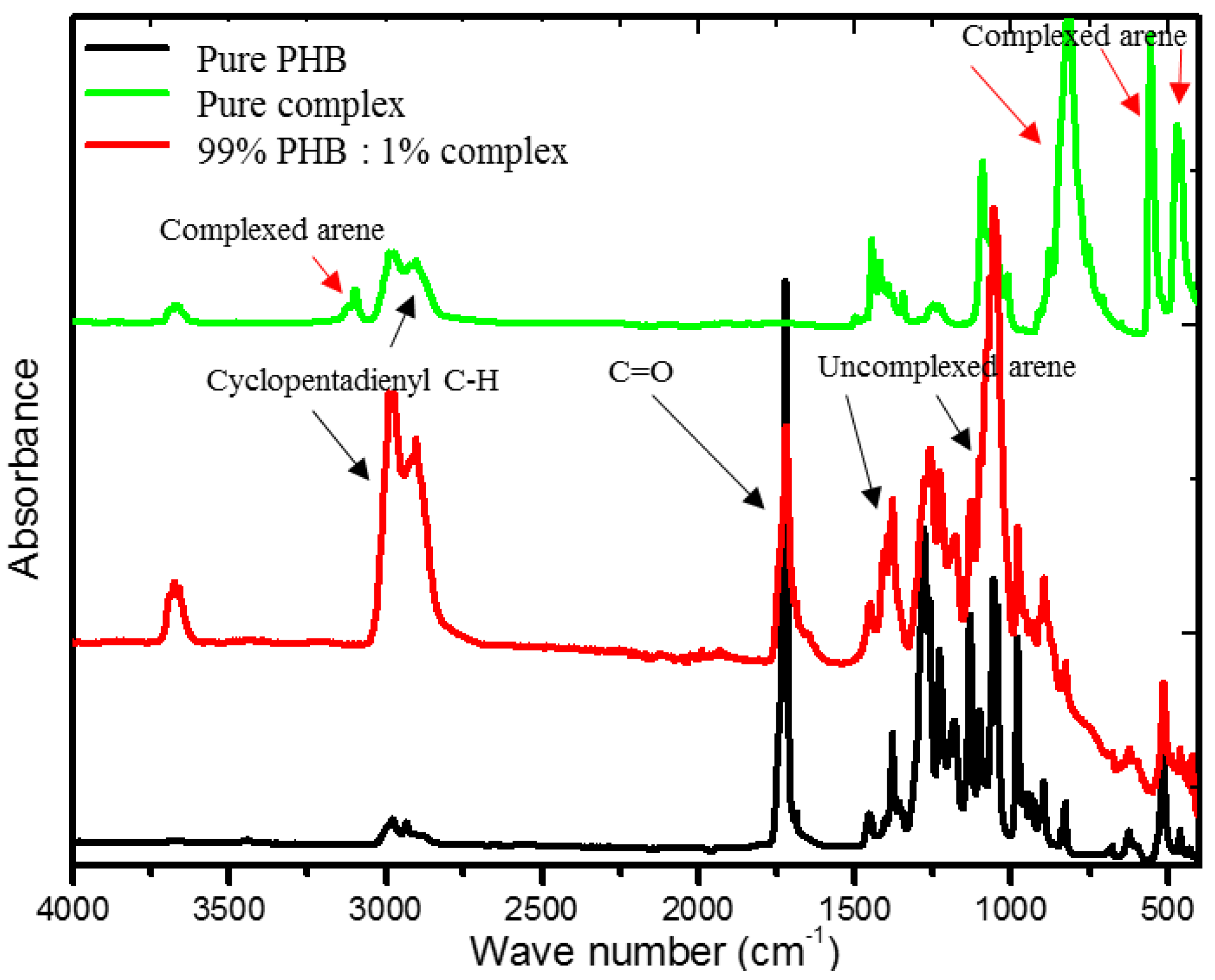
2.3. FTIR Analysis
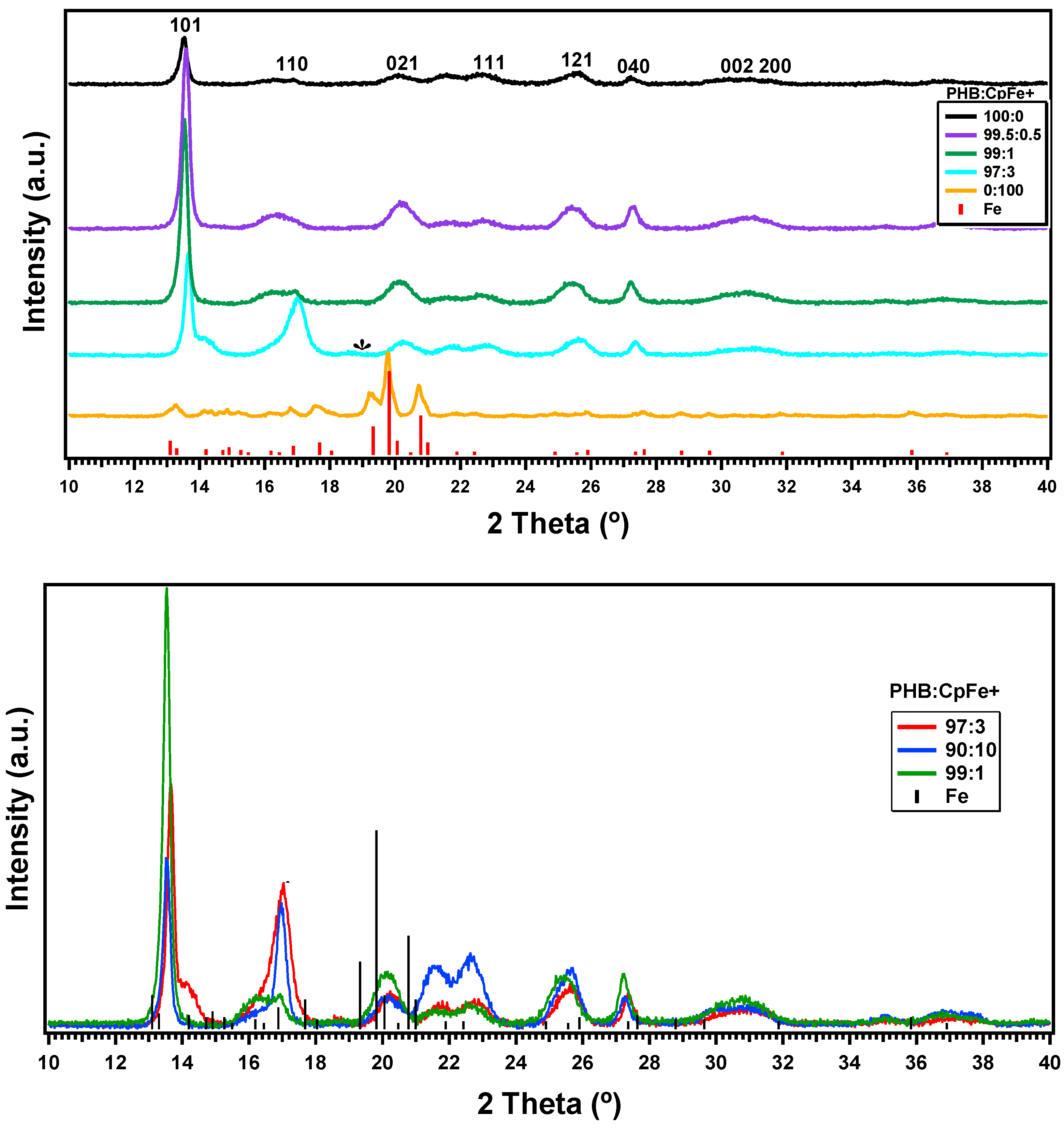
2.4. X-ray Results
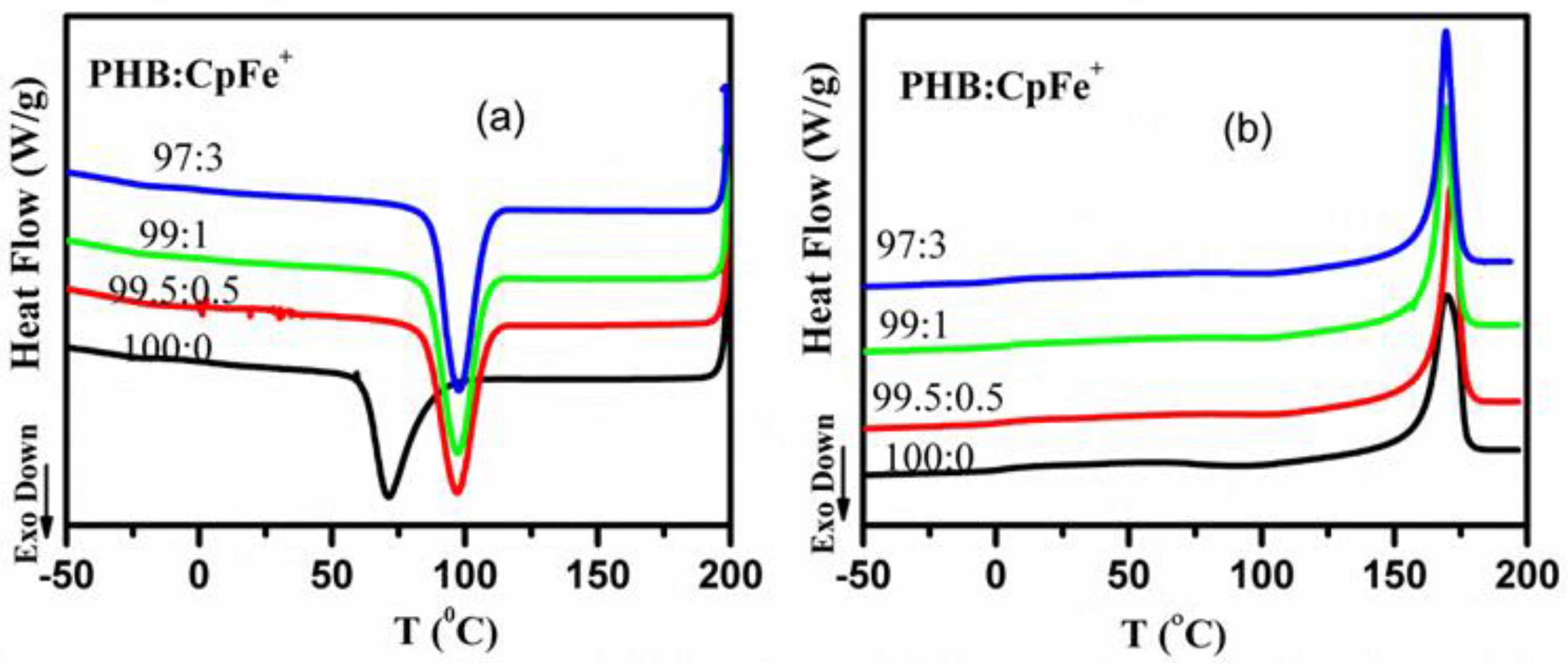
2.5. Thermal Properties
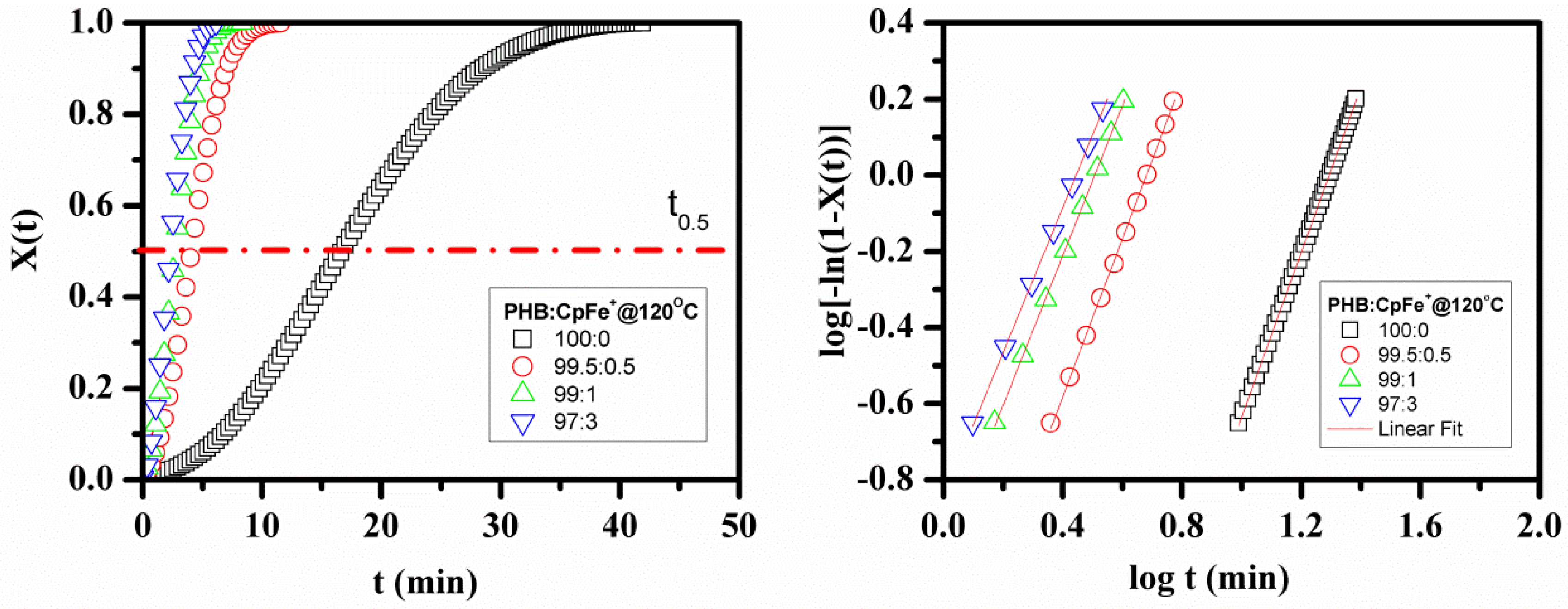
2.6. Isothermal Crystallization Kinetics
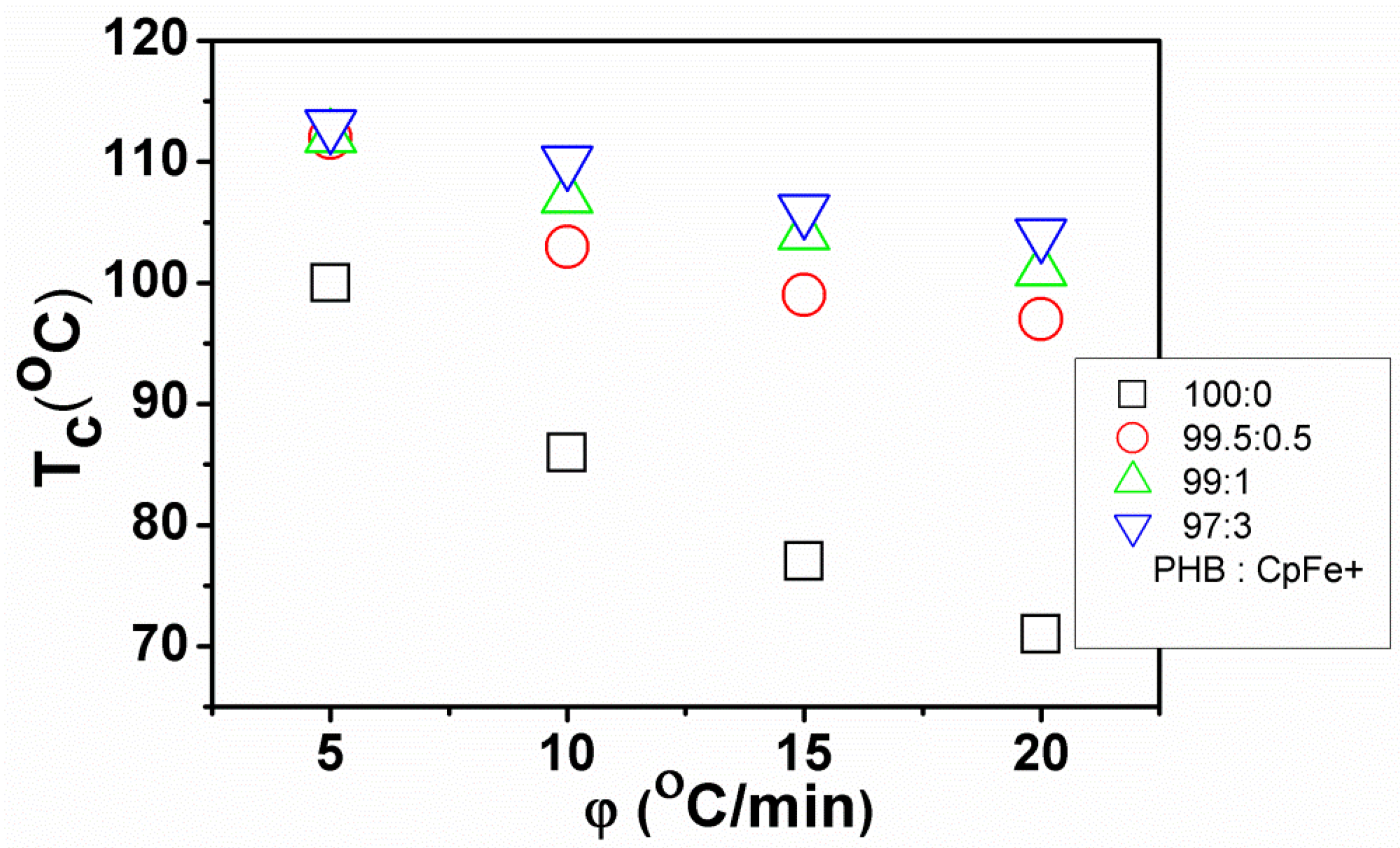
2.7. Non-Isothermal Crystallization Kinetics
2.8. An Effective Activation Energy of Non-Isothermal Crystallization
2.9. Thermal Gravimetric Analysis
3. Materials and Methods
3.1. Preparation of Blends
3.2. Characterization
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express. Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Chisti, Y. Recent Advances in the Production, Recovery and Applications of Polyhydroxyalkanoates. J. Polym. Environ. 2013, 21, 580–605. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J.; Freire, C.S.R.; Silvestre, A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014, 5, 3119–3141. [Google Scholar] [CrossRef]
- Lagarón, J.M.; López-Rubio, A.; José Fabra, M. Bio-based packaging. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Pan, Y.; Farmahini-Farahani, M.; O’Hearn, P.; Xiao, H.; Ocampo, H. An overview of bio-based polymers for packaging materials. J. Bioresour. Bioprod. 2016, 1, 106–113. [Google Scholar]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Kato, S.; Shintani, N.; Kamini, N.R.; Nakajima-Kambe, T. Microbial degradation of aliphatic and aliphatic-aromatic co-polyesters. Appl. Microbiol. Biotechnol. 2014, 98, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, B.S.; Kushwah, A.V.S.; Singh, V. Towards understanding polyhydroxyalkanoates and their use. J. Polym. Res. 2016, 23, 153. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J. Biodegradable Polymers and Polymer Blends. In Handbook of Biopolymers and Biodegradable Plastics; Elsevier: Oxford, UK; William Andrew: Waltham, MA, USA, 2013; pp. 109–128. ISBN 9781455728343. [Google Scholar]
- Jayanth, D.; Kumar, P.S.; Nayak, G.C.; Kumar, J.S.; Pal, S.K.; Rajasekar, R. A Review on Biodegradable Polymeric Materials Striving Towards the Attainment of Green Environment. J. Polym. Environ. 2018, 26, 838–865. [Google Scholar] [CrossRef]
- Ha, C.S.; Cho, W.J. Miscibility, properties, and biodegradability of microbial polyester containing blends. Prog. Polym. Sci. 2002, 27, 759–809. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Mitomo, H.; Barham, P.J.; Keller, A. Crystallization and Morphology of Poly(β-hydroxybutyrate) and Its Copolymer. Polym. J. 1987, 19, 1241–1253. [Google Scholar] [CrossRef]
- Tanadchangsaeng, N. Structure, chemomechanical properties and degradability of polyhydroxyalkanoates: A review. Bull. Health Sci. Technol. 2014, 12, 9–21. [Google Scholar]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part. A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Organ, S.J.; Barham, P.J. Nucleation of poly(hydroxy butyrate) by epitaxy on nitrogen-containing compounds. J. Mater. Sci. 1992, 27, 3239–3242. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Effect of MolecularWeight and Temperature on Physical Aging of ThinGlassy Poly(2,6-dimethyl-1,4-phenylene oxide) Films. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1390–1398. [Google Scholar] [CrossRef]
- Puente, J.A.S.; Esposito, A.; Chivrac, F.; Dargent, E. Effect of boron nitride as a nucleating agent on the crystallization of bacterial poly(3-hydroxybutyrate). J. Appl. Polym. Sci. 2013, 128, 2586–2594. [Google Scholar] [CrossRef]
- Öner, M.; Çöl, A.A.; Pochat-Bohatier, C.; Bechelany, M. Effect of incorporation of boron nitride nanoparticles on the oxygen barrier and thermal properties of poly(3-hydroxybutyrate-co-hydroxyvalerate). RSC Adv. 2016, 6, 90973–90981. [Google Scholar] [CrossRef]
- Vandewijngaarden, J.; Murariu, M.; Dubois, P.; Carleer, R.; Yperman, J.; D’Haen, J.; Peeters, R.; Buntinx, M. Effect of ultrafine talc on crystallization and end-use properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Kai, W.; He, Y.; Inoue, Y. Fast crystallization of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with talc and boron nitride as nucleating agents. Polym. Int. 2005, 54, 780–789. [Google Scholar] [CrossRef]
- Pan, P.; Shan, G.; Bao, Y.; Weng, Z. Crystallization kinetics of bacterial poly(3-hydroxylbutyrate) copolyesters with cyanuric acid as a nucleating agent. J. Appl. Polym. Sci. 2013, 129, 1374–1382. [Google Scholar] [CrossRef]
- He, Y.; Inoue, Y. Effect of α-cyclodextrin on the crystallization of poly(3-hydroxybutyrate). J. Polym. Sci. Part. B Polym. Phys. 2004, 42, 3461–3469. [Google Scholar] [CrossRef]
- Weihua, K.; He, Y.; Asakawa, N.; Inoue, Y. Effect of lignin particles as a nucleating agent on crystallization of poly(3-hydroxybutyrate). J. Appl. Polym. Sci. 2004, 94, 2466–2474. [Google Scholar] [CrossRef]
- Jing, X.; Qiu, Z. Effect of Low Thermally Reduced Graphene Loadings on the Crystallization Kinetics and Morphology of Biodegradable Poly(3-hydroxybutyrate). Ind. Eng. Chem. Res. 2012, 51, 13686–13691. [Google Scholar] [CrossRef]
- Shan, G.-F.; Gong, X.; Chen, W.-P.; Chen, L.; Zhu, M.-F. Effect of multi-walled carbon nanotubes on crystallization behavior of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Colloid Polym. Sci. 2011, 289, 1005–1014. [Google Scholar] [CrossRef]
- Lai, M.; Li, J.; Yang, J.; Liu, J.; Tong, X.; Cheng, H. The morphology and thermal properties of multi-walled carbon nanotube and poly(hydroxybutyrate-co-hydroxyvalerate) composite. Polym. Int. 2004, 53, 1479–1484. [Google Scholar] [CrossRef]
- Jaques, N.G.; Silva, I.D.D.S.; Barbosa Neto, M.D.C.; Diniz, R.K.M.; Wellen, R.M.R.; Canedo, E.L. Comparative study of the effect of TiO2 and ZnO on the crystallization of PHB. Matéria 2017, 22. [Google Scholar] [CrossRef]
- Montagna, L.S.; Montanheiro, T.L.D.A.; Machado, J.P.B.; Passador, F.R.; Lemes, A.P.; Rezende, M.C. Effect of Graphite Nanosheets on Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G.; Cohen, S.R.; Laikhtman, A.; Rapoport, L.; Zak, A. Novel poly(3-hydroxybutyrate) nanocomposites containing WS2 inorganic nanotubes with improved thermal, mechanical and tribological properties. Mater. Chem. Phys. 2014, 147, 273–284. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed poly(hydroxybutyrate-co-valerate) (PHBV)/poly(ε-caprolactone) (PCL) biodegradable polymer blend nanocomposites with TiO2 as filler. J. Mater. Sci. 2015, 50, 3812–3824. [Google Scholar] [CrossRef]
- Bekat, T.; Öner, M. Effects of surface modification and ultrasonic agitation on the properties of PHBV/ZnO nanocomposites. Pure Appl. Chem. 2016, 88, 1027–1035. [Google Scholar] [CrossRef]
- Gahleitner, M.; Wolfschwenger, J. Polymer Crystal Nucleating Agents. In Encyclopedia of Materials: Science and Technology; Elsevier: Oxford, UK, 2001; pp. 7239–7244. ISBN 978-0-08-043152-9. [Google Scholar]
- Martínez-Sanz, M.; Villano, M.; Oliveira, C.; Albuquerque, M.G.E.; Majone, M.; Reis, M.; Lopez-Rubio, A.; Lagaron, J.M. Characterization of polyhydroxyalkanoates synthesized from microbial mixed cultures and of their nanobiocomposites with bacterial cellulose nanowhiskers. New Biotechnol. 2014, 31, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.Y.; Tan, W.L.; Abu Bakar, M.; Ismail, J. Silver sulfide/poly(3-hydroxybutyrate) nanocomposites: Thermal stability and kinetic analysis of thermal degradation. Polym. Degrad. STable 2010, 95, 1299–1304. [Google Scholar] [CrossRef]
- Duangphet, S.; Szegda, D.; Tarverdi, K.; Song, J. Effect of Calcium Carbonate on Crystallization Behavior and Morphology of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate). Key Eng. Mater. 2017, 751, 242–251. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Zhou, W.-Y.; Cheung, W.-L. Nonisothermal melt-crystallization behavior of calcium phosphate/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposite microspheres. Polym. Eng. Sci. 2011, 51, 1580–1591. [Google Scholar] [CrossRef]
- Kaynak, C.; Erdogan, A.R. Mechanical and thermal properties of polylactide/talc microcomposites: Before and after accelerated weathering. Polym. Adv. Technol. 2016, 27, 812–822. [Google Scholar] [CrossRef]
- De O. Patrício, P.S.; Pereira, F.V.; dos Santos, M.C.; de Souza, P.P.; Roa, J.P.B.; Orefice, R.L. Increasing the elongation at break of polyhydroxybutyrate biopolymer: Effect of cellulose nanowhiskers on mechanical and thermal properties. J. Appl. Polym. Sci. 2013, 127, 3613–3621. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R. Morphology, thermal and barrier properties of biodegradable films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) containing cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2017, 93, 41–48. [Google Scholar] [CrossRef]
- Srithep, Y.; Ellingham, T.; Peng, J.; Sabo, R.; Clemons, C.; Turng, L.-S.; Pilla, S. Melt compounding of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/nanofibrillated cellulose nanocomposites. Polym. Degrad. STable 2013, 98, 1439–1449. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.-F.; Panaitescu, D.M. Morphology and thermal properties of PLA–cellulose nanofibers composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Öner, M.; Ilhan, B. Fabrication of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biocomposites with reinforcement by hydroxyapatite using extrusion processing. Mater. Sci. Eng. C 2016, 65, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Joraid, A.A.; Okasha, R.M.; Rock, C.L.; Abd-El-Aziz, A.S. A Nonisothermal Study of Organoiron Poly(alkynyl methacrylate) Coordinated to Dicobalt Hexacarbonyl Using Advanced Kinetics Modelling. J. Inorg. Organomet. Polym. Mater. 2014, 24, 121–127. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; May, L.J.; Hurd, J.A.; Okasha, R.M. First ring-opening metathesis polymerization of norbornenes containing cationic iron moieties. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2716–2722. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Todd, E.K.; Epp, K.M. Synthesis and Characterization of Novel Organoiron Polymers. J. Inorg. Organomet. Polym. 1998, 8, 127–133. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Okasha, R.M.; Shipman, P.O.; Afifi, T.H. Neutral and cationic cyclopentadienyliron macromolecules containing arylazo chromophores. Macromol. Rapid Commun. 2004, 25, 1497–1503. [Google Scholar] [CrossRef]
- Okasha, R.M. Proton Sensing Color Changing Organoiron and Organic Macromolecules. J. Inorg. Organomet. Polym. Mater. 2015, 25, 354–366. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Okasha, R.M.; Afifi, T.H.; Todd, E.K. A new class of cationic organoiron polynorbornenes containing azo dyes. Macromol. Chem. Phys. 2003, 204, 555–563. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Schriemer, D.C.; Denus, C.R. De Bis(cyclopentadienyliron)arene Complexes: A New Route to the Synthesis and Functionalization of Polyaromatic Ethers. Organometallics 1994, 13, 374–384. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; De Denus, C.R.; Zaworotko, M.J.; MacGillivray, L.R. Controlled design of oligomeric ethers with pendant cyclopentadienyliron moieties. J. Chem. Soc. Dalton Trans. 1995, 3375–3393. [Google Scholar] [CrossRef]
- Sato, H.; Ando, Y.; Mitomo, H.; Ozaki, Y. Infrared Spectroscopy and X-ray Diffraction Studies of Thermal Behavior and Lamella Structures of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(HB-co-HV)) with PHB-Type Crystal Structure and PHV-Type Crystal Structure. Macromolecules 2011, 44, 2829–2837. [Google Scholar] [CrossRef]
- Reusch, R. Biogenesis of Ion Channels. J. Biochem. Biophys. 2014, 24, 1–24. [Google Scholar] [CrossRef]
- Sato, H.; Nakamura, M.; Padermshoke, A.; Yamaguchi, H.; Terauchi, H.; Ekgasit, S.; Noda, I.; Ozaki, Y. Thermal Behavior and Molecular Interaction of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Studied by Wide-Angle X-ray Diffraction. Macromolecules 2004, 37, 3763–3769. [Google Scholar] [CrossRef]
- Scalioni, L.V.; Gutiérrez, M.C.; Felisberti, M.I. Green composites of poly(3-hydroxybutyrate) and curaua fibers: Morphology and physical, thermal, and mechanical properties. J. Appl. Polym. Sci. 2017, 134, 44676. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Silvestre, C. Non-isothermal crystallization of polymers. Prog. Polym. Sci. 1999, 24, 917–950. [Google Scholar] [CrossRef]
- Mansour, A.A.; Saad, G.R.; Hamed, A.H., II. Dielectric investigation of cold crystallization of poly(3- hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polymer 1999, 40, 5377–5391. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Hsu, S.F.; Wu, T.M.; Liao, C.S. Nonisothermal crystallization behavior and crystalline structure of poly(3-hydroxybutyrate)/layered double hydroxide nanocomposites. J. Polym. Sci. Part. B Polym. Phys. 2007, 45, 995–1002. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Polymers: The Decade Past. Macromol. Rapid Commun. 2017, 38, 1600615. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Vyazovkin, S. A time to search: Finding the meaning of variable activation energy. Phys. Chem. Chem. Phys. 2016, 18, 18643–18656. [Google Scholar] [CrossRef] [PubMed]
- De Lima Souza, J.; Kobelnik, M.; Ribeiro, C.A.; Capela, J.M.V.; Crespi, M.S. Kinetic study of crystallization of PHB in presence of hydroxy acids. J. Therm. Anal. Calorim. 2009, 97, 525–528. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Vol’kenau, N.A.; Bolesova, I.N. The interaction of ferrocene and its derivatives with aromatic compounds. Tetrahedron Lett. 1963, 4, 1725–1729. [Google Scholar] [CrossRef]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.-J. Calibration of Differential Scanning Calorimeters. In Differential Scanning Calorimetry; Springer: Berlin/Heidelberg, Germany, 2003; pp. 65–114. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


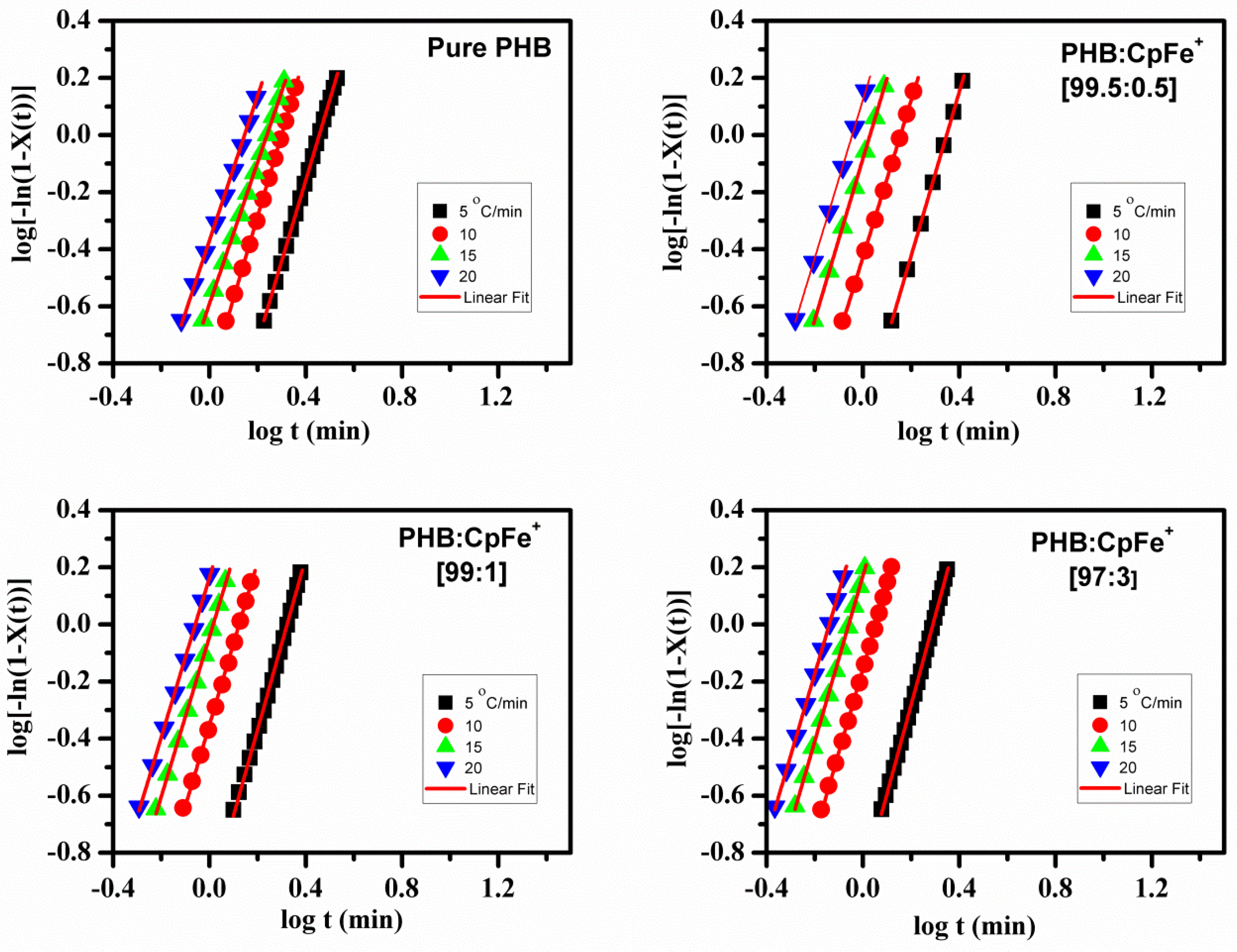
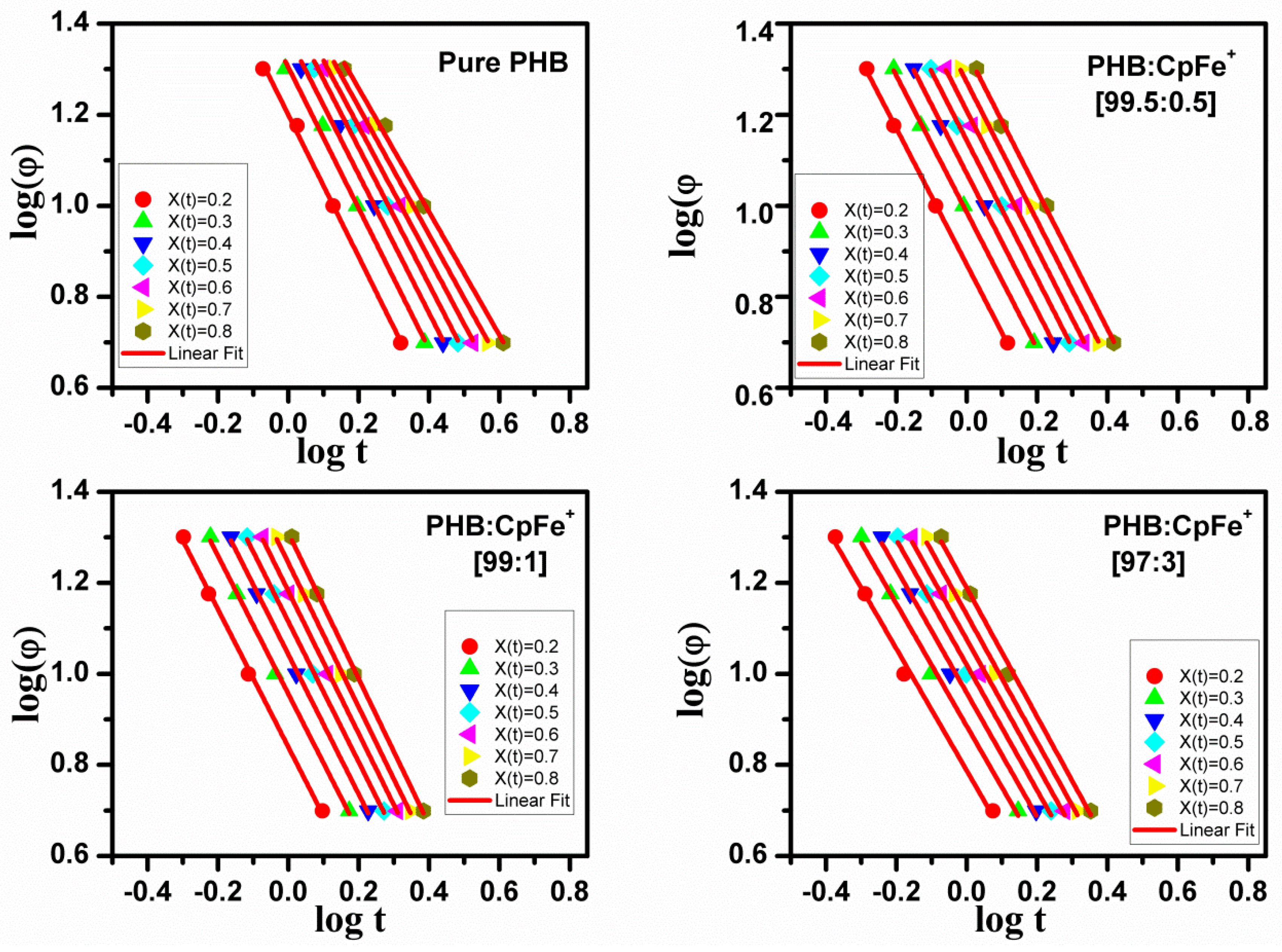
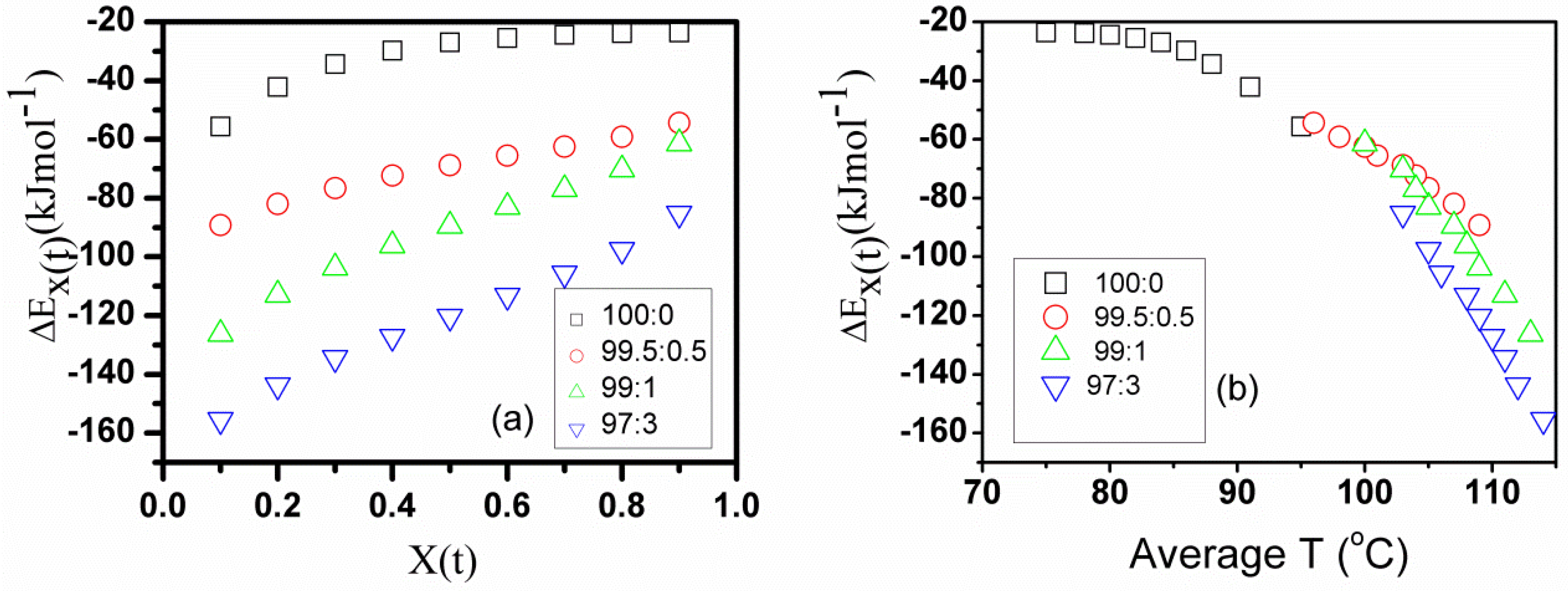
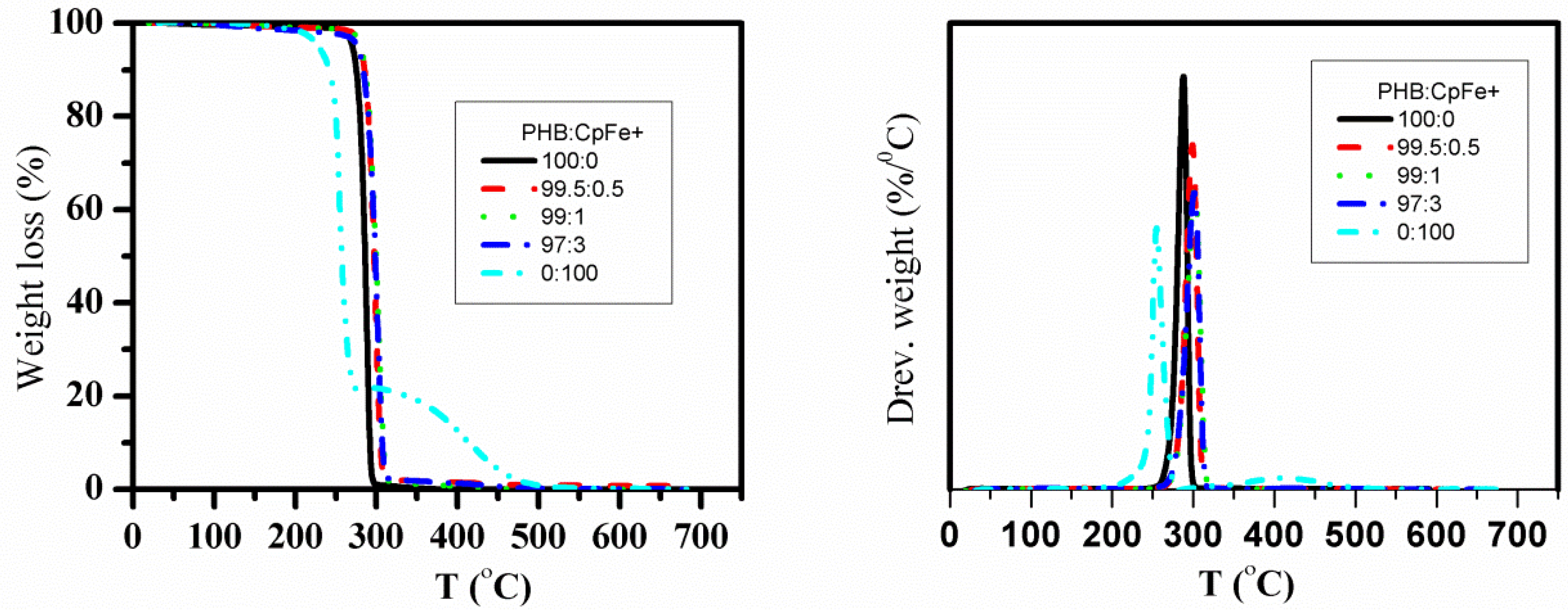
| Name of PHB Samples | Width | width | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | °C | J/g | °C | J/g°C | °C | J/g | °C | °C | J/g | % | ||
| PHB:CpFe+ | 100:0 | 71 | 59 | 3 | 0.21 | 95 | 8 | 170 | 72 | 50 | ||
| 99.5:0.5 | 97 | 13 | 72 | 2 | 0.16 | 105 | 4 | 170 | 7 | 87 | 60 | |
| 99:1 | 97 | 13 | 72 | 2 | 0.17 | 104 | 7 | 169 | 7 | 86 | 60 | |
| 97:3 | 97 | 12 | 73 | 2 | 0.16 | 105 | 4 | 169 | 6 | 87 | 61 | |
| Name of PHB Samples | |||||||
|---|---|---|---|---|---|---|---|
| °C | min−1 | Min | Min | ||||
| PHB:CpFe+ (100:0) | 90 | 3 | −0.23 | 59. 19 | 1 | 1.07 | 2.1 |
| 94 | 2 | −0.24 | 58.2 | 1 | 1.08 | 2.52 | |
| 100 | 2 | −0.62 | 24.01 | 1 | 1.71 | 4.05 | |
| 118 | 2 | −2.45 | 0. 35 | 1 | 11.5 | 29 | |
| 120 | 2 | −2.80 | 0.16 | 1 | 16.7 | 37.2 | |
| 122 | 2 | −2.80 | 0.16 | 1 | 18.6 | 37.7 | |
| 124 | 2 | −3.13 | 0.055 | 1 | 19.2 | 39.3 | |
| 99.5:0.5 | 120 | 2 | −1.41 | 3.88 | 1 | 4.05 | 9.63 |
| 124 | 2 | −2.29 | 0.52 | 1 | 9.75 | 21.4 | |
| 128 | 2 | −2.98 | 0.1 | 1 | 18.1 | 38.3 | |
| 99:1 | 120 | 2 | −0.99 | 10.06 | 1 | 2.69 | 6.58 |
| 124 | 2 | −1.63 | 2.33 | 1 | 5.43 | 13.3 | |
| 128 | 2 | −2.53 | 0.29 | 1 | 12.7 | 29 | |
| 97:3 | 120 | 2 | −0.85 | 14.23 | 1 | 2.31 | 5.53 |
| 124 | 2 | −1.62 | 2.37 | 1 | 5.4 | 13.2 | |
| 128 | 2 | −2.68 | 0.21 | 1 | 13 | 28.2 |
| Name of PHB Samples | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C /min | min | °C | J/g | min−1 | min−1 | Min | ||||||
| PHB:CpFe+ | 100:0 | 5 | 16.9 | 102 | 83.4 | 3 | −1.3 | 5.13 | 0.55 | 1 | 2.5 | 2.72 |
| 10 | 9.14 | 91 | 72.1 | 3 | −0.86 | 13.83 | 0.81 | 1 | 1.8 | |||
| 15 | 6.29 | 83 | 67.5 | 3 | −0.59 | 25.63 | 0.91 | 1 | 1.5 | |||
| 20 | 4.83 | 76 | 65.3 | 3 | −0.37 | 42.76 | 0.95 | 1 | 1.2 | |||
| 99.5:0.5 | 5 | 15.7 | 112 | 91.5 | 3 | −0.99 | 10.06 | 0.63 | 1 | 2 | 6.36 | |
| 10 | 8.09 | 109 | 87.9 | 3 | −0.24 | 58.16 | 0.91 | 1 | 1.1 | |||
| 15 | 5.53 | 105 | 85.4 | 3 | 0.11 | 131.2 | 0.99 | 1 | 0.8 | |||
| 20 | 4.24 | 102 | 83.3 | 3 | 0.35 | 224.4 | 1.01 | 1 | 0.7 | |||
| 99:1 | 5 | 15.6 | 112 | 93.8 | 3 | −0.97 | 10.71 | 0.64 | 1 | 1.9 | 5.12 | |
| 10 | 8.07 | 107 | 87.4 | 3 | −0.35 | 44.5 | 0.92 | 1 | 1.2 | |||
| 15 | 5.51 | 104 | 85.4 | 3 | −0.04 | 90.49 | 0.99 | 1 | 0.9 | |||
| 20 | 4.21 | 101 | 83.6 | 3 | 0.16 | 145.8 | 1.02 | 1 | 0.8 | |||
| 97:3 | 5 | 15.8 | 113 | 90.9 | 3 | −0.90 | 12.49 | 0.66 | 1 | 1.8 | 6.5 | |
| 10 | 8.06 | 110 | 85.8 | 3 | −0.15 | 70.08 | 0.97 | 1 | 1 | |||
| 15 | 5.5 | 106 | 84 | 3 | 0.17 | 148.3 | 1.02 | 1 | 0.8 | |||
| 20 | 4.21 | 104 | 81.9 | 3 | 0.4 | 251.7 | 1.05 | 1 | 0.6 | |||
| Name of PHB Samples | Kinetics Parameter | |||||
|---|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.6 | 0.8 | |||
| PHB:CpFe+ | 100:0 | 15.83 | 23.59 | 29.10 | 33.80 | |
| −1.56 | −1.52 | −1.45 | −1.36 | |||
| 0.997 | 0.993 | 0.993 | 0.995 | |||
| 99.5:0.5 | 7.239 | 8.903 | 10.047 | 11.122 | ||
| −1.496 | −1.509 | −1.522 | −1.522 | |||
| 0.999 | 0.999 | 0.999 | 0.998 | |||
| 99:1 | 6.978 | 8.669 | 9.825 | 10.889 | ||
| −1.512 | −1.535 | −1.565 | −1.602 | |||
| 0.998 | 0.999 | 0.999 | 0.999 | |||
| 97:3 | 6.546 | 7.965 | 8.940 | 9.882 | ||
| −1.339 | −1.362 | −1.384 | −1.414 | |||
| 0.993 | 0.994 | 0.995 | 0.995 | |||
| Name of PHB Samples | PHB Phase | |||
|---|---|---|---|---|
| PHB:CpFe+ | 100:0 | 280 | 288 | 293 |
| 99.5:0.5 | 287 | 297 | 303 | |
| 99:1 | 287 | 297 | 303 | |
| 97:3 | 286 | 298 | 305 | |
| 90:10 | 287 | 300 | 306 | |
| 0:100 | 200 354 | 243 410 | 255 455 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Taweel, S.H.; Al-Ahmadi, A.O.; Alhaddad, O.; Okasha, R.M. Cationic Cyclopentadienyliron Complex as a Novel and Successful Nucleating Agent on the Crystallization Behavior of the Biodegradable PHB Polymer. Molecules 2018, 23, 2703. https://doi.org/10.3390/molecules23102703
El-Taweel SH, Al-Ahmadi AO, Alhaddad O, Okasha RM. Cationic Cyclopentadienyliron Complex as a Novel and Successful Nucleating Agent on the Crystallization Behavior of the Biodegradable PHB Polymer. Molecules. 2018; 23(10):2703. https://doi.org/10.3390/molecules23102703
Chicago/Turabian StyleEl-Taweel, Safaa H., Arwa O. Al-Ahmadi, Omaima Alhaddad, and Rawda M. Okasha. 2018. "Cationic Cyclopentadienyliron Complex as a Novel and Successful Nucleating Agent on the Crystallization Behavior of the Biodegradable PHB Polymer" Molecules 23, no. 10: 2703. https://doi.org/10.3390/molecules23102703
APA StyleEl-Taweel, S. H., Al-Ahmadi, A. O., Alhaddad, O., & Okasha, R. M. (2018). Cationic Cyclopentadienyliron Complex as a Novel and Successful Nucleating Agent on the Crystallization Behavior of the Biodegradable PHB Polymer. Molecules, 23(10), 2703. https://doi.org/10.3390/molecules23102703