Enrichment and Purification of the Bioactive Flavonoids from Flower of Abelmoschus manihot (L.) Medic Using Macroporous Resins
Abstract
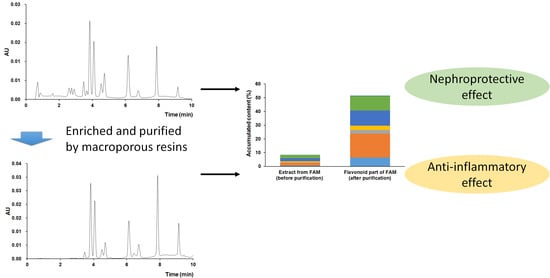
1. Introduction
2. Results and Discussion
2.1. Screening of Macroporous Resins
2.2. Adsorption Kinetics of FAM Flavonoids on HPD-100 Resins
2.3. Adsorption Isotherms of FAM Flavonoids on HPD-100 Resins
2.4. Optimization of Conditions for Purification of FAM Flavonoids by HPD-100 Resins
2.5. Process Scale-Up
2.6. Protective Effects on the Adriamycin-Damaged HK-2 Cells
2.7. Anti-Inflammatory Effects on the LPS-Stimulated Raw 264.7 Cells
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Pretreatment of Macroporous Resins
3.3. Static Adsorption and Desorption Tests
3.4. Adsorption Kinetics of Flavonoids from FAM on Macroporous Resin
3.5. Adsorption Isotherms of Flavonoids from FAM on Macroporous Resin
3.6. Optimization of Conditions for Removing Impurity by HPD-100 Resin
3.7. Optimization of Conditions for Desorption of FAM Flavonoids from HPD-100 Resin
3.8. Determination of the Total Flavonoid Content
3.9. Determination of Flavonoids by a Single Reference Standard
3.10. Cell Culture
3.11. Cell Viability Assay
3.12. Cell Viability on the Adriamycin-Damaged HK-2 Cells
3.13. NO production on the LPS-Stimulated Raw 264.7 Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rubiang-Yalambing, L.; Arcot, J.; Greenfield, H.; Holford, P. Aibika (Abelmoschus manihot L.): Genetic variation, morphology and relationships to micronutrient composition. Food Chem. 2016, 193, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Todarwal, A.; Jain, P.; Bari, S. Abelmoschus manihot Linn: Ethnobotany, phytochemistry and pharmacology. Asian J. Tradit. Med. 2011, 6, 1–7. [Google Scholar]
- Prabawardani, S.; Djuuna, I.A.F.; Asyerem, F.; Yaku, A.; Lyons, G. Morphological diversity and the cultivation practice of Abelmoschus manihot in West Papua, Indonesia. Biodiversitas 2016, 17, 894–999. [Google Scholar] [CrossRef]
- Du, L.; Qian, D.; Jiang, S.; Guo, J.; Su, S.; Duan, J. Comparative characterization of amino acids in Abelmoschus manihot roots, stems and leaves during different growth periods by UPLC-TQ-MS/MS. Anal. Methods 2015, 7, 10280–10290. [Google Scholar] [CrossRef]
- Du, L.; Qian, D.; Jiang, S.; Shang, E.; Guo, J.; Liu, P.; Su, S.; Duan, J.; Zhao, M. Comparative characterization of nucleotides, nucleosides and nucleobases in Abelmoschus manihot roots, stems, leaves and flowers during different growth periods by UPLC-TQ-MS/MS. J. Chromatogr. B 2015, 1006, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, D.; Xu, C.; Chen, Y. Research Progress of Abelmoschi Corolla. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 231–234. [Google Scholar]
- Zhang, L.; Li, P.; Xing, C.; Zhao, J.; He, Y.; Wang, J.; Wu, X.; Liu, Z.; Zhang, A.; Lin, H.; et al. Efficacy and Safety of Abelmoschus manihot for Primary Glomerular Disease: A Prospective, Multicenter Randomized Controlled Clinical Trial. Am. J. Kidney Dis. 2014, 64, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Shen, S.; Wan, Y.; Sun, W.; Chen, H.; Huang, M.; Yang, J.; Wu, W.; Tang, H.; Tang, R. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to alpha-lipoic acid. J. Ethnopharmacol. 2015, 173, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Sun, W.; Wan, Y.; Che, X.; Pu, H.; Yin, X.; Chen, H.; Meng, X.; Huang, Y.; Shi, X. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, ameliorates adriamycin-induced renal inflammation and glomerular injury via inhibiting p38MAPK signaling pathway activity in rats. J. Ethnopharmacol. 2013, 147, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Miao, J.; Sun, X.; Yu, J. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-alpha/gamma and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol. 2016, 189, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Jiang, S.; Guo, J.; Qian, D.; Duan, J.; Shang, E. Screening for in vitro metabolites of Abelmoschus manihot extract in intestinal bacteria by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2011, 879, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, S.; Zhou, L.; Gao, X.; Ju, W.; Tan, H.; Yang, C. Effects of HuangKui capsules on glibenclamide pharmacokinetics in rats. J. Ethnopharmacol. 2012, 139, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Guo, J.; Qian, D.; Duan, J.; Shang, E.; Shu, Y.; Lu, Y. Identification of the potential active components of Abelmoschus manihot in rat blood and kidney tissue by microdialysis combined with ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2011, 879, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, J.; Zhao, L.; Zou, H.; Yan, Y. Extraction process of total flavonoids of Abelmoschus manihot. J. Int. Pharm. Res. 2016, 43, 370–373. [Google Scholar]
- Yu, H.; Liu, H.; Liu, J.; Tang, H.; Zhang, P.; Zhang, J. Purification of total flavonoids from Abelmoschus manihot (L.) Medic by D-101 microporous resin. Chin. Tradit. Pat. Med. 2014, 36, 520–525. [Google Scholar]
- Zhang, H.; Zheng, W.; Wei, Y.; Zhang, J.; Li, C. Optimization of Purification Process of Total Flavonoids from Abelmoschi Corolla by Macroporous Resin. Chin. J. Exp. Tradit. Med Formulae 2015, 21, 28–31. [Google Scholar]
- Li, J.; Zhang, J.; Wang, M. Extraction of Flavonoids from the Flowers of Abelmoschus manihot (L.) Medic by Modified Supercritical CO2 Extraction and Determination of Antioxidant and Anti-Adipogenic Activity. Molecules 2016, 21, 810. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.Z.; Yue, J.H.; Shan, L.; Tao, G.J.; Wang, X.G.; Qiu, A.Y. Process research of macroporous resin chromotography for separation of N-(p-coumaroyl)serotonin and N-feruloylserotonin from Chinese safflower seed extracts. Sep. Purif. Technol. 2008, 62, 370–375. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, D.W.; Bao, T.; Xie, J.H.; Chen, W. A simple and rapid method for the preparation of pure delphinidin-3-O-sambubioside from Roselle and its antioxidant and hypoglycemic activity. J. Funct. Food. 2017, 39, 9–17. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Xiao, W.; Wang, Z.; Ding, G.; Zhao, L. Enrichment and Purification of Total Ginkgo Flavonoid O-Glycosides from Ginkgo Biloba Extract with Macroporous Resin and Evaluation of Anti-Inflammation Activities In Vitro. Molecules (Basel, Switzerland) 2018, 23, 1167. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, Y.; Chen, X.; Zhao, X. LC/MS guided approach to discovering nephroprotective substances from Huangkui capsule. J. Zhejiang Univ. (Med. Sci.) 2017, 46, 66–73. [Google Scholar]
- Cao, Q.; Harris, D.C.H.; Wang, Y. Macrophages in Kidney Injury, Inflammation, and Fibrosis. Physiology 2015, 30, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D. Nutritional strategies to reduce inflammation in chronic kidney disease patients. Nutrition 2015, 31, 1054. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Shin, H. Effects of 1,7-substituted methylxanthine derivatives on LPS-stimulated expression of cytokines and chemokines in Raw 264.7 and HK-2 cells. J. Microbiol. Biotechnol. 2015, 25, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zou, X.; Zhao, J.; Mel, H.; Wang, K.; Wang, X.; Chen, H. Determination of total flavonoids content in fresh Ginkgo biloba leaf with different colors using near infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 94, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, L.; Wu, M.; Liu, J.; Zhang, Y.; Jiang, K.; Wang, B. Antioxidant Activities and Total Flavonoid Content in the Peel of Leucaena leucocephala Fruits. Food Sci. 2015, 36, 187–190. [Google Scholar]
- Lu, L.; Qian, D.; Guo, J.; Xu, B.; Sha, M.; Duan, J. A quantitative method using one marker for simultaneous assay of seven flavonoids in the flowers of Abelmoschus manihot. Chin. J. Pharm. Anal. 2013, 33, 2082–2087. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |




| Macroporous Resin | Polarity | Material | Particle Size (mm) | Surface Area (m2/g) | Average Pore Diameter (Å) |
|---|---|---|---|---|---|
| HPD-100 | Non-polar | Polystyrene | 0.3~1.2 | 650~700 | 85~90 |
| D4020 | Non-polar | Polystyrene | 0.30~1.25 | 540~580 | 100~105 |
| ADS-8 | Non-polar | Polystyrene | 0.30~1.25 | 450~500 | 120~160 |
| AB-8 | Weak-polar | Polystyrene | 0.30~1.25 | 480~520 | 130~140 |
| ADS-17 | Moderately-polar | Polystyrene | 0.30~1.25 | 90~150 | 250~300 |
| NKA-II | Polar | Polystyrene | 0.30~1.25 | 160~200 | 145~155 |
| Temperature (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qm | Kl | R2 | 1/n | Kf | R2 | |
| 25 | 131.3420 | 0.0362 | 0.9948 | 0.4575 | 252.7008 | 0.9441 |
| 35 | 125.8467 | 0.0346 | 0.9826 | 0.4603 | 247.3965 | 0.9690 |
| 45 | 121.3182 | 0.0385 | 0.9913 | 0.4482 | 219.4200 | 0.9586 |
| Level | Factor | ||
|---|---|---|---|
| A (Ethanol Concentration) | B (Solvent Volume) | C (Flow Rate) | |
| 1 | 10% | 2 BV | 20 mL/(cm2·h) |
| 2 | 20% | 3 BV | 40 mL/(cm2·h) |
| 3 | 30% | 4 BV | 60 mL/(cm2·h) |
| Level | Factor | ||
|---|---|---|---|
| D (Ethanol Concentration) | E (Solvent Volume) | F (Flow Rate) | |
| 1 | 50% | 2 BV | 20 mL/(cm2·h) |
| 2 | 60% | 4 BV | 40 mL/(cm2·h) |
| 3 | 70% | 6 BV | 60 mL/(cm2·h) |
| Trial No. | A (Ethanol Concentration) | B (Solvent Volume) | C (Flow Rate) | Contents of 7 Main Flavonoids/% |
|---|---|---|---|---|
| 1 | 10% | 2 BV | 20 mL/(cm2·h) | 22.44 |
| 2 | 20% | 2 BV | 40 mL/(cm2·h) | 29.77 |
| 3 | 30% | 2 BV | 60 mL/(cm2·h) | 40.61 |
| 4 | 10% | 3 BV | 40 mL/(cm2·h) | 27.81 |
| 5 | 20% | 3 BV | 60 mL/(cm2·h) | 33.91 |
| 6 | 30% | 3 BV | 20 mL/(cm2·h) | 44.06 |
| 7 | 10% | 4 BV | 60 mL/(cm2·h) | 27.70 |
| 8 | 20% | 4 BV | 20 mL/(cm2·h) | 34.14 |
| 9 | 30% | 4 BV | 40 mL/(cm2·h) | 44.20 |
| k1 | 25.98 | 30.94 | 33.55 | |
| k2 | 32.61 | 35.26 | 33.93 | |
| k3 | 42.96 | 35.35 | 34.07 | |
| R | 16.97 | 4.40 | 0.53 |
| Trial No. | D (Ethanol Concentration) | E (Solvent Volume) | F (Flow Rate) | Contents of 7 Main Flavonoids/% |
|---|---|---|---|---|
| 1 | 50% | 2 BV | 20 mL/(cm2·h) | 43.23 |
| 2 | 60% | 2 BV | 40 mL/(cm2·h) | 43.32 |
| 3 | 70% | 2 BV | 60 mL/(cm2·h) | 44.79 |
| 4 | 50% | 4 BV | 40 mL/(cm2·h) | 50.37 |
| 5 | 60% | 4 BV | 60 mL/(cm2·h) | 49.37 |
| 6 | 70% | 4 BV | 20 mL/(cm2·h) | 51.02 |
| 7 | 50% | 6 BV | 60 mL/(cm2·h) | 53.72 |
| 8 | 60% | 6 BV | 20 mL/(cm2·h) | 51.51 |
| 9 | 70% | 6 BV | 40 mL/(cm2·h) | 48.52 |
| k1 | 49.11 | 43.78 | 48.59 | |
| k2 | 48.07 | 50.25 | 47.40 | |
| k3 | 48.11 | 51.25 | 49.29 | |
| R | 1.04 | 7.46 | 1.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Tang, H.; Shao, Q.; Bilia, A.R.; Wang, Y.; Zhao, X. Enrichment and Purification of the Bioactive Flavonoids from Flower of Abelmoschus manihot (L.) Medic Using Macroporous Resins. Molecules 2018, 23, 2649. https://doi.org/10.3390/molecules23102649
Yang Z, Tang H, Shao Q, Bilia AR, Wang Y, Zhao X. Enrichment and Purification of the Bioactive Flavonoids from Flower of Abelmoschus manihot (L.) Medic Using Macroporous Resins. Molecules. 2018; 23(10):2649. https://doi.org/10.3390/molecules23102649
Chicago/Turabian StyleYang, Zhenzhong, Haitao Tang, Qing Shao, Anna Rita Bilia, Yi Wang, and Xiaoping Zhao. 2018. "Enrichment and Purification of the Bioactive Flavonoids from Flower of Abelmoschus manihot (L.) Medic Using Macroporous Resins" Molecules 23, no. 10: 2649. https://doi.org/10.3390/molecules23102649
APA StyleYang, Z., Tang, H., Shao, Q., Bilia, A. R., Wang, Y., & Zhao, X. (2018). Enrichment and Purification of the Bioactive Flavonoids from Flower of Abelmoschus manihot (L.) Medic Using Macroporous Resins. Molecules, 23(10), 2649. https://doi.org/10.3390/molecules23102649