SIKVAV-Modified Chitosan Hydrogel as a Skin Substitutes for Wound Closure in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the SIKVAV-Modified Chitosan Hydrogel
2.3. In Vivo Studies of Skin Wound Healing in Mice Using the SIKVAV-Modified Chitosan Hydrogel
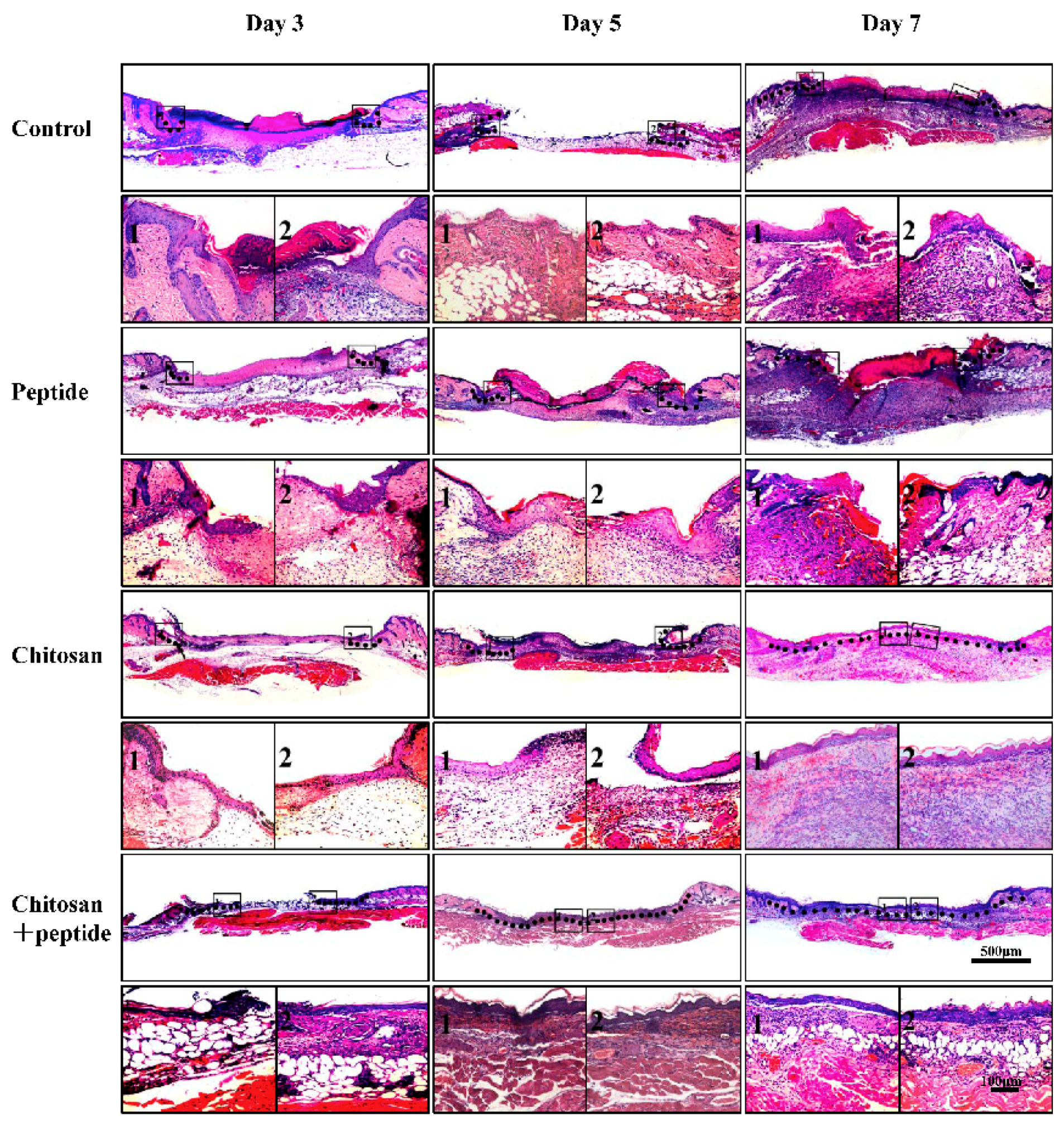
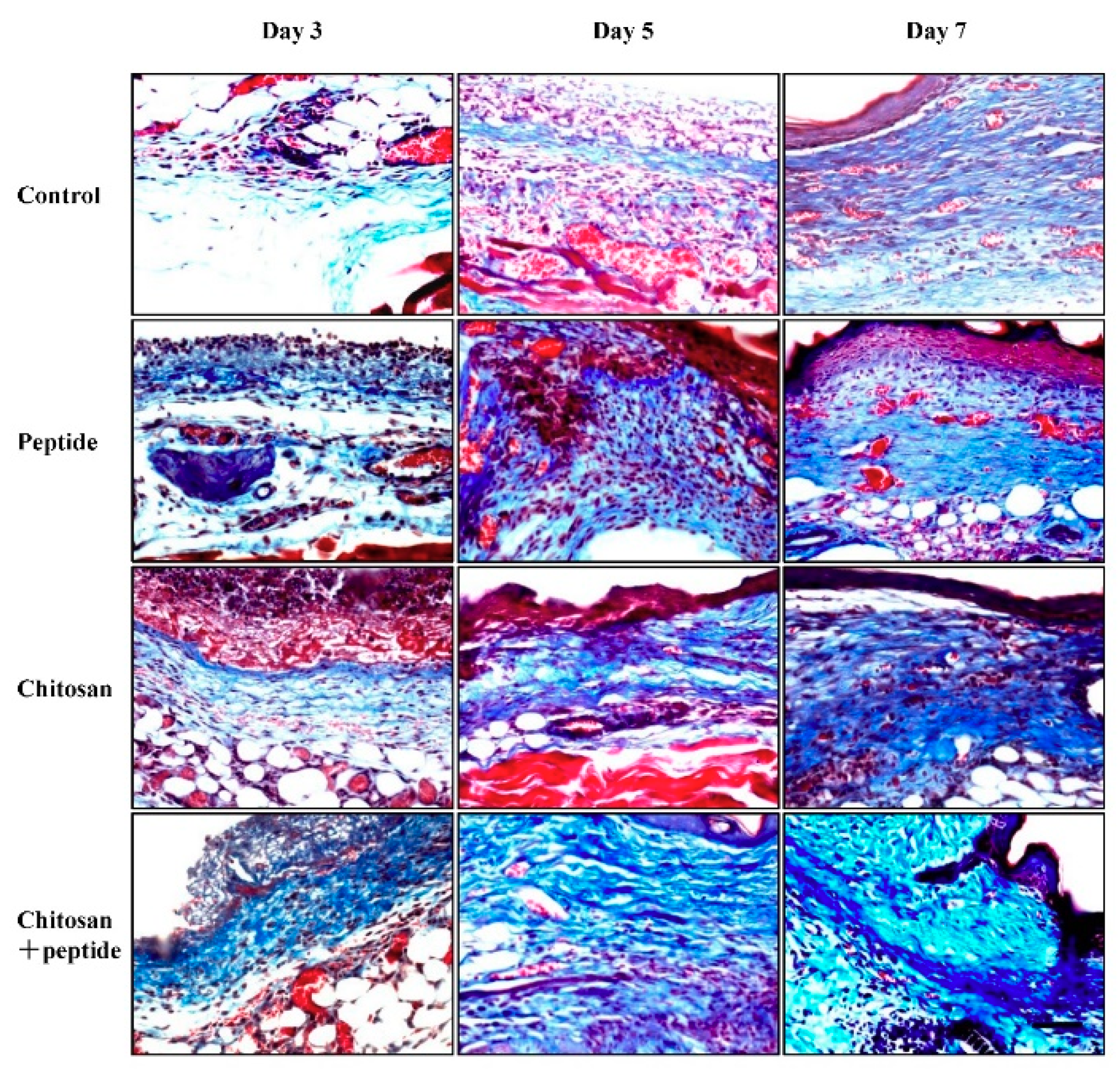
2.4. Histological Observations
2.5. Immunohistochemistry Assays
2.6. ELISA Assay
2.7. Statistical Analysis
3. Results
3.1. The SIKVAV-Modified Chitosan Hydrogel Promoted Skin Wound Contraction
3.2. The SIKVAV-Modified Chitosan Hydrogel Accelerated Skin Wound Re-Epithelialization
3.3. The SIKVAV-Modified Chitosan Hydrogel Promoted Skin Wound Angiogenesis
3.4. The SIKVAV-Modified Chitosan Hydrogel Promoted Skin Wound Collagen Synthesis
3.5. The SIKVAV-Modified Chitosan Hydrogel Promoted the Secretion of Growth Factors in Skin Wounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zoller, N.; Valesky, E.; Butting, M.; Hofmann, M.; Kippenberger, S.; Bereiter-Hahn, J.; Bernd, A.; Kaufmann, R. Clinical application of a tissue-cultured skin autograft: An alternative for the treatment of non-healing or slowly healing wounds? Dermatology 2014, 229, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bartolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Aoki, R. Roles of lactoferrin on skin wound healing. Biochem. Cell Biol. 2012, 90, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.; Lam, M.; Mitchell, R.; Black, D.; Taylor, C.; Dickson, C.; Jan, S.; Palmer, C.S.; Langcake, M.; Myburgh, J. Acute costs and predictors of higher treatment costs of trauma in New South Wales, Australia. Injury 2014, 45, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Santema, T.B.; Poyck, P.P.; Ubbink, D.T. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2016, 2, CD011255. [Google Scholar] [CrossRef] [PubMed]
- Seeger, M.A.; Paller, A.S. The roles of growth factors in keratinocyte migration. Adv. Wound Care 2015, 4, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Losi, P.; Briganti, E.; Errico, C.; Lisella, A.; Sanguinetti, E.; Chiellini, F.; Soldani, G. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013, 9, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Hakvoort, T.; Altun, V.; van Zuijlen, P.P.; de Boer, W.I.; van Schadewij, W.A.; van der Kwast, T.H. Transforming growth factor-beta(1), -beta(2), -beta(3), basic fibroblast growth factor and vascular endothelial growth factor expression in keratinocytes of burn scars. Eur. Cytokine Netw. 2000, 11, 233–239. [Google Scholar] [PubMed]
- Negrao, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Azevedo, I.; Soares, R. Different effects of catechin on angiogenesis and inflammation depending on VEGF levels. J. Nutr. Biochem. 2013, 24, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, J.; Durbeej, M. Laminin-211 in skeletal muscle function. Cell Adh. Migr. 2013, 7, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Pesapane, A.; Di Giovanni, C.; Rossi, F.W.; Alfano, D.; Formisano, L.; Ragno, P.; Selleri, C.; Montuori, N.; Lavecchia, A. Discovery of new small molecules inhibiting 67 kDa laminin receptor interaction with laminin and cancer cell invasion. Oncotarget 2015, 6, 18116–18133. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Fusaro, L.; Mosca, C.; Bosetti, M.; Chevallier, P.; Mantovani, D.; Cannas, M. The biological response of poly(l-lactide) films modified by different biomolecules: Role of the coating strategy. J. Biomed. Mater. Res. A 2012, 100, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y.; Suzuki, K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Kubinova, S.; Horak, D.; Vanecek, V.; Plichta, Z.; Proks, V.; Sykova, E. The use of new surface-modified poly(2-hydroxyethyl methacrylate) hydrogels in tissue engineering: Treatment of the surface with fibronectin subunits versus Ac-CGGASIKVAVS-OH, cysteine, and 2-mercaptoethanol modification. J. Biomed. Mater. Res. Part A 2014, 102, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liao, S.; Quan, D.; Ngiam, M.; Chan, C.K.; Ramakrishna, S.; Lu, J. The influence of laminin-derived peptides conjugated to Lys-capped PLLA on neonatal mouse cerebellum C17.2 stem cells. Biomaterials 2009, 30, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Gerente, C.; Lee, V.K.C.; Le Cloirec, P.; McKay, G. Application of chitosan for the removal of metals from wastewaters by adsorption—Mechanisms and models review. Crit. Rev. Env. Sci. Tech. 2007, 37, 41–127. [Google Scholar] [CrossRef]
- Harkins, A.L.; Duri, S.; Kloth, L.C.; Tran, C.D. Chitosan-cellulose composite for wound dressing material. Part 2. Antimicrobial activity, blood absorption ability, and biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Panzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lefler, A.; Ghanem, A. Development of bFGF-chitosan matrices and their interactions with human dermal fibroblast cells. J. Biomater. Sci. Polym. E 2009, 20, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.H.; Tegos, G.P.; Burkatovskaya, M.; Castano, A.P.; Hamblin, M.R. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob. Agents Chemother. 2009, 53, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Zhang, M.; Shao, X.B.; Wang, X.; Zhang, L.; Xu, P.C.; Zhong, W.; Zhang, L.; Xing, M.; Zhang, L. A laminin mimetic peptide SIKVAV-conjugated chitosan hydrogel promoting wound healing by enhancing angiogenesis, re-epithelialization and collagen deposition. J. Mater. Chem. B 2015, 3, 6798–6804. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhang, M.; Wang, X.E.; Chen, Y.H.; Yan, Y.; Zhang, L.; Zhang, L. Peptide-modified chitosan hydrogels promote skin wound healing by enhancing wound angiogenesis and inhibiting inflammation. Am. J. Trans. Res. 2017, 9, 2352–2362. [Google Scholar]
- Chaudhuri, V.; Zhou, L.; Karasek, M. Inflammatory cytokines induce the transformation of human dermal microvascular endothelial cells into myofibroblasts: A potential role in skin fibrogenesis. J. Cutan. Pathol. 2007, 34, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Servat-Medina, L.; Gonzalez-Gomez, A.; Reyes-Ortega, F.; Sousa, I.M.O.; Queiroz, N.D.A.; Zago, P.M.W.; Jorge, M.P.; Monteiro, K.M.; de Carvalho, J.E.; Roman, J.S.; et al. Chitosan-tripolyphosphate nanoparticles as arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int. J. Nanomed. 2015, 10, 3897–3909. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Ide, S.; Tokuyama, R.; Umeki, H.; Tatehara, S.; Kataoka, S.; Satomura, K. Leptin promotes wound healing in the skin. PLoS ONE 2015, 10, e0121242. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Singh, V.; Kumawat, S.; Kant, V.; Tandan, S.K.; Kumar, D. Bilirubin modulated cytokines, growth factors and angiogenesis to improve cutaneous wound healing process in diabetic rats. Int. Immunopharmacol. 2016, 30, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kibbey, M.C.; Grant, D.S.; Kleinman, H.K. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: An in vivo Matrigel model. J. National Cancer Inst. 1992, 84, 1633–1638. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial PECAM-1 and its function in vascular physiology and atherogenic pathology. Exp. Mol. Pathol. 2016, 100, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Koster, K.P.; Thomas, R.; Morris, A.W.; Tai, L.M. Epidermal growth factor prevents oligomeric amyloid-beta induced angiogenesis deficits in vitro. J. Cereb. Blood Flow Metab. 2016, 36, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Ju, Z.C.; Zhang, J.F.; Liu, X.C.; Tian, J.; Mu, G.Y. Effects of insulin-like growth factor 2 and its receptor expressions on corneal repair. Int. J. Clin. Exp. Pathol. 2015, 8, 10185–10191. [Google Scholar] [PubMed]
- Kanji, S.; Das, H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediat. Inflamm. 2017. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The materials generated during the study are available from the corresponding author on reasonable request. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Cao, X.; Jiang, H.; Che, X.; Xu, X.; Ma, B.; Zhang, J.; Huang, T. SIKVAV-Modified Chitosan Hydrogel as a Skin Substitutes for Wound Closure in Mice. Molecules 2018, 23, 2611. https://doi.org/10.3390/molecules23102611
Chen X, Cao X, Jiang H, Che X, Xu X, Ma B, Zhang J, Huang T. SIKVAV-Modified Chitosan Hydrogel as a Skin Substitutes for Wound Closure in Mice. Molecules. 2018; 23(10):2611. https://doi.org/10.3390/molecules23102611
Chicago/Turabian StyleChen, Xionglin, Xiaoming Cao, He Jiang, Xiangxin Che, Xiaoyuan Xu, Baicheng Ma, Jie Zhang, and Tao Huang. 2018. "SIKVAV-Modified Chitosan Hydrogel as a Skin Substitutes for Wound Closure in Mice" Molecules 23, no. 10: 2611. https://doi.org/10.3390/molecules23102611
APA StyleChen, X., Cao, X., Jiang, H., Che, X., Xu, X., Ma, B., Zhang, J., & Huang, T. (2018). SIKVAV-Modified Chitosan Hydrogel as a Skin Substitutes for Wound Closure in Mice. Molecules, 23(10), 2611. https://doi.org/10.3390/molecules23102611



