Establishing the Phenolic Composition of Olea europaea L. Leaves from Cultivars Grown in Morocco as a Crucial Step Towards Their Subsequent Exploitation
Abstract
:1. Introduction
2. Results and Discussion
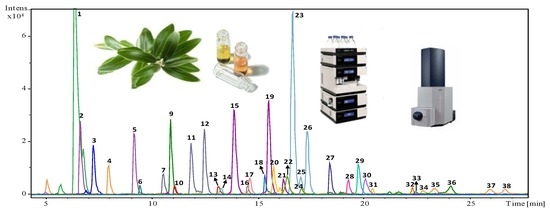
2.1. Profiling and Qualitative Characterization of the Phenolic Fraction of Olive Leaves from the Selected Eleven Cultivars
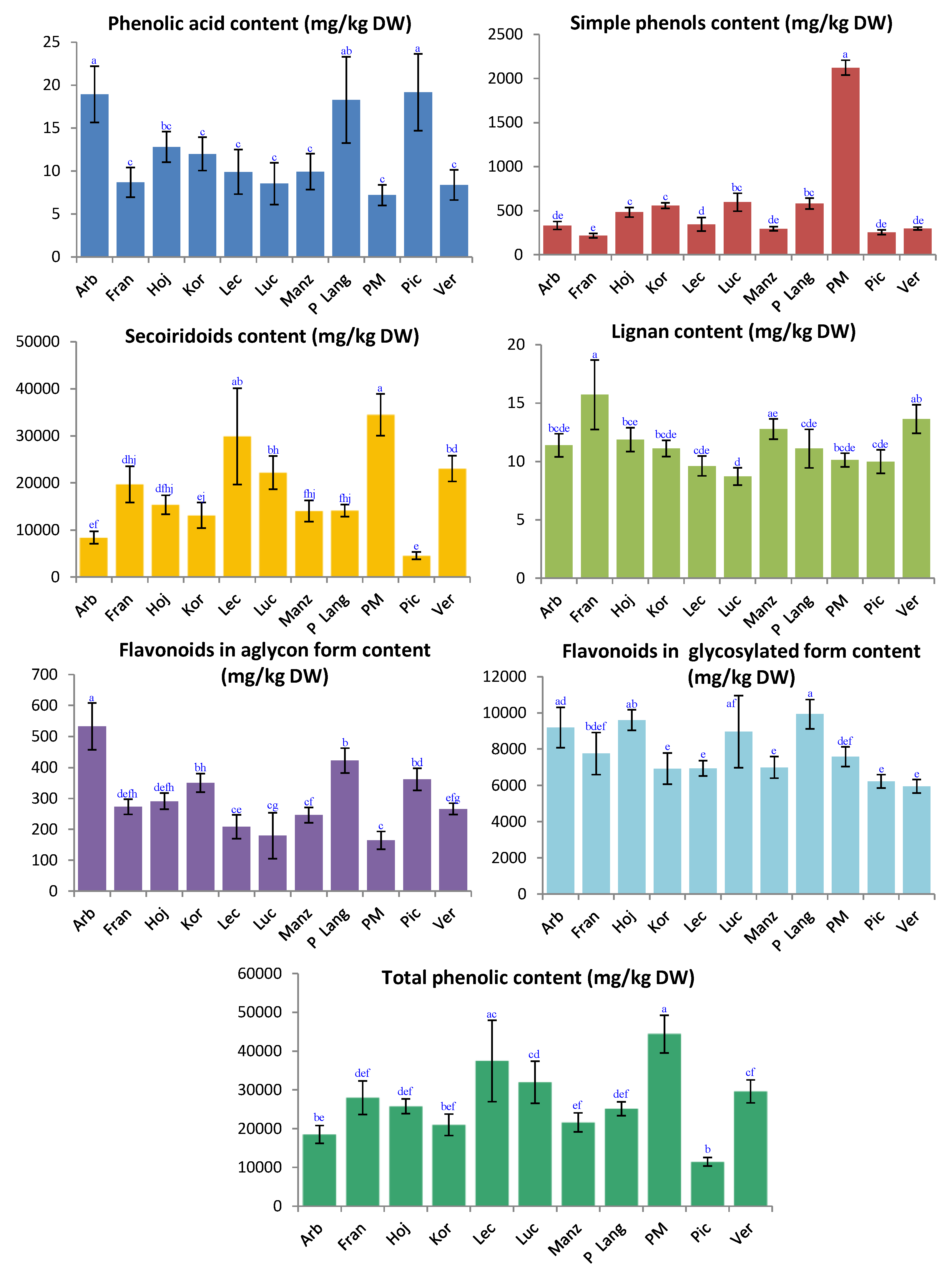
2.2. Phenolic Contents in Different Olive Leaves Cultivars
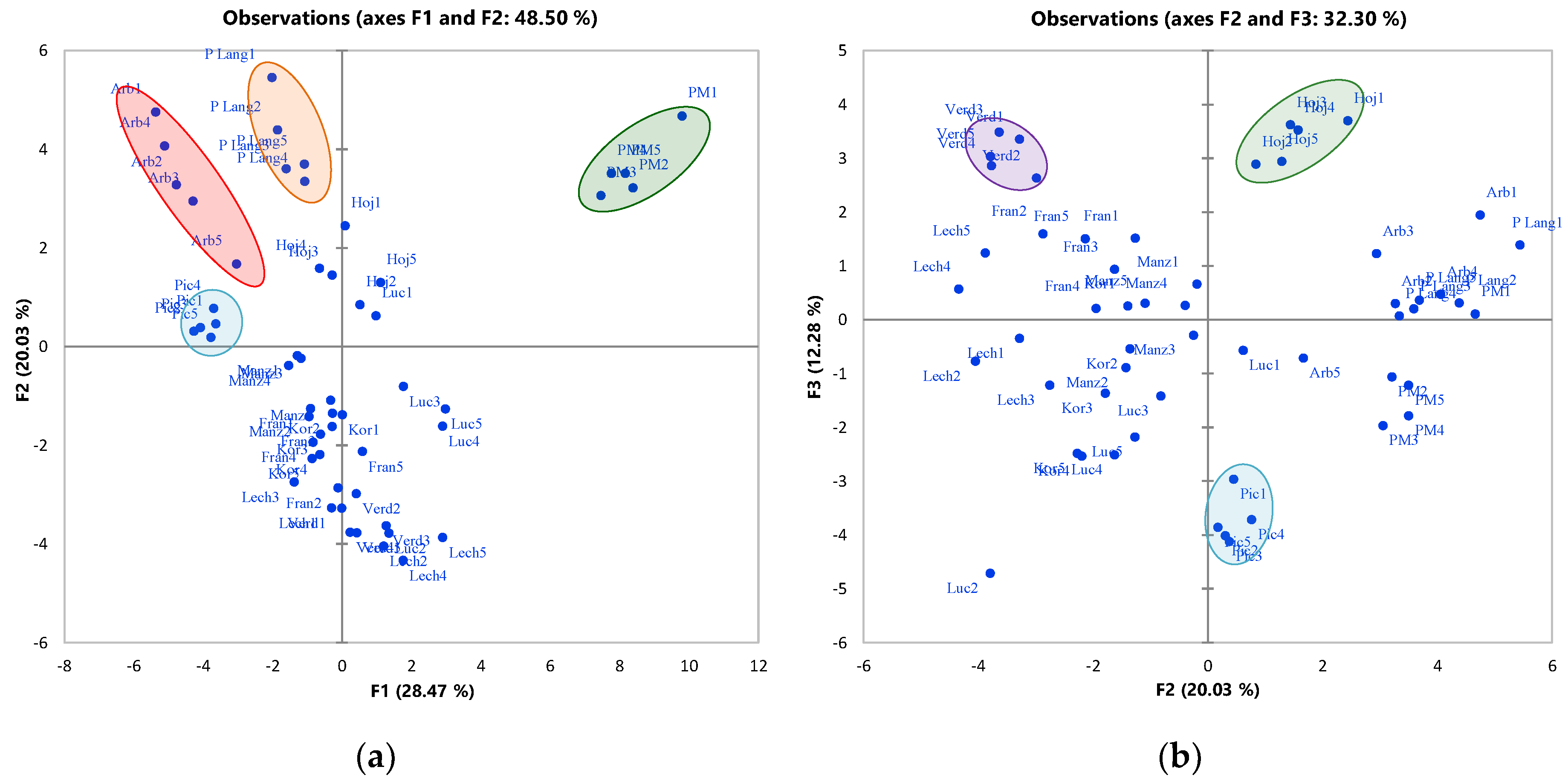
2.3. Varietal Discrimination
3. Materials and Methods
3.1. Olive Leaves Sampling and Preparation
3.2. Phenolic Compounds Profiling
3.2.1. Chemical and Reagents
3.2.2. Phenolic Compounds Extraction
3.2.3. Analytical Procedure and MS Conditions
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Olive Council. World Olive Oil Production Statistics. Available online: http://www.internationaloliveoil.org/estaticos/view/131-world-olive-oil-figures (accessed on 15 August 2018).
- Sarnari, T. La Scheda di Settore Olio di Oliva. Available online: http://www.cposalerno.it/file/2013/12/Scheda-di-settore-olio-da-olive-Ismea-2017.pdf. (accessed on 18 August 2018).
- Kostelenos, G.; Kiritsakis, A. Olive tree history and evolution. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing; Kiritsakis, A., Shahidi, F., Eds.; Wiley: Oxford, UK, 2017; ISBN 1119135338. [Google Scholar]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Zhao, X.-B.; Wang, L.; Liu, D.-H. Effect of several factors on peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2007, 82, 1115–1121. [Google Scholar] [CrossRef]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of agro-industrial by-products, effluents and waste: Concept, opportunities and the case of olive mill waste waters. J. Chem. Technol. Biotechnol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- Skaltsounis, A.L.; Argyropoulou, A.; Aligiannis, N.; Xynos, N. Recovery of high added value compounds from olive tree products and olive processing byproducts. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; Elsevier Inc.: Urbana, ID, USA, 2015; pp. 333–356. ISBN 9781630670429. [Google Scholar]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2017, 98, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Khemakhem, I.; Gargouri, O.D.; Dhouib, A.; Ayadi, M.A.; Bouaziz, M. Oleuropein rich extract from olive leaves by combining microfiltration, ultrafiltration and nanofiltration. Sep. Purif. Technol. 2017, 172, 310–317. [Google Scholar] [CrossRef]
- Baldino, L.; Della Porta, G.; Osseo, L.S.; Reverchon, E.; Adami, R. Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluids 2018, 133, 65–69. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Cittan, M.; Çelik, A. Development and Validation of an Analytical Methodology Based on Liquid Chromatography–Electrospray Tandem Mass Spectrometry for the Simultaneous Determination of Phenolic Compounds in Olive Leaf Extract. J. Chromatogr. Sci. 2018, 56, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; You, F.; Zhang, Y.L. Reduced pressure extraction of oleuropein from olive leaves (Olea europaea L.) with ultrasound assistance. Food Bioprod. Process. 2015, 93, 29–38. [Google Scholar] [CrossRef]
- Khemakhem, I.; Ahmad-Qasem, M.H.; Catalán, E.B.; Micol, V.; García-Pérez, J.V.; Ayadi, M.A.; Bouaziz, M. Kinetic improvement of olive leaves’ bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrason. Sonochem. 2017, 34, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.X.; Rico, D.; McHugh, T.H. Antimicrobial Olive Leaf Gelatin films for enhancing the quality of cold-smoked Salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Moudache, M.; Nerín, C.; Colon, M.; Zaidi, F. Antioxidant effect of an innovative active plastic film containing olive leaves extract on fresh pork meat and its evaluation by Raman spectroscopy. Food Chem. 2017, 229, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Peker, H.; Arslan, S. Effect of Olive Leaf Extract on the Quality of Low Fat Apricot Yogurt. J. Food Process. Preserv. 2017, 41, 1–10. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P.P. Olive by-products: Challenge application in cosmetic industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Moroccan Ministry of Agriculture and Marine Fisheries. Statistiques du Secteur Oléicole Marocain. Available online: http://www.agriculture.gov.ma/pages/rapports-statistiques/campagne-agricole-2015-2016 (accessed on 11 February 2018).
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of “Sikitita” olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents “Arbequina” and “Picual” olive leaves. LWT-Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; Roldán, C.; León, L.; De la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Chemometric Analysis for the Evaluation of Phenolic Patterns in Olive Leaves from Six Cultivars at Different Growth Stages. J. Agric. Food Chem. 2015, 63, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Shirzad, H.; Niknam, V.; Taheri, M.; Ebrahimzadeh, H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: A nutraceutical study using RSM and LC–ESI–DAD–MS. J. Food Sci. Technol. 2017, 54, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.; Contreras, M.D.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS based metabolic profiling of the potential Olea europaea by-product “wood” and its comparison with leaf counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Vezza, T.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Gálvez, J.; Segura-Carretero, A. Phenolic compounds and in vitro immunomodulatory properties of three Andalusian olive leaf extracts. J. Funct. Foods 2016, 22, 270–277. [Google Scholar] [CrossRef]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. Quantification of bioactive compounds in Picual and Arbequina olive leaves and fruit. J. Sci. Food Agric. 2017, 97, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Zun-Qiu, W.; Gui-Zhou, Y.; Qing-Ping, Z.; You-Jun, J.; Kai-Yu, T.; Hua-Ping, C.; Ze-Shen, Y.; Qian-Ming, H. Purification, Dynamic Changes and Antioxidant Activities of Oleuropein in Olive (Olea europaea L.) Leaves. J. Food Biochem. 2015, 39, 566–574. [Google Scholar] [CrossRef]
- Belaj, A.; Trujillo, I.; de la Rosa, R.; Rallo, L.; Gimenez, M.J. Polymorphism and discrimination capacity of randomly amplified polymorphic markers in an olive germplasm bank. J. Am. Soc. Hortic. Sci. 2001, 126, 64–71. [Google Scholar]
- Linos, A.; Nikoloudakis, N.; Katsiotis, A.; Hagidimitriou, M. Genetic structure of the Greek olive germplasm revealed by RAPD, ISSR and SSR markers. Sci. Hortic. (Amsterdam) 2014, 175, 33–43. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
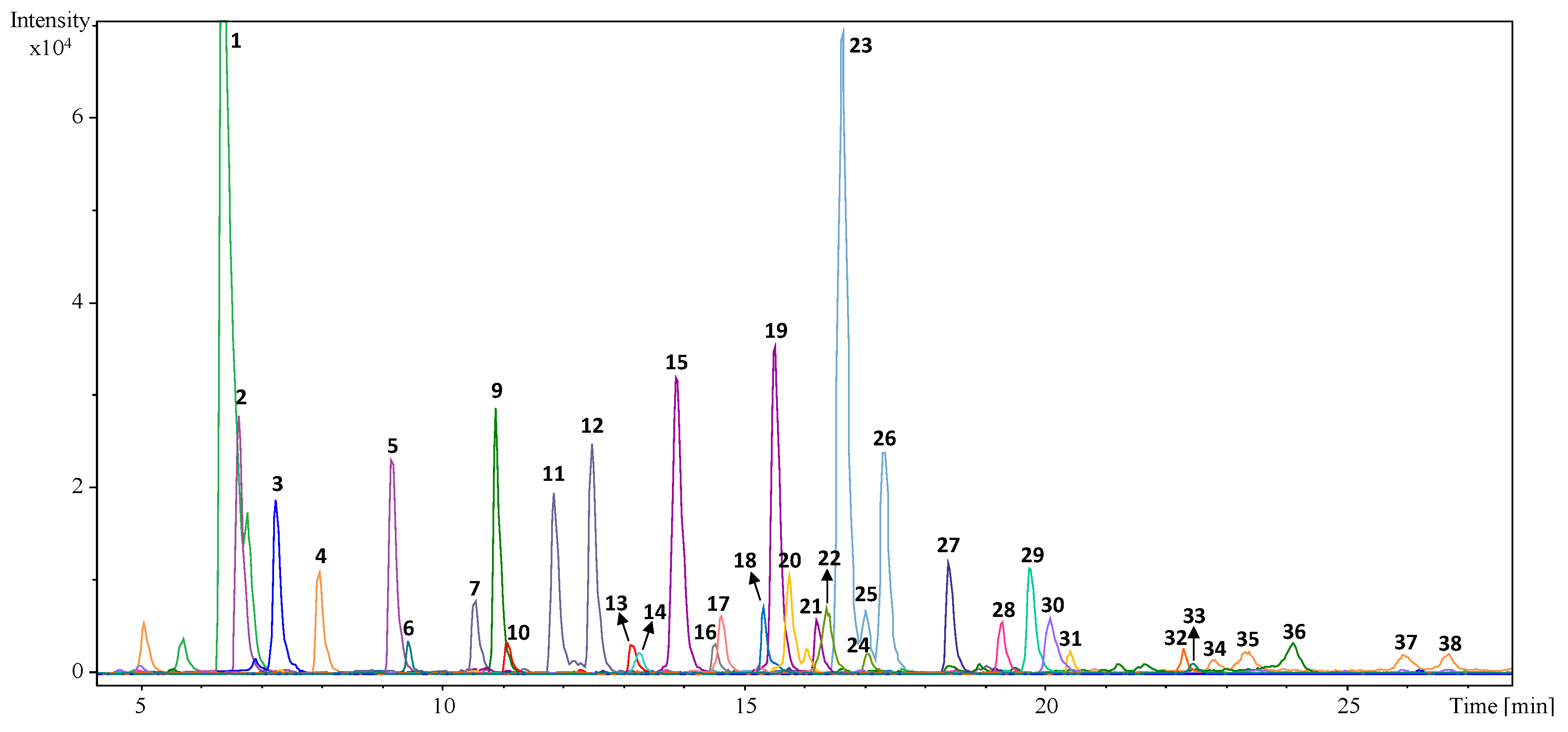
| Peak | Retention Time | Molecular Formula | Experimental m/z * | Calculated m/z | Error (ppm) | mSigma | Suggested Compound |
|---|---|---|---|---|---|---|---|
| 1 | 6.4 | C14H20O8 | 315.1083 | 315.1085 | 0.8 | 6.2 | Hydroxytyrosol glucoside |
| 2 | 6.7 | C16H22O11 | 389.1086 | 389.1089 | 1.0 | 5.2 | Secologanoside is. 1 |
| 3 | 7.3 | C8H10O3 | 153.0557 | 153.0557 | 0.1 | 8.7 | Hydroxytyrosol |
| 4 | 8.1 | C14H20O7 | 299.1131 | 299.1136 | 1.8 | 2.3 | Tyrosol glucoside |
| 5 | 9.2 | C16H22O11 | 389.1088 | 389.1089 | 0.3 | 18 | Secologanoside is. 2 |
| 6 | 9.4 | C8H10O2 | 137.0607 | 137.0608 | 1.0 | 8.2 | Tyrosol |
| 7 | 10.6 | C17H24O11 | 403.1247 | 403.1246 | −0.2 | 6.3 | Elenolic acid glucoside is. 1 |
| 8 | 10.8 | C8H8O4 | 167.0348 | 167.035 | 1.1 | 3.1 | Vanillic acid |
| 9 | 10.9 | C19H22O8 | 377.1446 | 377.1453 | 2.0 | 6.9 | Oleuropein aglycon is. 1 |
| 10 | 11.1 | C27H30O16 | 609.1468 | 609.1461 | −1.2 | 21.4 | Luteolin diglucoside |
| 11 | 11.9 | C17H24O11 | 403.1246 | 403.1246 | 0 | 15.5 | Elenolic acid glucoside is. 2 |
| 12 | 12.5 | C17H24O11 | 403.1239 | 403.1246 | 1.8 | 10.2 | Elenolic acid glucoside is. 3 |
| 13 | 13.2 | C27H30O16 | 609.146 | 609.1461 | 0.1 | 3.1 | Rutin |
| 14 | 13.3 | C25H32O14 | 555.1707 | 555.1719 | 2.2 | 6.2 | Hydroxyoleuropein |
| 15 | 13.9 | C21H20O11 | 447.0934 | 447.0933 | −0.3 | 11 | Luteolin-7-glucoside |
| 16 | 14.5 | C27H30O14 | 577.157 | 577.1563 | −1.3 | 19.3 | Apigenin rutinoside |
| 17 | 14.7 | C31H42O18 | 701.2299 | 701.2298 | 0 | 5.5 | Oleuropein diglucoside |
| 18 | 15. 5 | C21H20O10 | 431.0983 | 431.0984 | 0.2 | 4.9 | Apigenin-7-glucoside |
| 19 | 15.6 | C21H20O11 | 447.0938 | 447.0933 | −1.1 | 8.4 | Luteolin-glucoside is. 1 |
| 20 | 15.7 | C22H22O11 | 461.1086 | 461.1089 | 0.7 | 13.9 | Chrysoeriol-7-glucoside |
| 21 | 16.3 | C21H20O11 | 447.0941 | 447.0933 | −1.8 | 8.1 | Luteolin-glucoside is. 2 |
| 22 | 16.3 | C26H34O14 | 569.1869 | 569.1876 | 1.3 | 24.2 | 2″-methoxyoleuropein is. 1 |
| 23 | 16.7 | C25H32O13 | 539.1769 | 539.1770 | 0.2 | 12.6 | Oleuropein is. 1 |
| 24 | 17.0 | C26H34O14 | 569.1875 | 569.1876 | 0.1 | 2.6 | 2″-methoxyoleuropein is. 2 |
| 25 | 17.1 | C25H32O13 | 539.1766 | 539.1771 | 0.9 | 8.3 | Oleuropein is. 2 |
| 26 | 17.4 | C25H32O13 | 539.1765 | 539.1769 | 0.7 | 4.7 | Oleuropein is. 3 |
| 27 | 18.5 | C25H32O12 | 523.1812 | 523.1821 | 1.8 | 21.5 | Ligstroside |
| 28 | 19.3 | C19H22O7 | 361.1287 | 361.1293 | 1.5 | 2.7 | Ligtroside aglycone |
| 29 | 19.8 | C15H10O6 | 285.0399 | 285.0405 | 2.0 | 16.3 | Luteolin |
| 30 | 20.1 | C15H10O7 | 301.0354 | 301.0354 | 0 | 7.7 | Quercetin |
| 31 | 20.5 | C20H22O6 | 357.1355 | 357.1344 | −3.2 | 3 | Pinoresinol |
| 32 | 22.3 | C15H10O5 | 269.0456 | 269.0455 | −0.3 | 7.4 | Apigenin |
| 33 | 22.5 | C15H12O5 | 271.0612 | 271.0612 | −0.1 | 13.6 | Naringenin |
| 34 | 22.8 | C16H12O6 | 299.0564 | 299.0561 | −1.0 | 16.2 | Diosmetin |
| 35 | 23.3 | C15H8O7 | 299.0202 | 299.0197 | −1.4 | 12.3 | Uk is. 1 |
| 36 | 24.1 | C19 H22O8 | 377.1242 | 377.1242 | −0.1 | 17.1 | Oleuropein aglycon is. 2 |
| 37 | 26.0 | C15H8O7 | 299.0196 | 299.0197 | 0.4 | 6.1 | Uk is. 2 |
| 38 | 26.7 | C15H8O7 | 299.0200 | 299.0197 | −0.9 | 13.9 | Uk is. 3 |
| ‘Arbequina’ | ‘Frantoio’ | ‘Hojiblanca’ | ‘Koroneiki’ | |
|---|---|---|---|---|
| Hydroxytyrosol glucoside | 10 a ± 5 | 22 a ± 7 | 185 b ± 33 | 203 b ± 16 |
| Secologanoside is. 1 | 333 ab ± 28 | 754 e ± 120 | 844 ef ± 80 | 643 de ± 42 |
| Hydroxytyrosol | 209 a ± 39 | 119 b ± 14 | 147 ab ± 23 | 136 b ± 7 |
| Tyrosol glucoside | 61 f ± 5 | 48 ef ± 6 | 120 d ± 13 | 178 b ± 20 |
| Secologanoside is. 2 | 483 ac ± 67 | 1312 bd ± 80 | 1330 bd ± 69 | 769 ce ± 184 |
| Tyrosol | 53 ab ± 10 | 29 cd ± 5 | 31 cd ± 3 | 41 bd ± 7 |
| Elenolic acid glucoside is. 1 | 484 d ± 43 | 850 c ± 63 | 742 b ± 34 | 576 d ± 38 |
| Vanillic acid | 19 a ± 3 | 9 c ± 2 | 13 bc ± 2 | 12 c ± 2 |
| Oleuropein aglycon is. 1 | 48 a ± 11 | 422 b ± 60 | 206 cd ± 20 | 397 b ± 38 |
| Luteolin diglucoside | 626 a ± 79 | 421 c ± 66 | 240 b ± 26 | 355 bc ± 36 |
| Elenolic acid glucoside is. 2 | 95 b ± 13 | 468 ef ± 104 | 431 def ± 15 | 370 def ± 27 |
| Elenolic acid glucoside is. 3 | 73 c ± 4 | 174 e ± 14 | 140 de ± 17 | 135 de ± 20 |
| Rutin | 411 de ± 34 | 542 ce ± 113 | 489 ce ± 53 | 1099 a ± 223 |
| Hydroxyoleuropein | 525 cf ± 54 | 758 de ± 90 | 757 de ± 48 | 843 d ± 43 |
| Luteolin-7-glucoside | 3324 ab ± 375 | 2527 c ± 408 | 3708 a ± 322 | 2632 c ± 191 |
| Apigenin rutinoside | 431 def ± 60 | 354 bdf ± 36 | 542 a ± 65 | 312 bcf ± 46 |
| Oleuropein diglucoside | 94 c ± 19 | 249 efh ± 36 | 458 a ± 24 | 301 df ± 38 |
| Apigenin-7-glucoside | 65 bc ± 12 | 65 bc ± 6 | 246 ad ± 13 | 158 c ± 23 |
| Luteolin-glucoside is. 1 | 3428 ac ± 542 | 3013 abc ± 555 | 3584 c ± 172 | 1630 de ± 513 |
| Chrysoeriol-7-glucoside | 606 b ± 44 | 496 cd ± 27 | 552 bc ± 24 | 387 a ± 41 |
| Luteolin-glucoside is. 2 | 295 cdfgh ± 32 | 341 dgh ± 49 | 230 cbf ± 12 | 347 degh ± 50 |
| 2″-methoxyoleuropein is.1 | 1499 bd ± 194 | 2329 a ± 231 | 2063 ad ± 159 | 1642 bd ± 510 |
| Oleuropein is. 1 | 3465 e ± 960 | 10,959 cdf ± 3283 | 6923 def ± 1813 | 6023 def ± 1679 |
| 2″-methoxyoleuropein is. 2 | 130 e ± 31 | 100 de ± 16 | 176 a ± 22 | 128 e ± 31 |
| Oleuropein is. 2 | 57 ce±21 | 159 def ± 51 | 130 cf ± 54 | 139 cf ± 60 |
| Oleuropein is. 3 | 234 cf ± 50 | 336 cf ± 106 | 440 df ± 116 | 375 ef ± 96 |
| Ligstroside | 505 df ± 92 | 343 cd ± 51 | 406 cd ± 37 | 496 de ± 130 |
| Ligstroside aglycon | 334 bc ± 93 | 142 c ± 104 | 312 c ± 41 | 278 c ± 33 |
| Luteolin | 373 a ± 63 | 189 e ± 23 | 175 de ± 20 | 279 b ± 34 |
| Quercetin | 41 a ± 11 | 14 b ± 3 | 14 b ± 1 | 9 b ± 5 |
| Pinoresinol | 11 bcde ± 1 | 16 a ± 3 | 12 bce ± 1 | 11.1 bcde ± 0.7 |
| Apigenin | 21 bc ± 6 | 12 acdf ± 2 | 17 bde ± 2 | 24 b ± 11 |
| Naringenin | 7 ac ± 1 | 5.4 c ± 0.7 | 6 bc ± 1 | 5.3 c ± 0.4 |
| Diosmetin | 27 a ± 7 | 14 cd ± 2 | 6.2 b ± 0.7 | 15 cd ± 2 |
| Unknown is. 1 | 13 efg ± 3 | 13 ef ± 2 | 21 d ± 2 | 3 b ± 3 |
| Oleuropein aglycon is. 2 | 60 e ± 36 | 359 bc ± 66 | 18 e ± 5 | 13 e ± 5 |
| Unknown is. 2 | 36 a ± 6 | 14 cd ± 2 | 28 a ± 2 | 8 bc ± 4 |
| Unknown is. 3 | 14 a ± 4 | 12 a ± 2 | 24 a ± 3 | 6 bc ± 1 |
| ‘Lechin’ | ‘Lucque’ | ‘Manzanilla’ | ‘Picholine de Languedoc’ | |
|---|---|---|---|---|
| Hydroxytyrosol glucoside | 39 a ± 19 | 316 c ± 64 | 48 a ± 7 | 186 b ± 18 |
| Secologanoside is. 1 | 876 cef ± 198 | 1018 cf ± 91 | 507 bd ± 74 | 608 de ± 49 |
| Hydroxytyrosol | 147 ab ± 42 | 143 ab ± 58 | 144 ab ± 20 | 202 a ± 46 |
| Tyrosol glucoside | 122 cd ± 28 | 114 cd ± 22 | 60 efg ± 2 | 160 b ± 19 |
| Secologanoside is. 2 | 1455 b ± 298 | 854 e ± 132 | 746 ce ± 125 | 572 ace ± 61 |
| Tyrosol | 38 d ± 4 | 23 c ± 6 | 44 bd ± 4 | 33 cd ± 4 |
| Elenolic acid glucoside is. 1 | 799 bc ± 99 | 507 d ± 48 | 513 d ± 15 | 494 d ± 24 |
| Vanillic acid | 10 c ± 3 | 9 c ± 2 | 10 c ± 2 | 18 ab ± 5 |
| Oleuropein aglycon is. 1 | 143 de ± 37 | 244 c ± 57 | 202 cd ± 12 | 164 de ± 25 |
| Luteolin diglucoside | 393 c ± 48 | 302 bc ± 104 | 344 bc ± 31 | 607 a ± 102 |
| Elenolic acid glucoside is. 2 | 323 df ± 95 | 346 cdef ± 49 | 226 cd ± 29 | 426 f ± 27 |
| Elenolic acid glucoside is. 3 | 93 cd ± 12 | 153 de ± 22 | 156 e ± 18 | 264 a ± 33 |
| Rutin | 294 de ± 26 | 2436 b ± 320 | 384 de ± 67 | 689 c ± 82 |
| Hydroxyoleuropein | 577 fg ± 41 | 551 cf ± 83 | 643 ef ± 17 | 490 cg ± 33 |
| Luteolin-7-glucoside | 2715 bc ± 101 | 2258 c ± 561 | 2561 c ± 223 | 3548 a ± 358 |
| Apigenin rutinoside | 275 b ± 15 | 385 cdef ± 56 | 471 aef ± 32 | 396 cdef ± 39 |
| Oleuropein diglucoside | 164 cg ± 38 | 312 dfh ± 60 | 219 eg ± 27 | 354 d ± 20 |
| Apigenin-7-glucoside | 93 f ± 3 | 221 ae ± 42 | 157 d ± 5 | 135 df ± 6 |
| Luteolin-glucoside is. 1 | 2289 bde ± 250 | 2598 ab ± 965 | 2425 bde ± 271 | 3687 c ± 266 |
| Chrysoeriol-7-glucoside | 532 bc ± 30 | 498 cd ± 54 | 437 ad ± 83 | 547 bc ± 23 |
| Luteolin-glucoside is. 2 | 350 degh ± 43 | 267 fg ± 21 | 209 bf ± 24 | 317 gh ± 40 |
| 2″-methoxyoleuropein is.1 | 1588 bd ± 255 | 761 ce ± 314 | 1162 bc ± 213 | 928 ce ± 86 |
| Oleuropein is. 1 | 20,645 ab ± 8348 | 1535 bc ± 2708 | 7696 def ± 1583 | 8176 def ± 895 |
| 2″-methoxyoleuropein is. 2 | 67 bd ± 8 | 54 bc ± 14 | 100 de ± 21 | 133 e ± 10 |
| Oleuropein is. 2 | 247 bd ± 85 | 301 b ± 54 | 115 cf ± 42 | 174 df ± 22 |
| Oleuropein is. 3 | 638 bd ± 197 | 873 b ± 207 | 397 df ± 107 | 597 de ± 67 |
| Ligstroside | 653 d ± 147 | 425 cd ± 28 | 575 d ± 31 | 185 cef ± 22 |
| Ligstroside aglycon | 979 a ± 494 | 400 bc ± 113 | 526 bc ± 185 | 447 bc ± 104 |
| Luteolin | 169 de ± 35 | 113 cd ± 57 | 157 de ± 18 | 276 b ± 32 |
| Quercetin | 3.9 b ± 0.6 | 7 b ± 4 | 10 b ± 2 | 19 b ± 3 |
| Pinoresinol | 9.6 cde ± 0.8 | 8.7 d ± 0.7 | 12.8 ae ± 0.9 | 11 cde ± 2 |
| Apigenin | 11 aef ± 2 | 11 aef ± 2 | 16 ab ± 2 | 17 bf ± 3 |
| Naringenin | 7 ac ± 1 | 5.0 c ± 0.5 | 8 ab ± 2 | 8.7 a ± 0.8 |
| Diosmetin | 6 b ± 2 | 6 b ± 4 | 6 b ± 2 | 20 d ± 4 |
| Unknown is. 1 | 4 bc ± 2 | 9 ce ± 3 | 16 df ± 2 | 29 a ± 2 |
| Oleuropein aglycon is. 2 | 666 a ± 260 | 53 e ± 17 | 262 cd ± 100 | 132 de ± 19 |
| Unknown is. 2 | 4 b ± 1 | 18 d ± 6 | 18 d ± 2 | 30 a ± 3 |
| Unknown is. 3 | 3 b ± 2 | 11 cd ± 3 | 14 d ± 2 | 22.6 a ± 0.7 |
| ‘Picholine Marocaine’ | ‘Picual’ | ‘Verdal’ | |
|---|---|---|---|
| Hydroxytyrosol glucoside | 1510 d ± 67 | 11 a ± 6 | 15 a ± 9 |
| Secologanoside is. 1 | 1059 c ± 50 | 182 a ± 38 | 1005 cf ± 112 |
| Hydroxytyrosol | 323 c ± 22 | 155 ab ± 14 | 140 b ± 11 |
| Tyrosol glucoside | 237 a ± 13 | 62 efg ± 10 | 82 cefg ± 6 |
| Secologanoside is. 2 | 1199 bd ± 226 | 376 a ± 94 | 1100 de ± 144 |
| Tyrosol | 54 ab ± 10 | 28 cd ± 4 | 61 a ± 8 |
| Elenolic acid glucoside is. 1 | 342 a ± 29 | 266 a ± 42 | 787 bc ± 58 |
| Vanillic acid | 7 c ± 1 | 19 a ± 4 | 8 c ± 2 |
| Oleuropein aglycon is. 1 | 437 b ± 37 | 105 ae ± 41 | 173 cde ± 23 |
| Luteolin diglucoside | 395 c ± 31 | 353 bc ± 38 | 294 bc ± 27 |
| Elenolic acid glucoside is. 2 | 887 a ± 95 | 85 b ± 19 | 402 cdef ± 65 |
| Elenolic acid glucoside is. 3 | 989 b ± 75 | 114 ce ± 11 | 127 ce ± 17 |
| Rutin | 554 ce ± 45 | 161 d ± 28 | 362 de ± 17 |
| Hydroxyoleuropein | 147 a ± 16 | 420 c ± 70 | 1027 b ± 140 |
| Luteolin-7-glucoside | 2800 bc ± 232 | 2284 c ± 152 | 2662 bc ± 292 |
| Apigenin rutinoside | 456 ade ± 32 | 395 cdef ± 80 | 327 f ± 45 |
| Oleuropein diglucoside | 623 b ± 47 | 94 c ± 38 | 243 efg ± 28 |
| Apigenin-7-glucoside | 148 df ± 18 | 114 f ± 7 | 202 e ± 11 |
| Luteolin-glucoside is. 1 | 2471 bd ± 228 | 2132 de ± 130 | 1494 e ± 115 |
| Chrysoeriol-7-glucoside | 480 cd ± 26 | 424 ad ± 14 | 495 ad ± 12 |
| Luteolin-glucoside is. 2 | 277 befgh ± 36 | 364 h ± 58 | 116 a ± 7 |
| 2″-methoxyoleuropein is.1 | 572 e ± 48 | 611 ce ± 188 | 2241 a ± 384 |
| Oleuropein is. 1 | 23,963 a ± 3513 | 1632 e ± 437 | 12,443 cf ± 2403 |
| 2″-methoxyoleuropein is. 2 | 127 e ± 10 | 52 b ± 19 | 95 cde ± 16 |
| Oleuropein is. 2 | 434 a ± 47 | 42 c ± 15 | 193 df ± 54 |
| Oleuropein is. 3 | 2249 a ± 126 | 114 c ± 40 | 419 df ± 87 |
| Ligstroside | 1118 a ± 358 | 129 c ± 55 | 1608 b ± 260 |
| Ligstroside aglycon | 209 c ± 20 | 298 c ± 39 | 730 ab ± 300 |
| Luteolin | 49 c ± 8 | 265 b ± 28 | 184 de ± 16 |
| Quercetin | 50 ± 14 | 7 b ± 2 | 7 b ± 2 |
| Pinoresinol | 10.1 bcde ± 0.6 | 10 cde ± 1 | 14 ab ± 1 |
| Apigenin | 7.5 ac ± 0.7 | 21 b ± 2 | 19 bf ± 3 |
| Naringenin | 5.2 c ± 0.5 | 6.6 ac ± 0.5 | 6.3 ac ± 0.3 |
| Diosmetin | 4 b ± 1 | 16 cd ± 2 | 13 c ± 2 |
| Unknown is. 1 | 19 dg ± 4 | 16 df ± 2 | 10 e ± 2 |
| Oleuropein aglycon is. 2 | 125 de ± 18 | 32 e ± 12 | 465 b ± 91 |
| Unknown is. 2 | 15 cd ± 3 | 18 d ± 3 | 16 d ± 2 |
| Unknown is. 3 | 15 d ± 2 | 13 d ± 1 | 12 cd ± 2 |
| Confusion Matrix for the Training Sample | |||||||||||||
| Variety/Classified as | Arbequina | Frantoio | Hojiblanca | Koroneiki | Lechín | Lucque | Manzanilla | Picholine Marocaine | Picholine de Languedoc | Picual | Verdal | Total | % Correct |
| Arbequina | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Frantoio | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Hojiblanca | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Koroneiki | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Lechín | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Lucque | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Manzanilla | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Picholine Marocaine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 100.0 |
| Picholine de Languedoc | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 | 100.0 |
| Picual | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 5 | 100.0 |
| Verdal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 5 | 100.0 |
| Total | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 55 | 100.0 |
| Confusion Matrix for the Cross-Validation Results | |||||||||||||
| Variety/Classified as | Arbequina | Frantoio | Hojiblanca | Koroneiki | Lechín | Lucque | Manzanilla | Picholine Marocaine | Picholine de Languedoc | Picual | Verdal | Total | % Correct |
| Arbequina | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Frantoio | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Hojiblanca | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Koroneiki | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Lechín | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Lucque | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Manzanilla | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 5 | 100.0 |
| Picholine Marocaine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 100.0 |
| Picholine de Languedoc | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 | 100.0 |
| Picual | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 5 | 100.0 |
| Verdal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 5 | 100.0 |
| Total | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 55 | 100.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmo-García, L.; Bajoub, A.; Benlamaalam, S.; Hurtado-Fernández, E.; Bagur-González, M.G.; Chigr, M.; Mbarki, M.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Establishing the Phenolic Composition of Olea europaea L. Leaves from Cultivars Grown in Morocco as a Crucial Step Towards Their Subsequent Exploitation. Molecules 2018, 23, 2524. https://doi.org/10.3390/molecules23102524
Olmo-García L, Bajoub A, Benlamaalam S, Hurtado-Fernández E, Bagur-González MG, Chigr M, Mbarki M, Fernández-Gutiérrez A, Carrasco-Pancorbo A. Establishing the Phenolic Composition of Olea europaea L. Leaves from Cultivars Grown in Morocco as a Crucial Step Towards Their Subsequent Exploitation. Molecules. 2018; 23(10):2524. https://doi.org/10.3390/molecules23102524
Chicago/Turabian StyleOlmo-García, Lucía, Aadil Bajoub, Sara Benlamaalam, Elena Hurtado-Fernández, María Gracia Bagur-González, Mohammed Chigr, Mohamed Mbarki, Alberto Fernández-Gutiérrez, and Alegría Carrasco-Pancorbo. 2018. "Establishing the Phenolic Composition of Olea europaea L. Leaves from Cultivars Grown in Morocco as a Crucial Step Towards Their Subsequent Exploitation" Molecules 23, no. 10: 2524. https://doi.org/10.3390/molecules23102524
APA StyleOlmo-García, L., Bajoub, A., Benlamaalam, S., Hurtado-Fernández, E., Bagur-González, M. G., Chigr, M., Mbarki, M., Fernández-Gutiérrez, A., & Carrasco-Pancorbo, A. (2018). Establishing the Phenolic Composition of Olea europaea L. Leaves from Cultivars Grown in Morocco as a Crucial Step Towards Their Subsequent Exploitation. Molecules, 23(10), 2524. https://doi.org/10.3390/molecules23102524