Antioxidant Activity and Phytochemical Characterization of Senecio clivicolus Wedd.
Abstract
1. Introduction
2. Results and Discussion
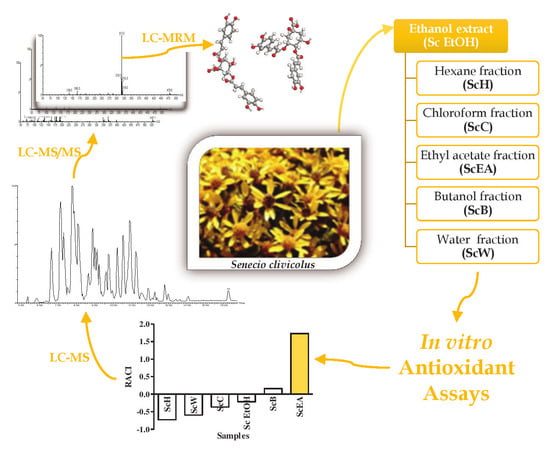
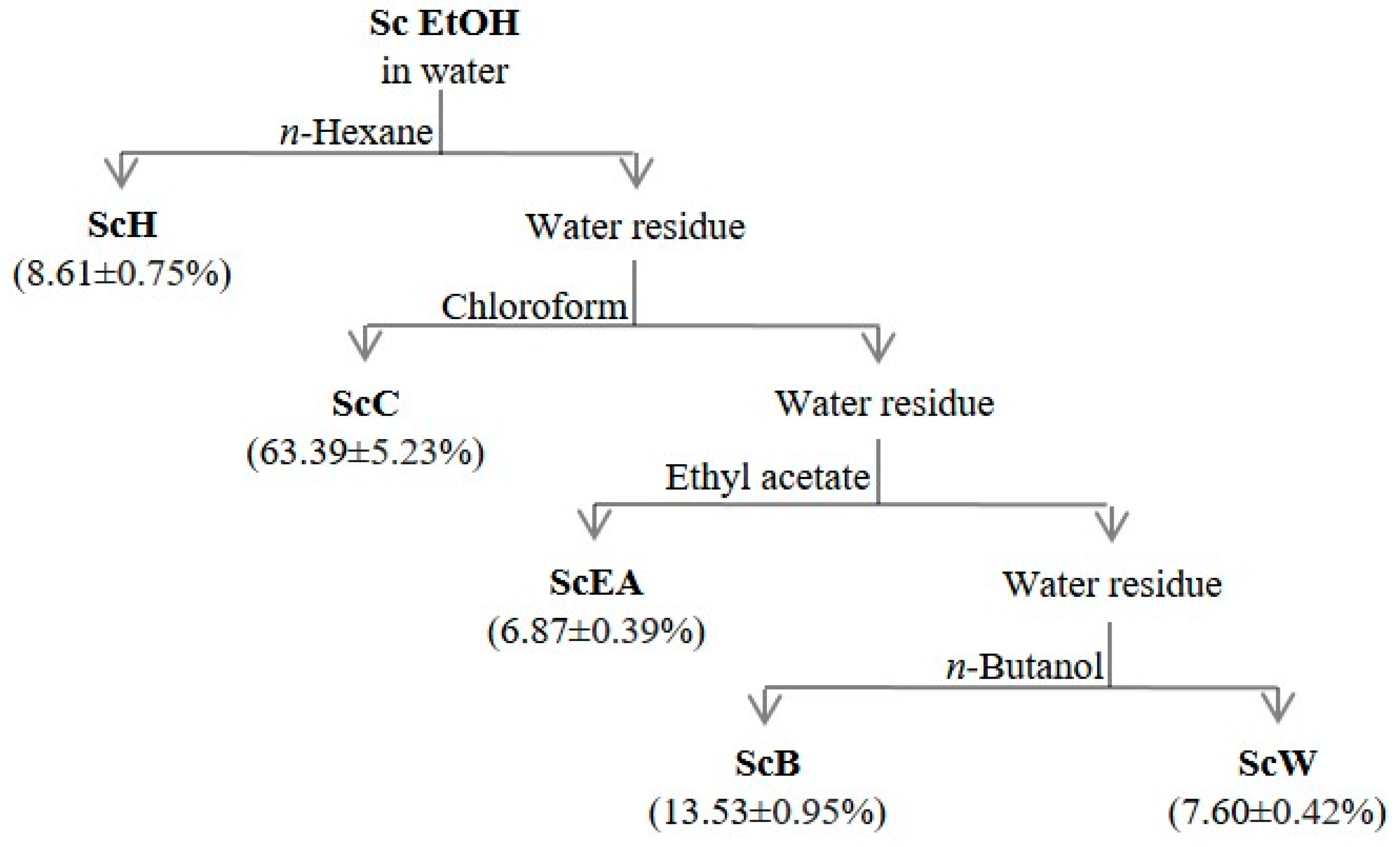
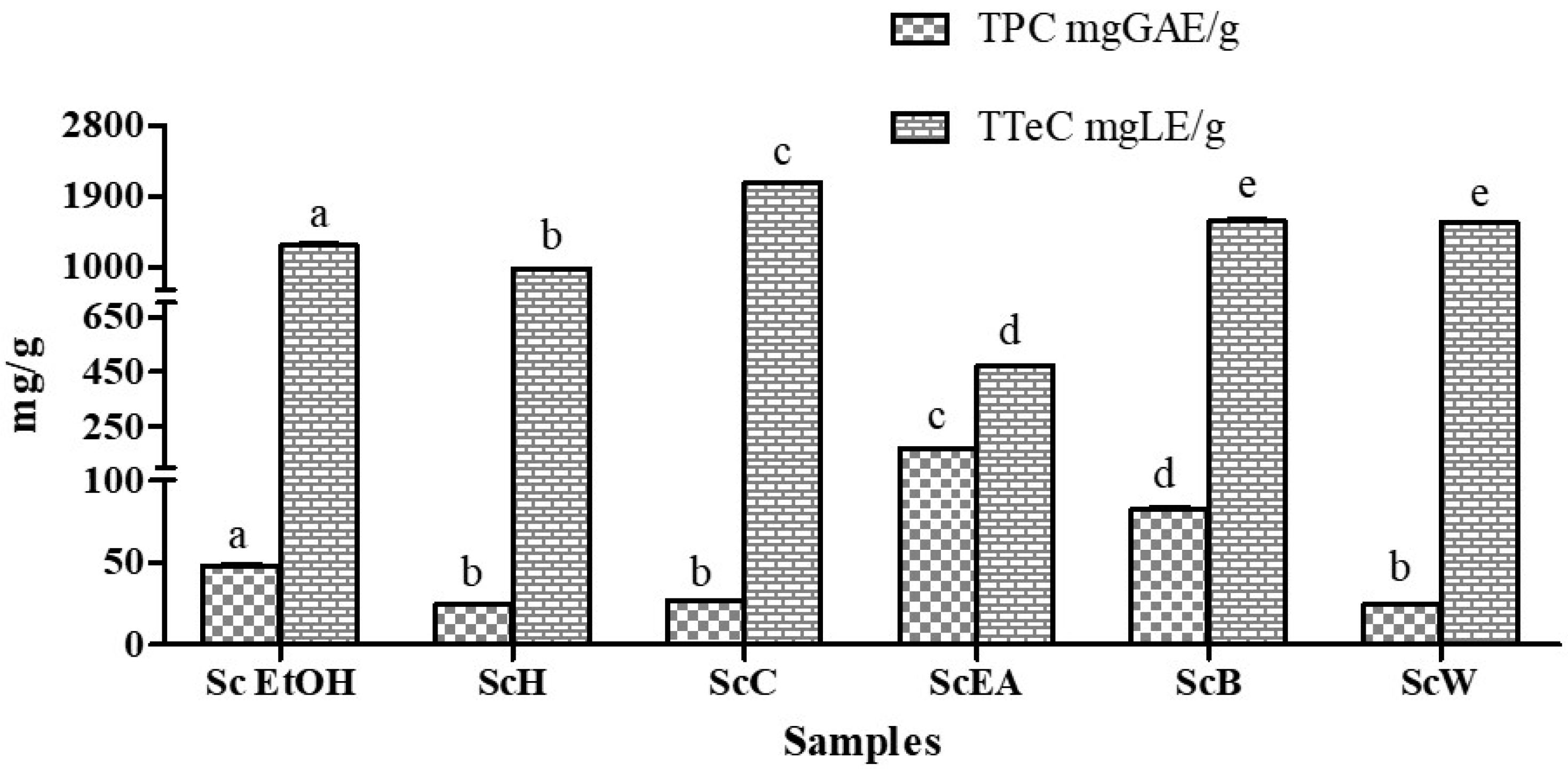
2.1. The Extraction Yield and the Influence of Solvents on Total Polyphenolic and Terpenoid Content
2.2. Antioxidant Activity
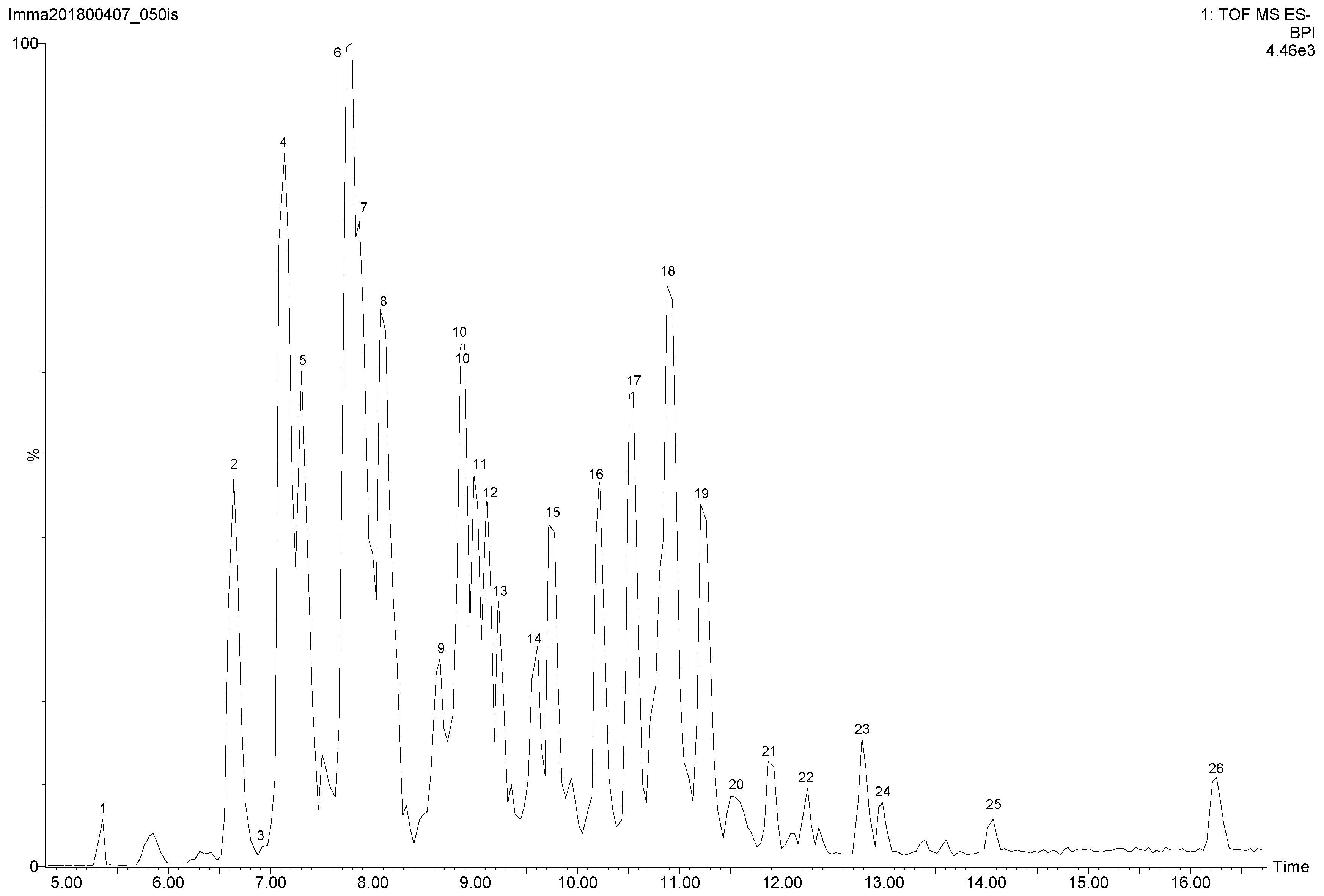
2.3. Identification and Quantification of Polyphenols by Mass Spectrometry
3. Materials and Methods
3.1. Chemicals, Reagents and Equipment
3.2. Plant Material and Sample Preparation
3.3. Total Phenolic Content (TPC)
3.4. Total Terpenoid Content (TTeC)
3.5. Radical-Scavenging Activity
3.5.1. ABTS Assay
3.5.2. DPPH Assay
3.5.3. Super Oxide Anion Scavenging Activity (SO)
3.5.4. Nitric Oxide Radical Scavenging Activity (NO)
3.6. Ferric Reducing Antioxidant Power Assay (FRAP)
3.7. β-Carotene Bleaching Assay (BCB)
3.8. Identification and Quantification by Liquid Chromatography Mass Spectrometry
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patel Rajesh, M.; Patel Natvar, J. In vitro antioxidant activity of coumarin compounds by DPPH, super oxide and nitric oxide free radical scavenging methods. J. Adv. Pharm. Educ. Res. 2011, 1, 52–68. [Google Scholar]
- Milutinović, V.; Niketić, M.; Ušjak, L.; Nikolić, D.; Krunić, A.; Zidorn, C.; Petrović, S. Methanol extracts of 28 Hieracium species from the Balkan Peninsula–Comparative LC–MS analysis, chemosystematic evaluation of their flavonoid and phenolic acid profiles and antioxidant potentials. Phytochem. Anal. 2018, 29, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, J.G.; Folch, J.; Vazquez De la Torre, A.; Verdaguer, E.; Junyent, F.; Jordán, J.; Pallas, M.; Camins, A. Oxidative stress-induced DNA damage and cell cycle regulation in B65 dopaminergic cell line. Free Radic. Res. 2009, 43, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Satoh, K.; Hatano, T.; Yoshida, T.; Okuda, T. Possible role of radical intensity and oxidation potential for gallic acid-induced apoptosis. Anticancer Res. 1997, 17, 377–380. [Google Scholar] [PubMed]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Araujo, N.; Mü Ller, R.; Nowicki, C.; Ibisch, P. Análisis de Vacíos de Representatividad del Sistema Nacional de Áreas Protegidas; FAN: Santa Cruz, CA, USA, 2005. [Google Scholar]
- Deharo, E.; Bourdy, G.; Quenevo, C.; Munoz, V.; Ruiz, G.; Sauvain, M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part V. Evaluation of the antimalarial activity of plants used by the Tacana Indians. J. Ethnopharmacol. 2001, 77, 91–98. [Google Scholar] [CrossRef]
- Muñoz, V.; Sauvain, M.; Bourdy, G.; Callapa, J.; Bergeron, S.; Rojas, I.; Bravo, J.; Balderrama, L.; Ortiz, B.; Gimenez, A. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach: Part I. Evaluation of the antimalarial activity of plants used by the Chacobo Indians. J. Ethnopharmacol. 2000, 69, 127–137. [Google Scholar] [CrossRef]
- Bourdy, G.; Oporto, P.; Gimenez, A.; Deharo, E. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach: Part VI. Evaluation of the antimalarial activity of plants used by Isoceno-Guaranı Indians. J. Ethnopharmacol. 2004, 93, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Macía, M.J.; García, E.; Vidaurre, P.J. An ethnobotanical survey of medicinal plants commercialized in the markets of La Paz and El Alto, Bolivia. J. Ethnopharmacol. 2005, 97, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Semo, L.; Morales, M.; Noza, Z.; Nuñez, H.; Cayuba, A.; Noza, M.; Humaday, N.; Vaya, J.; Van Damme, P. Ethnomedicinal practices and medicinal plant knowledge of the Yuracarés and Trinitarios from indigenous territory and national park Isiboro-Sécure, Bolivian Amazon. J. Ethnopharmacol. 2011, 133, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, Z.; Hohmann, J. An ethnopharmacological survey of the traditional medicine utilized in the community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. J. Ethnopharmacol. 2012, 139, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Fournet, A.; Barrios, A.A.; Munoz, V. Leishmanicidal and trypanocidal activities of Bolivian medicinal plants. J. Ethnopharmacol. 1994, 41, 19–37. [Google Scholar] [CrossRef]
- Bustamante, G.; Escalante, L.; Mejia, U.; Valdivia, M.; Soria, I. Estudio Etnobotánico y Actividad Antimicrobiana de las Plantas Medicinales de los Valles Bajos de Cochabamba; Universidad Mayor de San Simón: Cochabamba, Bolivia, 2001; Volume 78. [Google Scholar]
- Pelser, P.B.; Nordenstam, B.; Kadereit, J.W.; Watson, L.E. An ITS phylogeny of tribe Senecioneae (Asteraceae) and a new delimitation of Senecio L. Taxon 2007, 56, 1077–1104. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, B.A.; Aguilar, M.I.; King-Díaz, B.; Rivero, J.F.; Lotina-Hennsen, B. The sesquiterpenes β-caryophyllene and caryophyllene oxide isolated from Senecio salignus act as phytogrowth and photosynthesis inhibitors. Molecules 2012, 17, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Oladipupo, L.; Adebola, O. Chemical composition of the essential oils of the flowers, leaves and stems of two Senecio polyanthemoides Sch. Bip. samples from South Africa. Molecules 2009, 14, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Balzaretti, V.; Arancibia, A.; Marchiaro, A.; Arce, M.; Feijóo, M. Variation in the composition of the essential oil of Senecio filaginoides DC. Molecules 2000, 5, 459–461. [Google Scholar] [CrossRef]
- Krasovskaya, N.; Kulesh, N.; Denisenko, V. Natural antioxidants. Furanoeremophilanes from Cacalia roots. Chem. Nat. Compd. 1989, 25, 545–548. [Google Scholar] [CrossRef]
- Milella, L.; Bader, A.; De Tommasi, N.; Russo, D.; Braca, A. Antioxidant and free radical-scavenging activity of constituents from two Scorzonera species. Food Chem. 2014, 160, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Saltos, M.B.V.; Puente, B.F.N.; Faraone, I.; Milella, L.; De Tommasi, N.; Braca, A. Inhibitors of α-amylase and α-glucosidase from Andromachia igniaria Humb. & Bonpl. Phytochem. Lett. 2015, 14, 45–50. [Google Scholar] [CrossRef]
- Fraige, K.; Dametto, A.C.; Zeraik, M.L.; de Freitas, L.; Saraiva, A.C.; Medeiros, A.I.; Castro-Gamboa, I.; Cavalheiro, A.J.; Silva, D.H.S.; Lopes, N.P. Dereplication by HPLC-DAD-ESI-MS/MS and screening for biological activities of Byrsonima species (Malpighiaceae). Phytochem. Anal. 2018, 29, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Dávila, M.; Sterner, O.; Hinojosa, N. Furanoeremophilanes from Senecio clivicolus Wedd. In Revista Boliviana de Química; Universidad Mayor de San Andrés: San Andrés, Bolivia, 2013; Volume 30, pp. 80–83. ISSN 0250-5460. [Google Scholar]
- Lienou, L.L.; Telefo, P.B.; Bayala, B.; Yemele, D.M.; Lemfack, M.C.; Mouokeu, C.; Goka, S.C.; Tagnie, R.S.; Fewou, P. Effect of ethanolic extract of Senecio biafrae on puberty onset and fertility in immature female rat. Cameroon J. Exp. Biol. 2010, 6, 101–109. [Google Scholar] [CrossRef]
- Hassan, W.; Gendy, A.; Al-youssef, H.; El-Shazely, A. Chemical constituents and biological activities of Senecio aegyptius var. discoideus Boiss. J. Biosci. C 2012, 67, 144–150. [Google Scholar] [CrossRef]
- Conforti, F.; Marrelli, M.; Statti, G.; Menichini, F. Antioxidant and cytotoxic activities of methanolic extract and fractions from Senecio gibbosus subsp. gibbosus (GUSS) DC. Nat. Prod. Res. 2006, 20, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Hariprasath, L.; Raman, J.; Nanjian, R. Gastroprotective effect of Senecio candicans DC on experimental ulcer models. J. Ethnopharmacol. 2012, 140, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Saada, M.; Falleh, H.; Catarino, M.D.; Cardoso, S.M.; Ksouri, R. Plant growth modulates metabolites and biological activities in Retama raetam (Forssk.) Webb. Molecules 2018, 23, 2177. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ra, J.H.; Jee, Y.; Kim, J.S. Impact of different partitioned solvents on chemical composition and bioavailability of Sasa quelpaertensis Nakai leaf extract. J. Food Drug Anal. 2017, 25, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Bośko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive phytochemicals and antioxidant properties of the grains and sprouts of colored wheat genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef] [PubMed]
- Mariod, A.; Matthaeus, B. Fatty acids, tocopherols, sterols, phenolic profiles and oxidative stability of Cucumis melo var. agrestis oil. J. Food Lip. 2008, 15, 56–67. [Google Scholar] [CrossRef]
- Conforti, F.; Loizzo, M.R.; Statti, G.A.; Houghton, P.J.; Menichini, F. Biological properties of different extracts of two Senecio species. Int. J. Food Sci. Nutr. 2006, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Novel antioxidant compounds from Tart Cherries (Prunus c erasus). J. Nat. Prod. 1999, 62, 86–88. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.; Thuong, P.T.; Su, N.D.; Sok, D.; Song, K.S.; Seong, Y.H.; Bae, K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Razavi, S.M.; Zahri, S.; Zarrini, G.; Nazemiyeh, H.; Mohammadi, S. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. Russ. J. Bioorg. Chem. 2009, 35, 376–378. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.R.; Guo, D.A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Yépez, A.M.; de Ugaz, O.L.; Alvarez, C.M.; De Feo, V.; Aquino, R.; De Simone, F.; Pizza, C. Quinovic acid glycosides from Uncaria guianensis. Phytochemistry 1991, 30, 1635–1637. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of two new derivatives of chlorogenic acids in Arnica (Arnica montana L.) flowers by high-performance liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 4033–4039. [Google Scholar] [CrossRef] [PubMed]
- Ibdah, M.; Zhang, X.H.; Schmidt, J.; Vogt, T. A novel Mg2+-dependent O-methyltransferase in the phenylpropanoid metabolism of Mesembryanthemum crystallinum. J. Biol. Chem. 2003, 278, 43961–43972. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Desta, K.T.; Kim, G.S.; Lee, S.J.; Lee, W.S.; Kim, Y.H.; Jin, J.S.; Abd El-Aty, A.M.; Shin, H.C.; Shim, J.H.; et al. Polyphenolic profile and antioxidant effects of various parts of Artemisia annua L. Biomed. Chromatogr. 2016, 30, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Keller, P.; Streker, P.; Klaiber, I.; Carle, R. Detection of isorhamnetin glycosides in extracts of apples (Malus domestica cv.“Brettacher”) by HPLC-PDA and HPLC-APCI-MS/MS. Phytochem. Anal. 2002, 13, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska-Kowalczyk, J.; Pecio, Ł.; Mołdoch, J.; Ludwiczuk, A.; Kowalczyk, M. Novel phenolic constituents of Pulmonaria officinalis L. LC-MS/MS Comparison of Spring and Autumn metabolite profiles. Molecules 2018, 23, 2277. [Google Scholar] [CrossRef] [PubMed]
- Todaro, L.; Russo, D.; Cetera, P.; Milella, L. Effects of thermo-vacuum treatment on secondary metabolite content and antioxidant activity of poplar (Populus nigra L.) wood extracts. Ind. Crops Prod. 2017, 109, 384–390. [Google Scholar] [CrossRef]
- Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of total Terpenoids concentration in plant tissues using a monoterpene, linalool as standard reagent. Protoc. Exch. 2012, 5. [Google Scholar] [CrossRef]
- Armentano, M.F.; Bisaccia, F.; Miglionico, R.; Russo, D.; Nolfi, N.; Carmosino, M.; Andrade, P.B.; Valentão, P.; Diop, M.S.; Milella, L. Antioxidant and proapoptotic activities of Sclerocarya birrea [(A. Rich.) Hochst.] methanolic root extract on the hepatocellular carcinoma cell line HepG2. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Valentão, P.; Andrade, P.B.; Fernandez, E.C.; Milella, L. Evaluation of antioxidant, antidiabetic and anticholinesterase activities of Smallanthus sonchifolius landraces and correlation with their phytochemical profiles. Int. J. Mol. Sci. 2015, 16, 17696–17718. [Google Scholar] [CrossRef] [PubMed]
- Dekdouk, N.; Malafronte, N.; Russo, D.; Faraone, I.; De Tommasi, N.; Ameddah, S.; Severino, L.; Milella, L. Phenolic compounds from Olea europaea L. possess antioxidant activity and inhibit carbohydrate metabolizing enzymes in vitro. Evid. Based Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, Q.C.; Faraone, I.; Russo, D.; Aragão Catunda, F.E.; Vignola, L.; de Carvalho, M.G.; de Tommasi, N.; Milella, L. Chemical and biological insights of Ouratea hexasperma (A. St.-Hil.) Baill.: A source of bioactive compounds with multifunctional properties. Nat. Prod. Res. 2018, 1–4. [Google Scholar] [CrossRef] [PubMed]
- MikaMi, I.; YaMaguChi, M.; Shinmoto, H.; Tsushida, T. Development and validation of a microplate-based β-carotene bleaching assay and comparison of antioxidant activity (AOA) in several crops measured by β-carotene bleaching, DPPH and ORAC assays. Food Sci. Technol. Res. 2009, 15, 171–178. [Google Scholar] [CrossRef]
- Rico, D.; Diana, A.B.M.; Milton-Laskibar, I.; Fernández-Quintela, A.; Silván, J.M.; Rai, D.K.; Choudhary, A.; Peñas, E.; de Luis, D.A.; Martínez-Villaluenga, C. Characterization and in vitro evaluation of seaweed species as potential functional ingredients to ameliorate metabolic syndrome. J. Funct. Foods 2018, 46, 185–194. [Google Scholar] [CrossRef]
- Choudhary, A.; Naughton, L.M.; Dobson, A.D.; Rai, D.K. HPLC-ESI-MS/MS characterisation of metabolites produced by Pseudovibrio sp. W64, a marine sponge-derived bacterium isolated from Irish waters. Rapid Commun. Mass Spectrom. 2018, 32, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Senecio clivicolus are available from the authors and stored as reported above. |

| Samples | ABTS (mgTE/g) | DPPH (mgTE/g) | SO (IC25 mg/mL) | FRAP (mgTE/g) | BCB %AA |
|---|---|---|---|---|---|
| Sc EtOH | 137.87 ± 1.45 d | 63.42 ± 0.78 b | 0.37 ± 0.02 d | 93.08 ± 1.12 d | 53.11 ± 0.45 d,e |
| ScH | 28.94 ± 2.50 a | nc | 0.16 ± 0.01 b,c | 12.98 ± 1.04 a | 4.75 ± 0.23 a |
| ScC | 55.93 ± 2.24 b | 12.70 ± 0.94 a | 0.14 ± 0.01 b | 23.90 ± 1.32 b | 44.58 ± 0.96 c |
| ScEA | 409.53 ± 9.53 f | 317.53 ± 5.81 d | 0.08 ± 0.00 a | 507.66 ± 5.26 f | 55.82 ± 2.22 e |
| ScB | 208.37 ± 3.21 e | 119.54 ± 6.71 c | 0.20 ± 0.01 c | 184.18 ± 4.59 e | 51.70 ± 1.97 d |
| ScW | 107.01 ± 0.94 c | 55.58 ± 1.07 b | 0.64 ± 0.04 e | 53.41 ± 2.55 c | 23.28 ± 0.70 b |
| TPC | TTeC | ABTS | DPPH | SO | NO | FRAP | BCB | |
|---|---|---|---|---|---|---|---|---|
| TPC | 1.00 | |||||||
| TTeC | −0.69 | 1.00 | ||||||
| ABTS | 0.98 | −0.64 | 1.00 | |||||
| DPPH | 0.98 | −0.68 | 0.99 | 1.00 | ||||
| SO | 0.77 | −0.56 | 0.65 | 0.70 | 1.00 | |||
| NO | 0.92 | −0.75 | 0.89 | 0.93 | 0.84 | 1.00 | ||
| FRAP | 0.99 | −0.70 | 0.99 | 1.00 | 0.75 | 0.94 | 1.00 | |
| BCB | 0.61 | 0.01 | 0.65 | 0.59 | 0.29 | 0.41 | 0.58 | 1.00 |
| Peak No. | RT (min) | ESI (−) MS Observed | ESI (−) MS Calc. | Molecular Formula | MS/MS | Tentative Identity | mg/g DW | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.39 | 137.0242 | 137.0239 | C7H5O3 | 93 | 4-Hydroxybenzoic acid | 2.25 ± 1.31 | [36] |
| 2 | 6.64 | 479.0779 | 479.0826 | C21H19O13 | 317, 166, 139 | Quercetagetin-O-glucoside | nq | [47] |
| 3 | 6.92 | 367.1029 | 367.1029 | C17H19O9 | 161, 85 | Chlorogenic acid methylester | 2.57 ± 0.07 | [36] |
| 4 | 7.17 | 493.0982 | 493.0982 | C22H21O13 | 478, 331, 315, 287, 271, 244, 166 | Mearnsetin-O-glucoside isomer | nq | [48] |
| 609.1461 | 609.1456 | C27H29O16 | 300, 285, 271, 255, 179, 151 | Rutin | 0.16 ± 0.02 | [36] | ||
| 5 | 7.25 | 463.0894 | 463.0877 | C21H19O12 | 300, 271, 255, 179, 151 | Quercetin-3-O-glucoside | 0.84 ± 0.05 | [36,51] |
| 6 | 7.80 | 477.1067 | 477.1033 | C22H21O12 | 462, 315, 299, 271, 254, 243, 227, 151 | Isorhamnetin glycoside | nq | [49] |
| 7 | 7.91 | 515.1174 | 515.1190 | C25H23O12 | 179, 135 | 3,5-di-O-caffeoyl quinic acid | 45.44 ± 0.91 | [36] |
| 8 | 8.13 | 515.1169 | 515.1190 | C25H23O12 | 179, 135 | 3,4-di-O-caffeoyl quinic acid | 19.27 ± 0.68 | [36] |
| 9 | 8.66 | 529.1370 | 529.1346 | C26H25O12 | 367, 349, 191, 179, 173, 161, 135, 133, 101, 93 | Feruloyl-caffeoylquinic acid isomer | nq | [43,44] |
| 10 | 8.86 | 179.0353 | 179.0344 | C9H7O4 | 135, 79 | Caffeic acid | 1.88 ± 0.13 | [36] |
| 529.1370 | 529.1346 | C26H25O12 | 367, 349, 191, 179, 173, 161, 135, 101 | Feruloyl-caffeoylquinic acid isomer | nq | [43] | ||
| 11 | 8.99 | 519.1039 | 519.1139 | C24H23O13 | 504, 315, 299, 285, 271, 243, 191 | Isorhamnetin-acetyl-glucoside | nq | [50] |
| 12 | 9.12 | 529.1370 | 529.1346 | C26H25O12 | 367, 349, 191, 179, 173, 161, 135, 101 | Feruloyl-caffeoylquinic acid isomer | nq | [43] |
| 13 | 9.23 | 529.1370 | 529.1346 | C26H25O12 | 367, 349, 191, 179, 173, 161, 135, 101 | Feruloyl-caffeoylquinic acid isomer | nq | [43] |
| 793.4029 | 793.4010 | C41H61O15 | 529, 397, 353, 219, 191, 179, 173, 161, 101, 71 | Chlorogenic acid methylester hexoside derivative | nq | [45] | ||
| 14 | 9.61 | 493.0982 | 493.0982 | C22H21O13 | 331, 316, 179, 161, 135, 133, 101 | Mearnsetin-O-glucoside isomer | nq | [48] |
| 15/16 | 9.72/10.21 | 353.0885 | 353.0873 | C16H17O9 | 191, 179, 173, 93, 85 | Chlorogenic acid | 2.33 ± 0.45 | [36] |
| 617.2367 | 617.2387 | C35H37O10 | 353, 245, 191, 179, 173, 161, 135 | Chlorogenic acid derivative | nq | [46] | ||
| 601.2267 | 601.2285 | C31H37O12 | 439, 353, 263, 191, 179, 173, 161, 135, 85 | Dicaffeoyl-methoxyoxaloylquinic acids | nq | [46] | ||
| 17 | 10.55 | 779.2360 | 779.2340 | C43H39O14 | 515, 375, 353, 335, 191, 179, 173, 161, 155, 135, 111, 93 | Chlorogenic acid derivative | nq | [46] |
| 18/19 | 10.88/11.21 | 763.2333 | 763.2332 | C50H35O8 | 515, 353, 191, 179, 173, 161, 135, 110 | Dicaffeoylquinic acid derivative | nq | [46] |
| 20 | 11.50 | 529.1370 | 529.1346 | C26H25O12 | 367, 353, 293, 193, 191, 179, 173, 161, 134, 111 | Feruloyl-caffeoylquinic acid isomer | nq | [43] |
| 807.3002 | 807.3017 | C46H47O13 | 353, 335, 191, 179, 173, 161, 155, 135 | Chlorogenic acid derivative | nq | [46] | ||
| 21 | 11.87 | 793.2888 | 793.2860 | C45H45O13 | 353, 191, 179, 173, 161, 155, 135 | Chlorogenic acid derivative | nq | [45] |
| 22 | 12.25 | 819.2629 | 819.2618 | C28H51O27 | 353, 335, 191, 179, 173, 161, 155, 135 | Chlorogenic acid derivative | nq | [46] |
| 23 | 12.79 | 807.3002 | 807.3017 | C46H47O13 | 353, 335, 191, 179, 173, 161, 155, 135 | Chlorogenic acid derivative | nq | [46] |
| 24 | 12.99 | 735.3240 | 735.3228 | C37H51O15 | 353, 335, 191, 179, 173, 161, 135 | Chlorogenic acid derivative | nq | [46] |
| 25 | 14.70 | 675.3726 | 675.3744 | C37H55O11 | 415, 397, 277, 235, 161, 143, 125, 119, 113, 101, 89 | unknown | nq | |
| 26 | 16.25 | 480.3110 | 480.3087 | C27H44O7 | 255, 242, 224, 168, 153, 79 | unknown | nq |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraone, I.; Rai, D.K.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Prinzo, F.; Milella, L. Antioxidant Activity and Phytochemical Characterization of Senecio clivicolus Wedd. Molecules 2018, 23, 2497. https://doi.org/10.3390/molecules23102497
Faraone I, Rai DK, Chiummiento L, Fernandez E, Choudhary A, Prinzo F, Milella L. Antioxidant Activity and Phytochemical Characterization of Senecio clivicolus Wedd. Molecules. 2018; 23(10):2497. https://doi.org/10.3390/molecules23102497
Chicago/Turabian StyleFaraone, Immacolata, Dilip K. Rai, Lucia Chiummiento, Eloy Fernandez, Alka Choudhary, Flavio Prinzo, and Luigi Milella. 2018. "Antioxidant Activity and Phytochemical Characterization of Senecio clivicolus Wedd." Molecules 23, no. 10: 2497. https://doi.org/10.3390/molecules23102497
APA StyleFaraone, I., Rai, D. K., Chiummiento, L., Fernandez, E., Choudhary, A., Prinzo, F., & Milella, L. (2018). Antioxidant Activity and Phytochemical Characterization of Senecio clivicolus Wedd. Molecules, 23(10), 2497. https://doi.org/10.3390/molecules23102497