Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers
Abstract
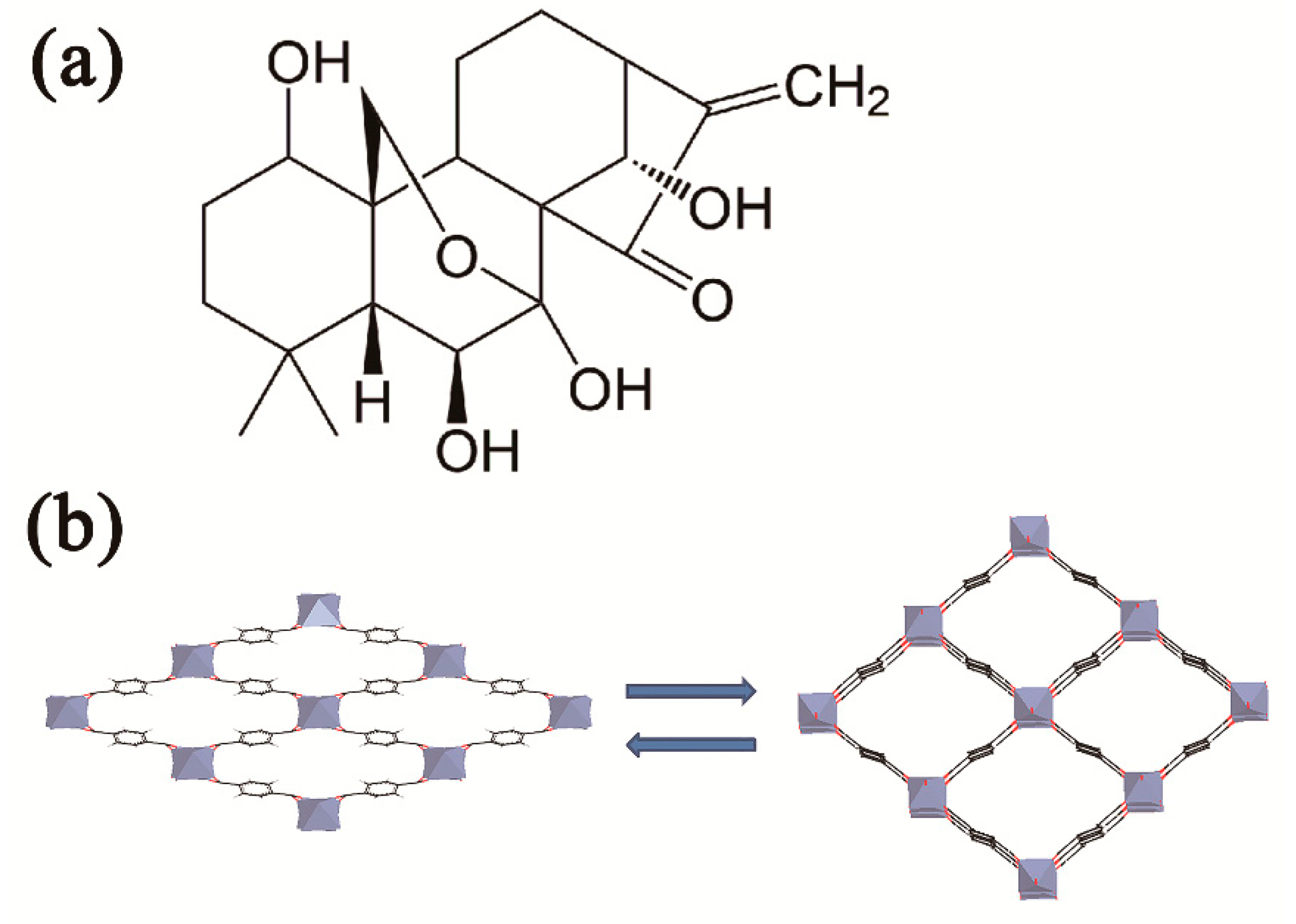
1. Introduction
2. Results
2.1. Preparation and Characteristion of MIL-53(Fe)
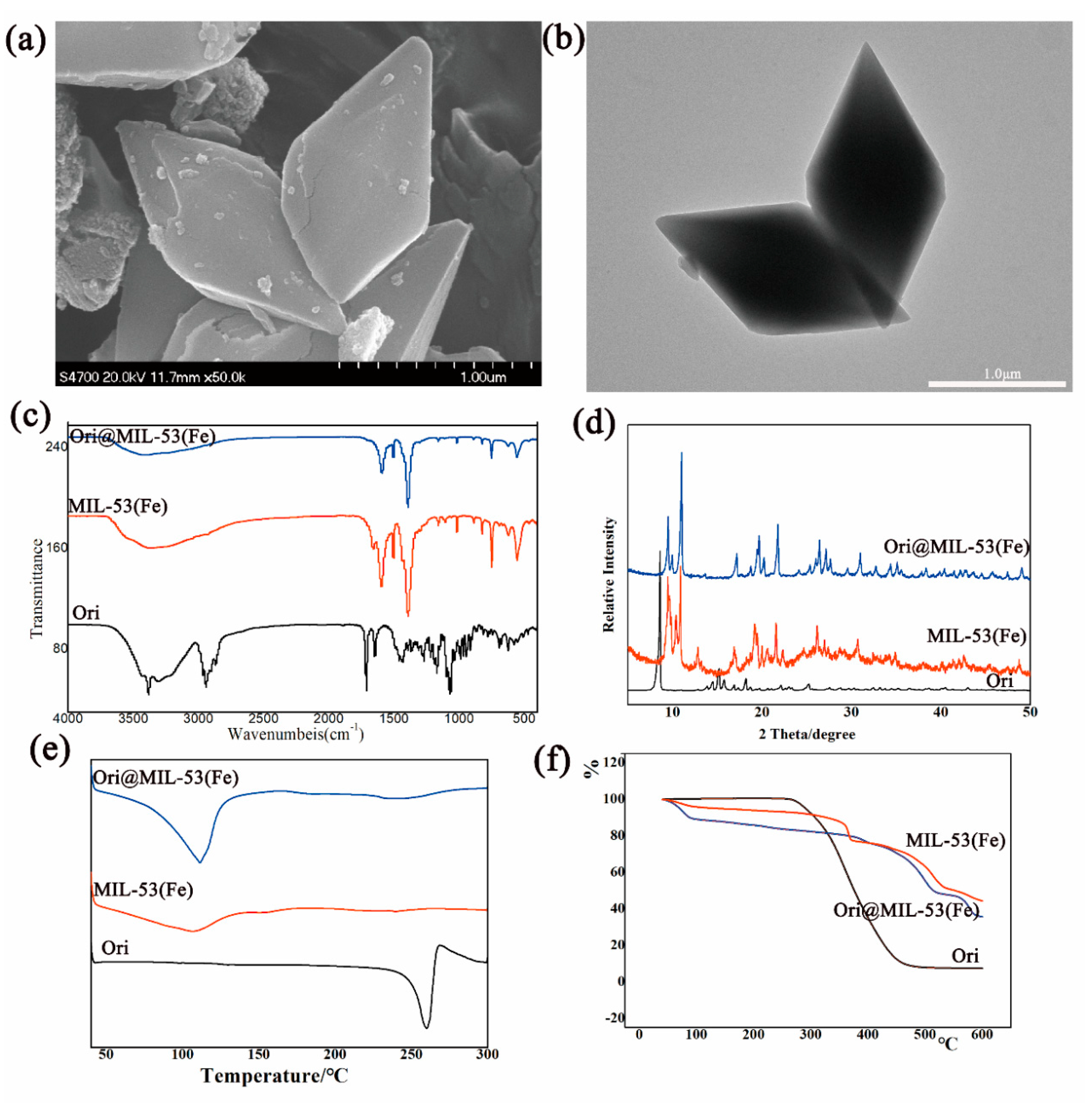
2.2. Optimal Loading Process and Characterization of Ori@MIL-53(Fe)
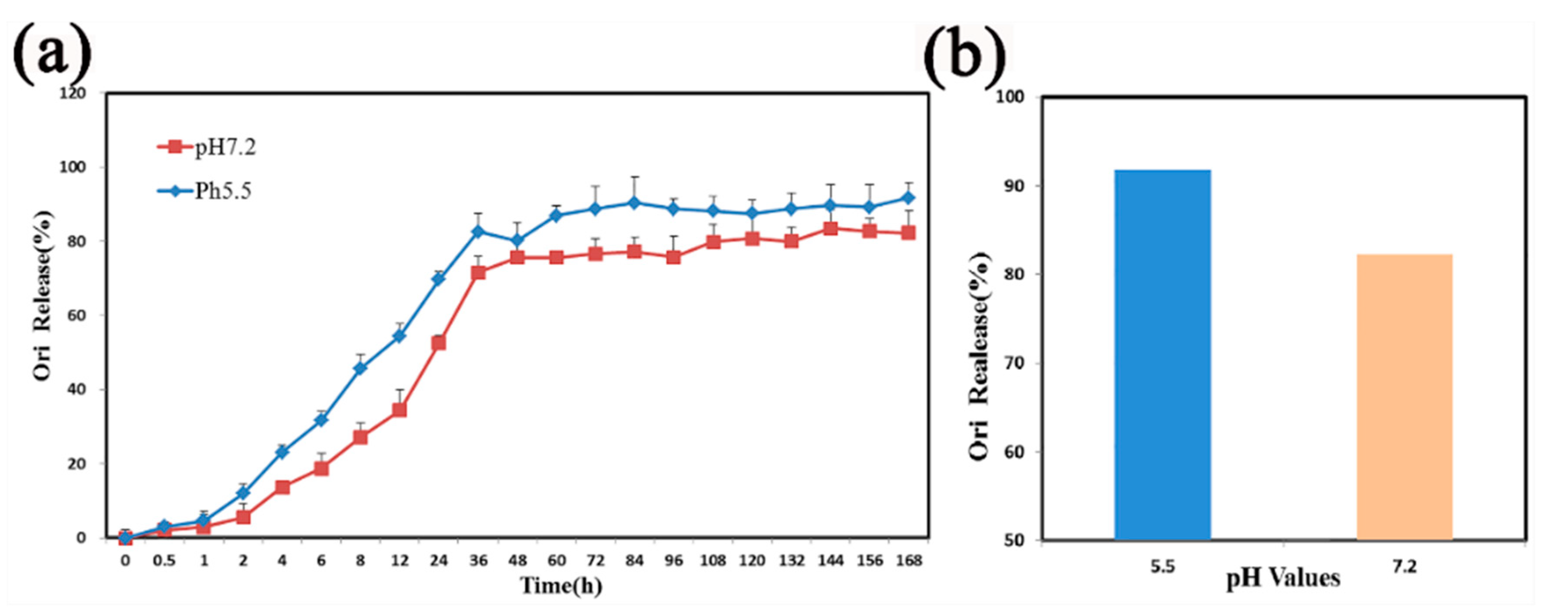
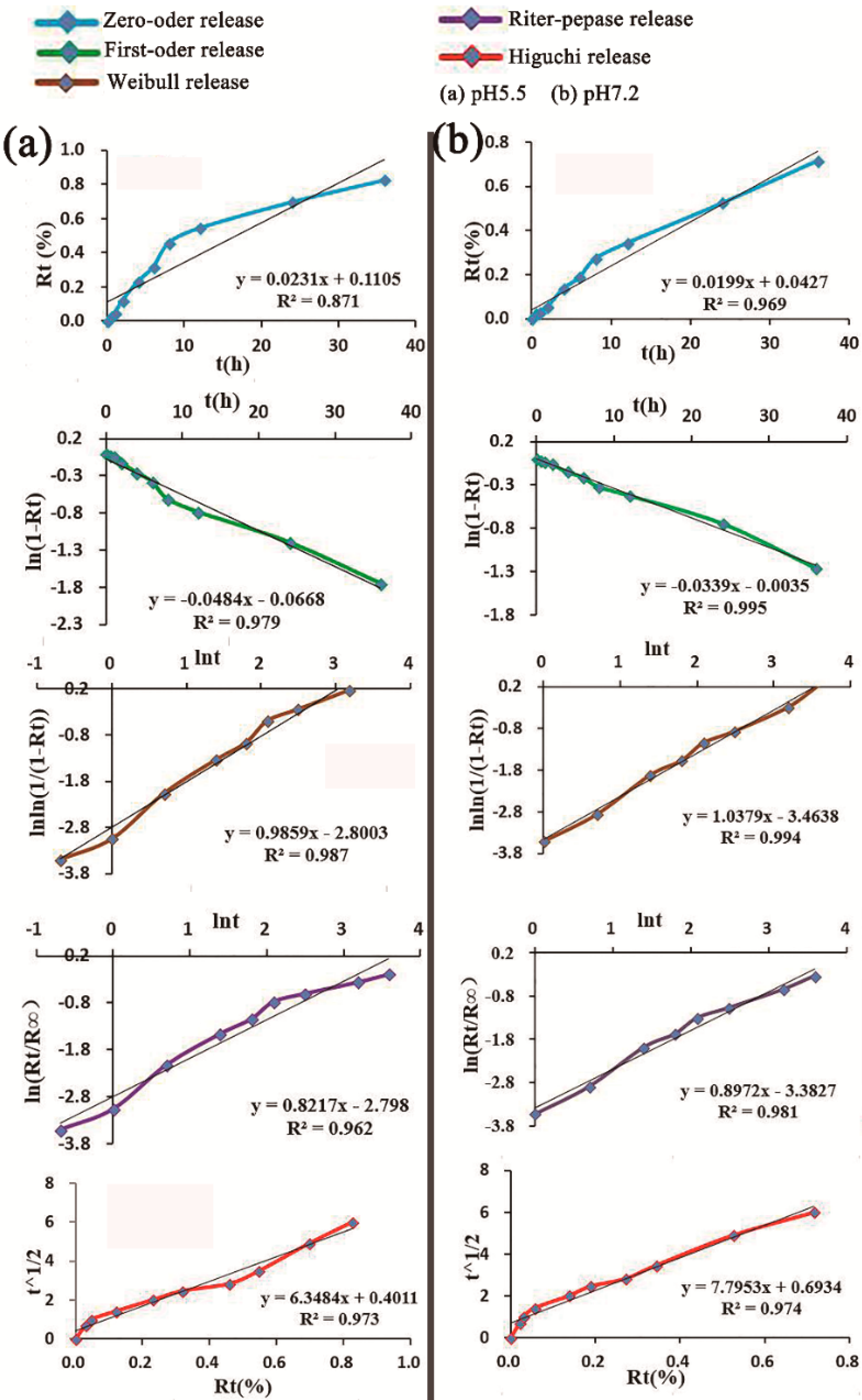
2.3. In Vitro Release Studies
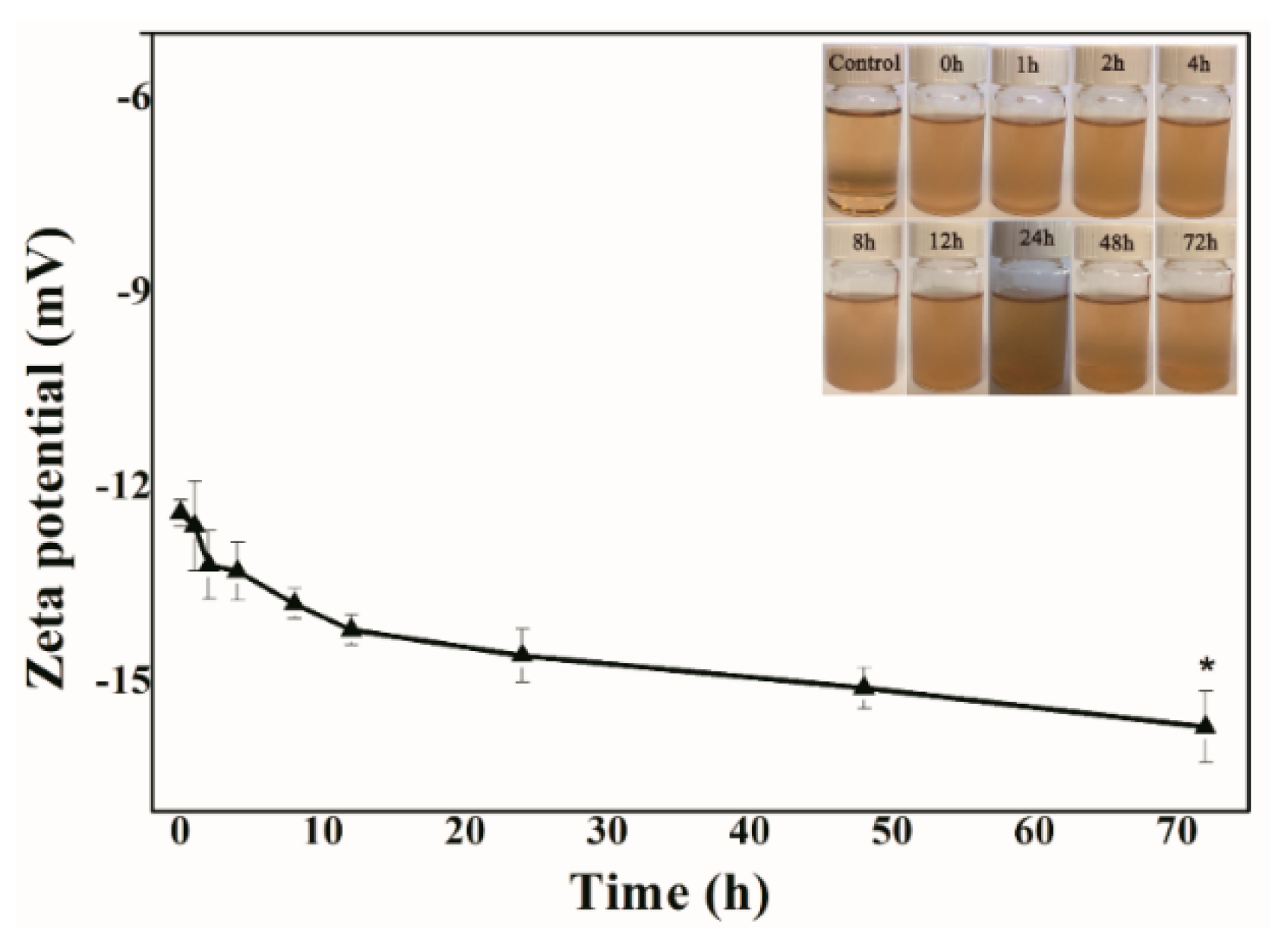
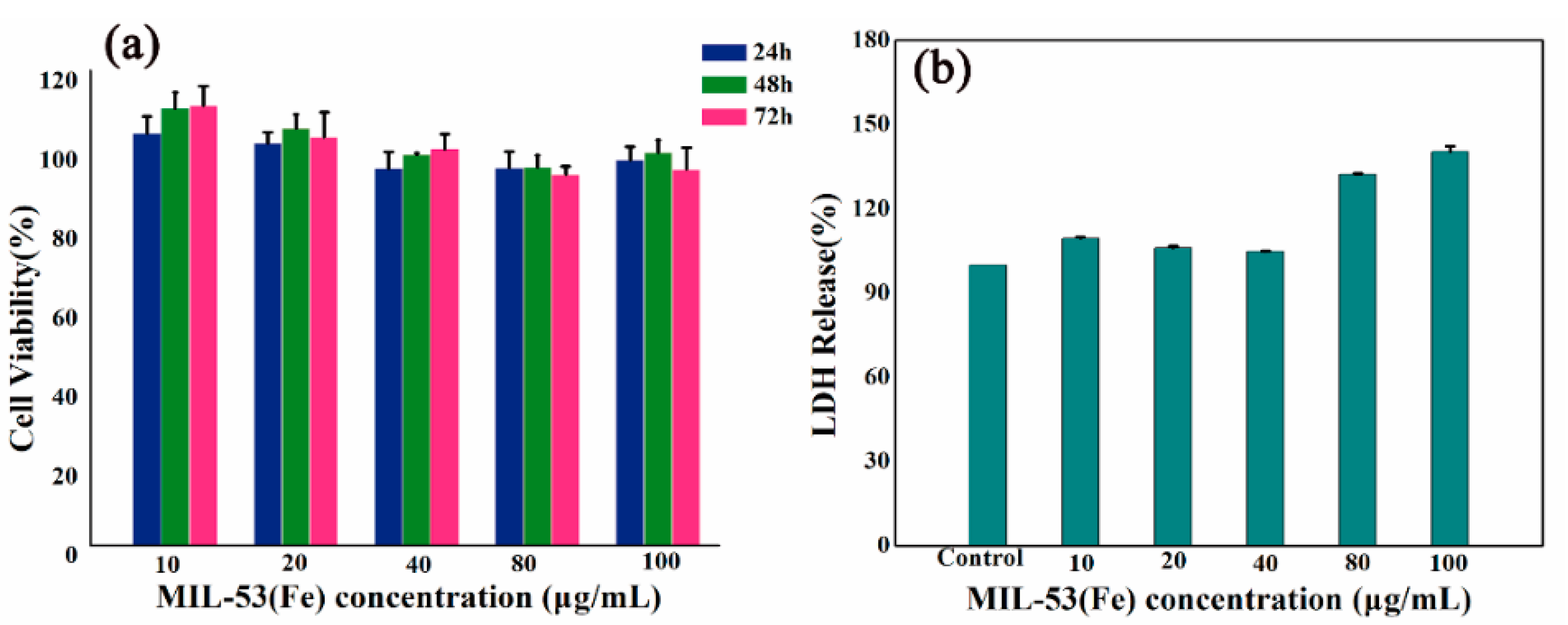
2.4. Safety Evaluation of MIL-53(Fe)
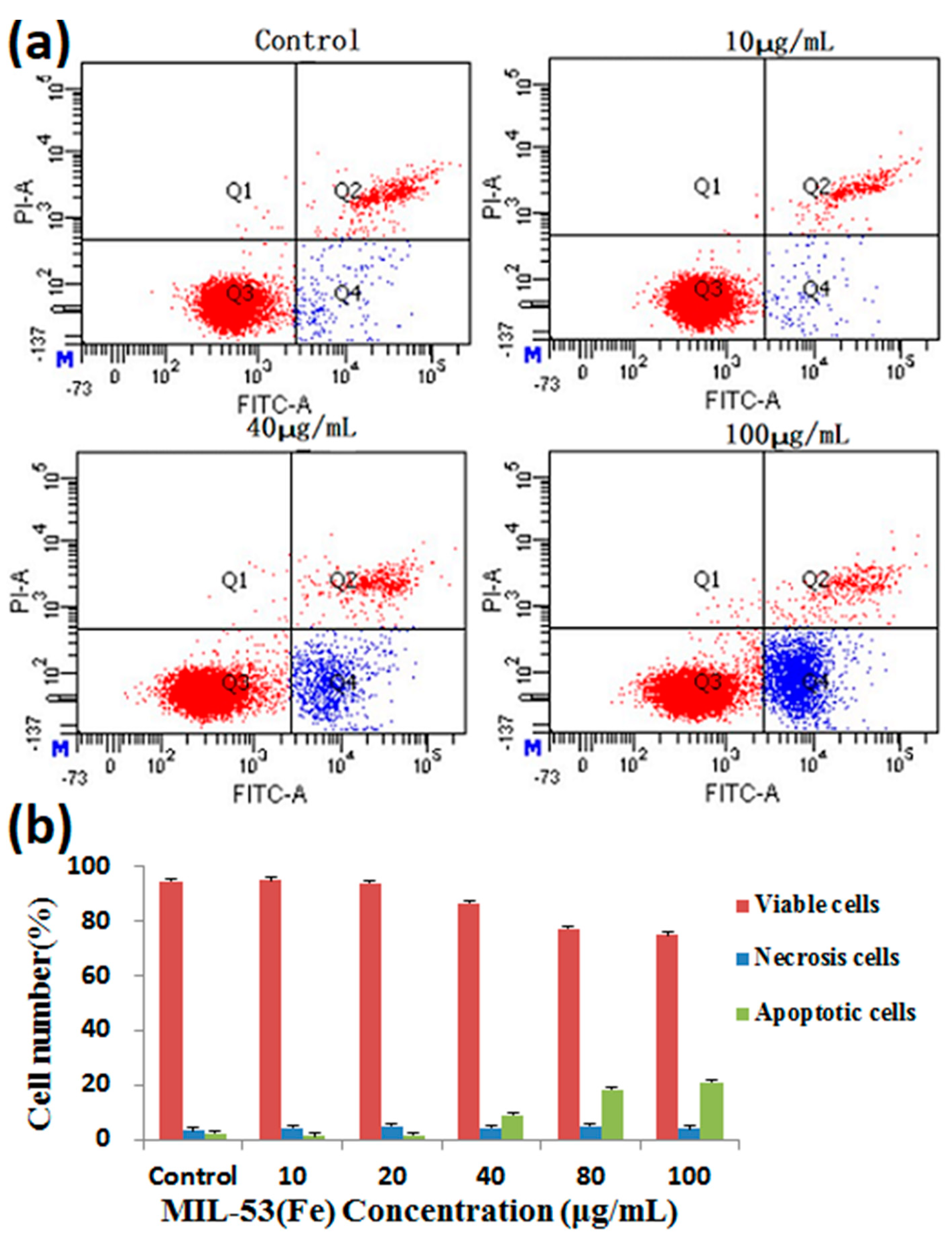
2.5. Cytotoxic Effect of Ori@MIL-53(Fe) on HepG2 Cells
3. Discussion and Conclusions
4. Methods
4.1. Synthesis and Characterization of MIL-53(Fe)
4.2. Stability of MIL-53(Fe) in FBS
4.3. Encapsulation and Evaluation of Ori@MIL-53(Fe)
4.4. In Vitro Release Studies
4.5. Cell Culture
4.6. Cytotoxicity Assay
4.7. LDH Assay
4.8. Annexin V/PI Double-Staining Assay
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.Q.; Zhang, H.M.; Sun, X.E. Inhibitory effects and mechanism of 5-fluorouracil combined with celecoxib on human gastric cancer xenografts in nude mice. Exp. Ther. Med. 2015, 9, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xie, K.; Sheng, D.; Wan, X.; Zhu, G. Antiangiogenic effects of oridonin. BMC Complem. Altern. Med. 2017, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhang, P.; Li, Q.X.; Pan, Q.; Zheng, H.L.; Zhao, S.R. Effect of Oridonin on apoptosis and intracellular reactive oxygen species level in triple-negative breast cancer MDA-MB-231 cells. Zhongguo Zhong Yao Za Zhi. 2017, 42, 2361–2365. [Google Scholar] [PubMed]
- Xia, S.X.; Zhang, X.L.; Li, C.H.; Guan, H.L. Oridonin inhibits breast cancer growth and metastasis through blocking the Notch signaling. Saudi Pharm. J. 2017, 25, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhang, S.F.; Bao, J.T.; Liu, F.Y. Oridonin synergizes with Nutlin-3 in osteosarcoma cells by modulating the levels of multiple Bcl-2 family proteins. Tumour Biol. 2017, 39, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, F.; Wu, K.R.; Ye, Z.P.; Li, N. Oridonin enhances in vitro anticancer effects of lentinan in SMMC-7721 human hepatoma cells through apoptotic genes. Exp. Ther. Med. 2017, 14, 5129–5134. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, X.; Qu, S.; Yao, B.; Xu, Y.; Wu, J.; Jin, Y.; Ma, C. Oridonin induces G2/M cell cycle arrest and apoptosis via the PI3K/Akt signaling pathway in hormone-independent prostate cancer cells. Oncol. Lett. 2017, 13, 2838–2846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, M.; He, P.; Zhao, J.; Chen, Y.; Qi, J.; Wang, Y. Proteomic analysis of oridonin-induced apoptosis in multiple myeloma cells. Mol. Med. Rep. 2017, 15, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, Y.; Chui, J.H.; Zhu, G.Y.; Li, Y.W.; Fong, D.W.; Yu, Z.L. Oridonin induces G2/M cell cycle arrest and apoptosis through MAPK and p53 signaling pathways in HepG2 cells. Oncol. Rep. 2010, 24, 647–651. [Google Scholar] [PubMed]
- Wang, H.; Ye, Y.; Yu, Z.L. Proteomic and functional analyses demonstrate the involvement of oxidative stress in the anticancer activities of oridonin in HepG2 cells. Oncol. Rep. 2014, 31, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.Q.; Liu, G.P.; Guo, H.J.; Li, C.Y.; Zhang, Y.C.; Zhang, F.; Zhao, Z.X.; Cheng, H.L. Novel galactosylated biodegradable nanoparticles for hepatocyte-delivery of oridonin. Int. J. Pharm. 2016, 502, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Hu, B. The Anti-tumor Effect of Folate-targeted Liposome Microbubbles Loaded with Oridonin as Ultrasound-triggered Tumor-targeted Therapeutic Carrier System. J. Drug Target. 2017, 25, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.Q.; Guo, J.H.; He, Y.F.; Song, P.F.; Xiong, Y.B.; Wang, R.M. Fabrication of dual-sensitive keratin-based polymer hydrogels and their controllable release behaviors. J. Biomater. Sci. Polym. Ed. 2016, 27, 1926–1940. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Bellia, F.; Viale, M.; Maric, I.; Vecchio, G. Linear polymers of β and γ cyclodextrins with a polyglutamic acid backbone as carriers for doxorubicin. Carbohyd. Polym. 2017, 177, 355–360. [Google Scholar] [CrossRef] [PubMed]
- De Moraes Nogueira, A.O.; de Sousa, R.S.; Pereira, L.S.; Mallmann, C.; da Silva Ferreira, A.; Clementin, R.M.; de Lima, V.R. Physicochemical interactions among α-eleostearic acid-loaded liposomes applied to the development of drug delivery systems. J. Mol. Struct. 2018, 1154, 248–255. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.W.; Yuan, X.Z.; Wang, J.F.; Zhang, L.J. Folic acid grafted and tertiary amino based pH-responsive pentablock polymeric micelles for targeting anticancer drug delivery. Mater. Sci. Eng. R 2018, 82, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Huang, C.Y.; Chen, Y.L.; Ger, T.R. pH-responsive magnetic micelles gelatin-g-poly (NIPAAm-co-DMAAm-co-UA)-g-dextran/Fe3O4 as a hydrophilic drug carrier. RSC Adv. 2018, 7, 28207–28212. [Google Scholar] [CrossRef]
- Tian, Z.F.; Yu, X.; Ruan, Z.J.; Zhu, M.; Zhu, Y.F.; Hanagata, N. Magnetic mesoporous silica nanoparticles coated with thermo-responsive copolymer for potential chemo- and magnetic hyperthermia therapy. Microporous Microporous Mater. 2018, 256, 1–9. [Google Scholar] [CrossRef]
- Tran, V.A.; Lee, S.W. A prominent anchoring effect on the kinetic control of drug release from mesoporous silica nanoparticles (MSNs). J. Colloid Interface Sci. 2018, 510, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Duo, Y.H.; Li, Y.; Chen, C.K.; Liu, B.Y.; Wang, X.Y.; Zeng, X.W.; Chen, H.B. DOX-loaded pH-sensitive mesoporous silica nanoparticles coated with PDA and PEG induce pro-death autophagy in breast cancer. RSC. Adv. 2017, 7, 39641–39650. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, C.Y.; Jiang, Y.; Liu, T.; Yang, J.Y.; Lin, R.; Zhang, T. Phosphorylcholine oligomer-grafted graphene oxide for tumor-targeting doxorubicin delivery. RSC. Adv. 2017, 7, 41675–41685. [Google Scholar] [CrossRef]
- Yan, L.; Chen, X.F.; Wang, Z.G.; Zhang, X.J.; Zhu, X.Y.; Zhou, M.J.; Chen, W.; Huang, L.B.; Roy, V.A.L.; Yu, P.K.N.; et al. Size Controllable and Surface Tunable Zeolitic Imidazolate Framework-8–Poly (acrylic acid sodium salt) Nanocomposites for pH Responsive Drug Release and Enhanced in Vivo Cancer Treatment. ACS Appl. Mater. Interface 2017, 9, 32990–33000. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, L.; Ladaviere, C. A close collaboration of chitosan with lipid colloidal carriers for drug delivery applications. J. Control. Release 2017, 256, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Recent advances in porous nanoparticles for drug delivery in antitumoral applications: Inorganic nanoparticles and nanoscale metal-organic frameworks. Expert Opin. Drug Deliv. 2017, 14, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Tavra, C.; Baxter, E.F.; Tian, T.; Bennett, T.D.; Slater, N.K.; Cheetham, A.K.; Fairen-Jimenez, D. Amorphous metal-organic frameworks for drug delivery. Chem. Commun. 2015, 51, 13878–13881. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhang, L.; Hu, Q.; Zhang, Q.; Lin, W.X.; Cui, Y.J.; Yang, Y.; Qian, G.D. Thermal Stimuli-Triggered Drug Release from a Biocompatible Porous Metal-Organic Framework. Chem. Eur. J. 2017, 23, 10215–10221. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.Q.; Li, Y.; Mao, L.Y.; Chen, Q.; Lin, N. A series of porous metal–organic frameworks with hendecahedron cage: Structural variation and drug slow release properties. J. Solid State Chem. 2018, 257, 58–63. [Google Scholar] [CrossRef]
- Wang, J.; Ma, D.Y.; Liao, W.L.; Li, S.J.; Huang, M.F.; Liu, H.M.; Wang, Y.F.; Xie, R.; Xu, J. A hydrostable anionic zinc-organic framework carrier with a bcu topology for drug delivery. Crystengcomm 2017, 19, 5244–5250. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.M.; Tan, L.; Cui, Z.D.; Yang, X.J.; Yeung, K.W.K.; Pan, H.B.; Wu, S.L. Porous Iron-Carboxylate Metal-Organic Framework: A Novel Bioplatform with Sustained Antibacterial Efficacy and Nontoxicity. ACS Appl. Mater. Interfaces 2017, 9, 19248–19257. [Google Scholar] [CrossRef] [PubMed]
- Pereacachero, A.; Romero, E.; Ariso, C.T. Insight into the reversible structural crystalline-state transformation from MIL-53(Al) to MIL-68(Al). Crystengcomm 2018, 20, 402–406. [Google Scholar] [CrossRef]
- Gordon, J.; Kazemian, H.; Rohani, S. MIL-53(Fe), MIL-101, and SBA-15 porous materials: Potential platforms for drug delivery. Mater. Sci. Eng. C Mater. 2015, 47, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev. 2009, 38, 1380–1399. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Guillaume, M.; Naseem, R.; Maria, V.R.; Sebban, M.; Francis, T.; Gerard, F. Flexible Porous Metal-Organic-Frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 435, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Tamames, T.C.; García-Márquez, A.; Blanco-Prieto, M.J. MOFs in pharmaceutical technology. Bio-Bioinspired Nanomater. 2014, 83–112. [Google Scholar] [CrossRef]
- Alkordi, M.H.; Belmabkhout, Y.; Cairns, A.; Eddaoudi, M. Metal-organic frameworks for H2 and CH4 storage: Insights on the pore geometry-sorption energetics relationship. IUCrJ 2017, 4, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Q. Vapochromic behavior of MOF for selective sensing of ethanol. Spectrochim. Acta A 2017, 184, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Mínguez, E.G.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Sabouni, R.; Husseini, G.A. Anti-cancer drug delivery using metal organic frameworks (MOFs). Curr. Med. Chem. 2017, 24, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.N.; Xu, X.Y.; Lian, X.; Zhang, C.; Yan, B. A Luminescent 3d-4f-4d MOF Nanoprobe as a Diagnosis Platform for Human Occupational Exposure to Vinyl Chloride Carcinogen. Inorg. Chem. 2017, 56, 11176–11183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, W.; Liu, R.; Zhang, J.; Zhang, D.; Li, Z. Rational Design of Metal Organic Framework Nanocarrier-Based Codelivery System of Doxorubicin Hydrochloride/Verapamil Hydrochloride for Overcoming Multidrug Resistance with Efficient Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 19687–19697. [Google Scholar] [CrossRef] [PubMed]
- Ananthoji, R.; Eubank, J.F.; Nouar, F.; Mouttaki, H. Symbiosis of zeolite-like metal–organic frameworks (rho-ZMOF) and hydrogels: Composites for controlled drug release. J. Mater. Chem. 2011, 21, 587–9594. [Google Scholar] [CrossRef]
- Motakef, K.N.; Shojaosadati, S.A.; Morsali, A. Evaluation of the effect of nanoporous nanorods Zn 2 (bdc) 2 (dabco) dimension on ibuprofen loading and release. J. Iranian Chem. Soc. 2016, 13, 1205–1212. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Helan, X.; Yang, Y. Quantitative correlation between cross-linking degrees and mechanical properties of protein films modified with polycarboxylic acids. Macromol. Mater. Eng. 2015, 300, 1133–1140. [Google Scholar] [CrossRef]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef] [PubMed]
- Sumit, A.; Jyutika, M.R.; Kishore, M.P. Nanotoxicology and in vitro studies: The need of the hour. Toxicol. Appl. Pharmacol. 2012, 258, 151–165. [Google Scholar]
- Cao, Y.; Yang, J. Development of a Folate Receptor (FR)-Targeted Indenoisoquinoline Using a pH-Sensitive N-Ethoxybenzylimidazole (NEBI) Bifunctional Cross-Linker. Bioconjugate Chem. 2014, 25, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Jing, F.; Yin, X. Induction of Apoptosis in HepaRG Cell Line by Aloe Emodin through Ceneration of Reactive Oxygen Species and the Mitochondrial Pathway. Cell. Physiol. Biochem. 2017, 42, 685–696. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, X.; Dong, X.; Wang, W.; Sai, N.; Yang, C.; You, L.; Huang, H.; Yin, X.; Ni, J. Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers. Molecules 2018, 23, 2490. https://doi.org/10.3390/molecules23102490
Leng X, Dong X, Wang W, Sai N, Yang C, You L, Huang H, Yin X, Ni J. Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers. Molecules. 2018; 23(10):2490. https://doi.org/10.3390/molecules23102490
Chicago/Turabian StyleLeng, Xin, Xiaoxv Dong, Wenping Wang, Na Sai, Chunjing Yang, Longtai You, Hongliang Huang, Xingbin Yin, and Jian Ni. 2018. "Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers" Molecules 23, no. 10: 2490. https://doi.org/10.3390/molecules23102490
APA StyleLeng, X., Dong, X., Wang, W., Sai, N., Yang, C., You, L., Huang, H., Yin, X., & Ni, J. (2018). Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers. Molecules, 23(10), 2490. https://doi.org/10.3390/molecules23102490




