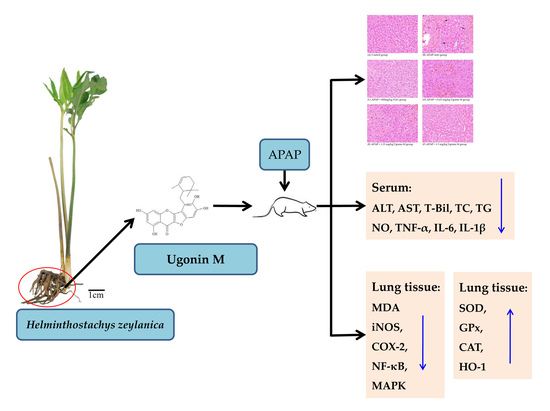
Hepatoprotective Effect of Ugonin M, A Helminthostachys zeylanica Constituent, on Acetaminophen-Induced Acute Liver Injury in Mice
Abstract
1. Introduction
2. Results
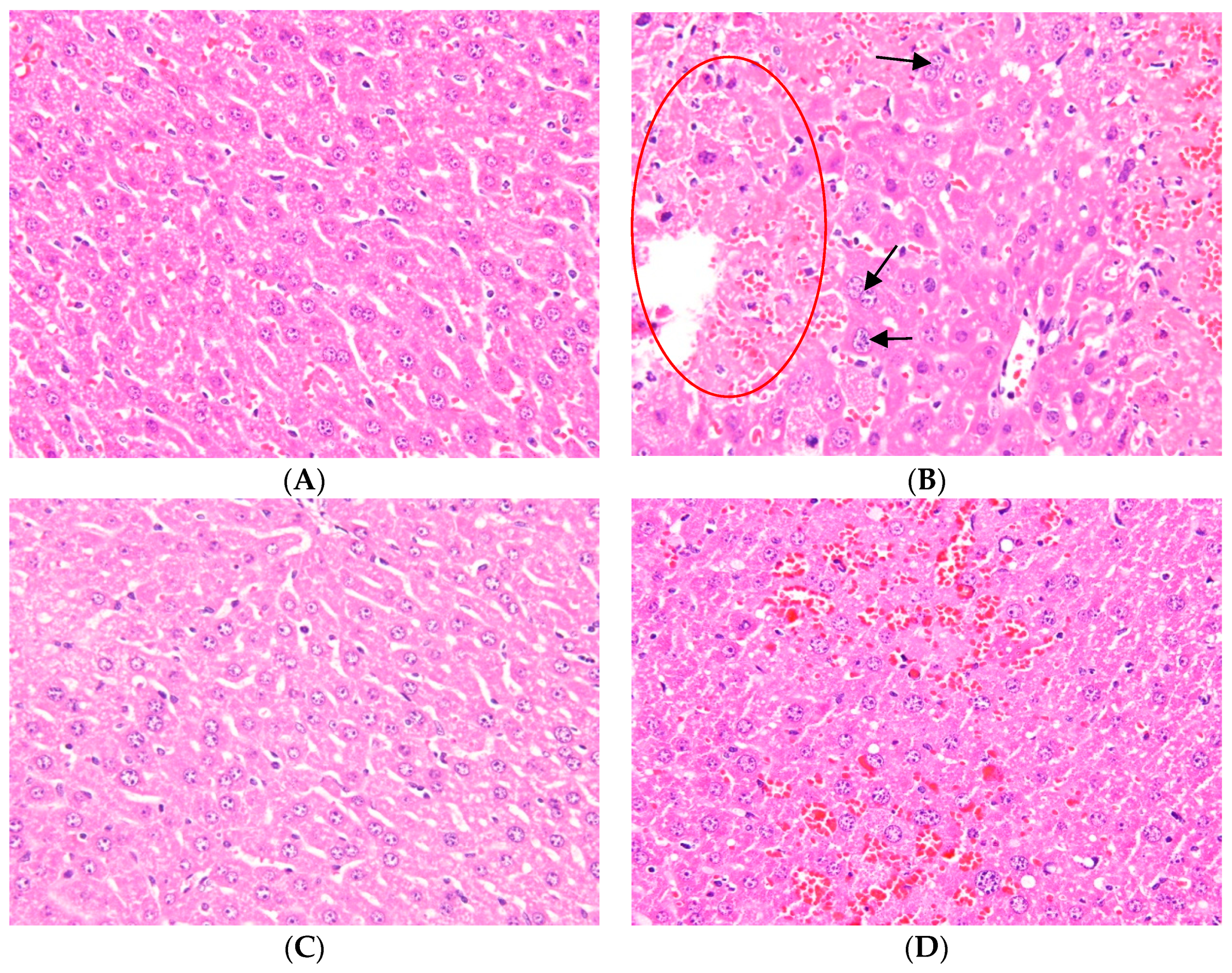
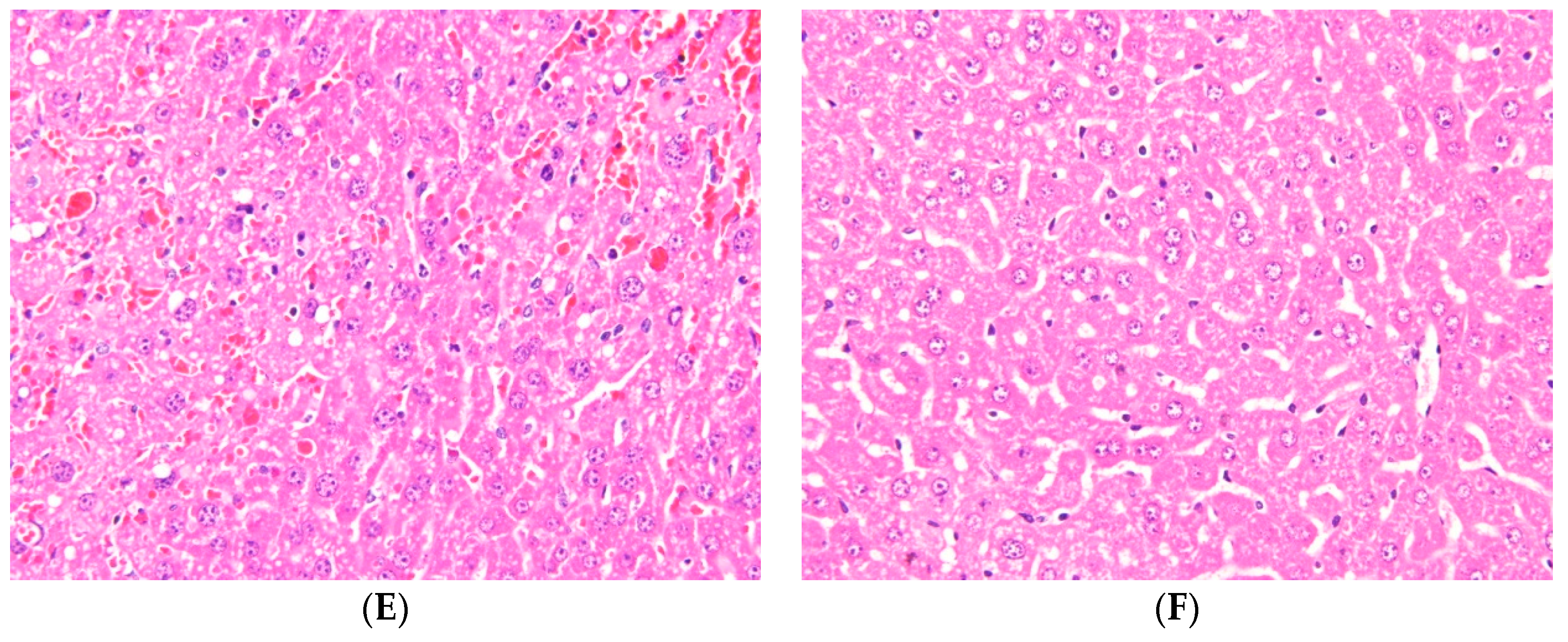
2.1. Effects of Ugonin M on APAP-Induced Liver Injury
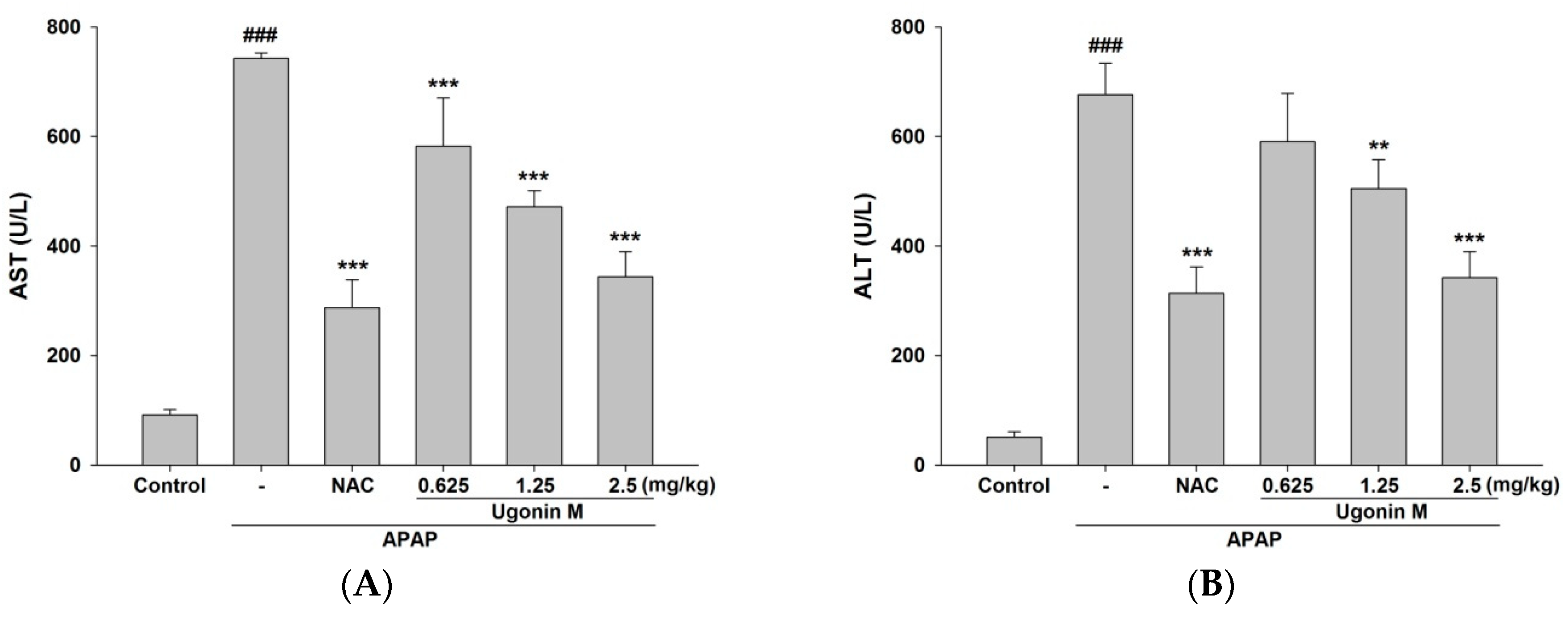
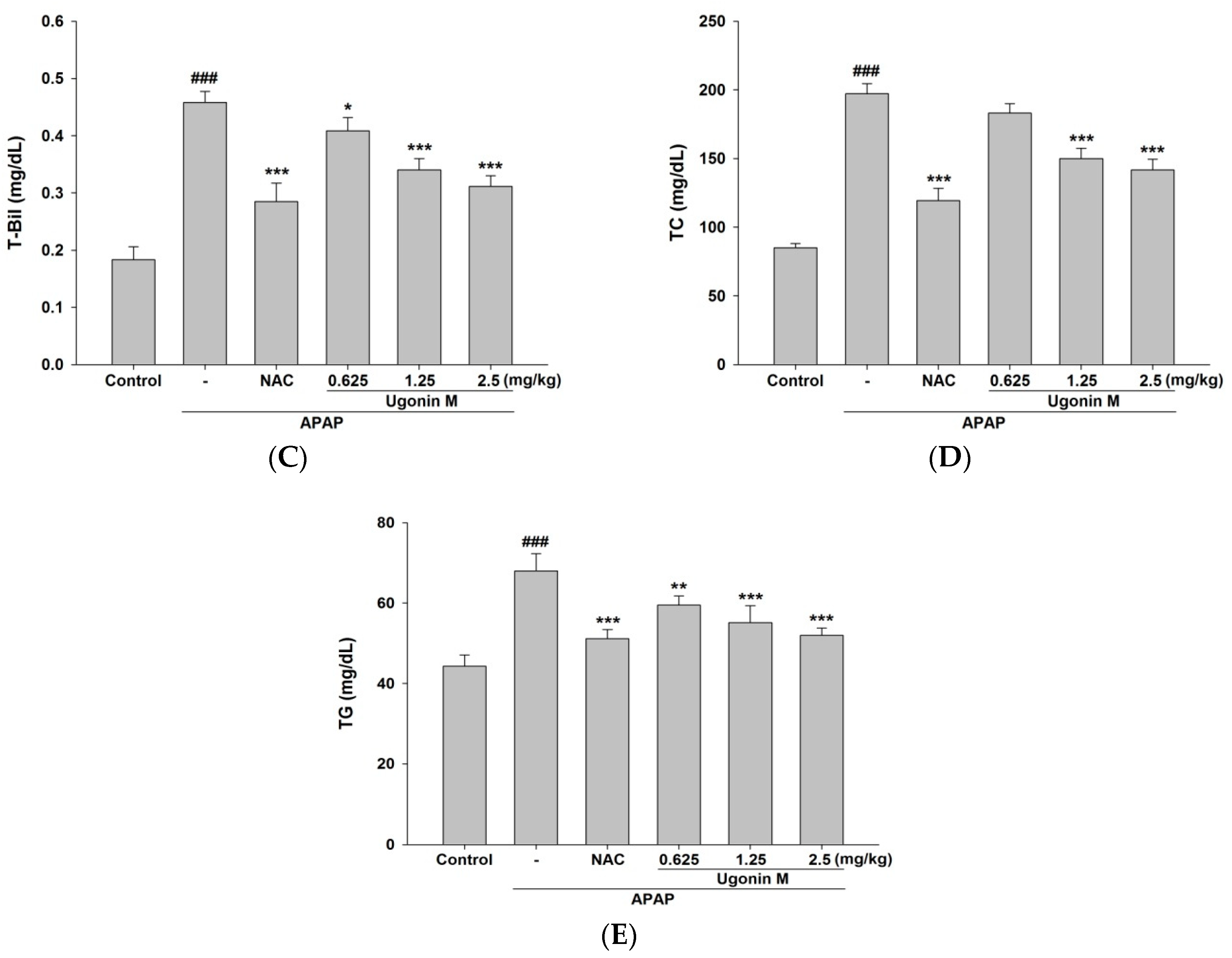
2.2. Effects of Ugonin M on Liver Functions
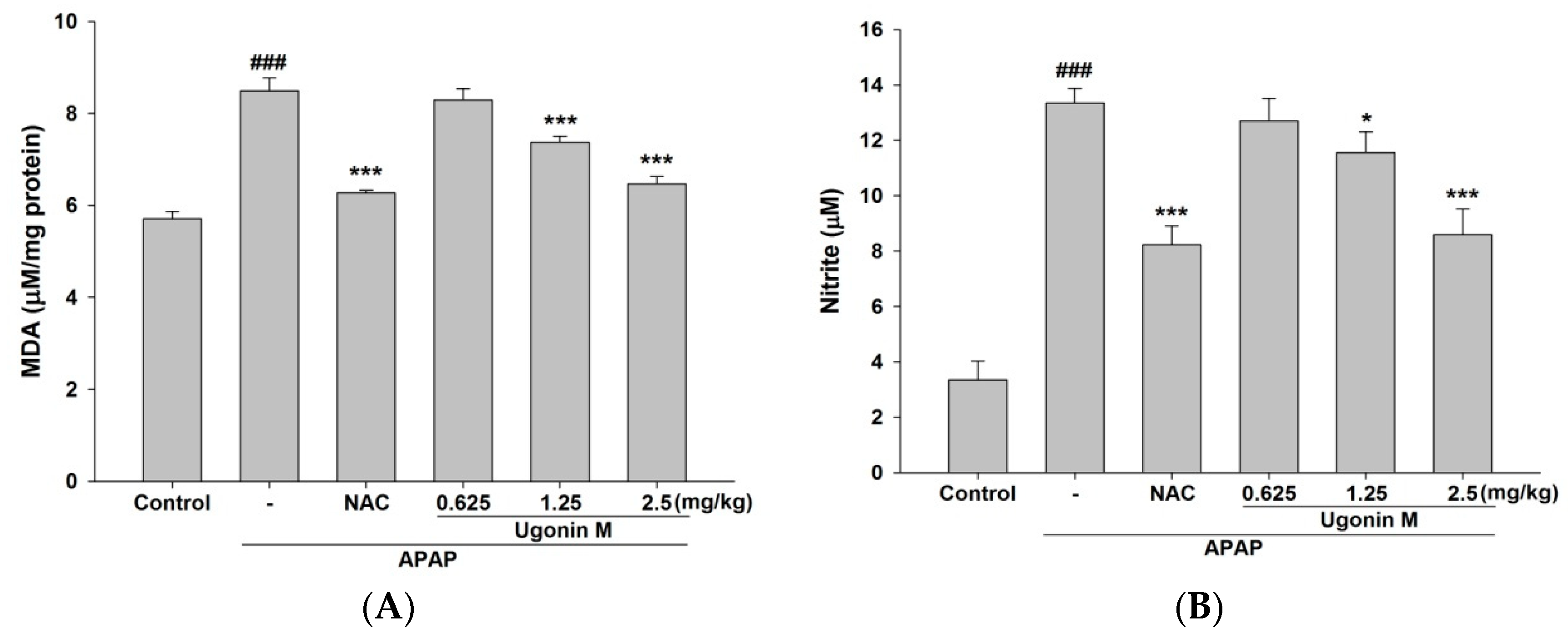
2.3. Effects of Ugonin M on Lipid Peroxidation in Liver Tissue
2.4. Effects of Ugonin M on Serum Nitric Oxide (NO) Levels
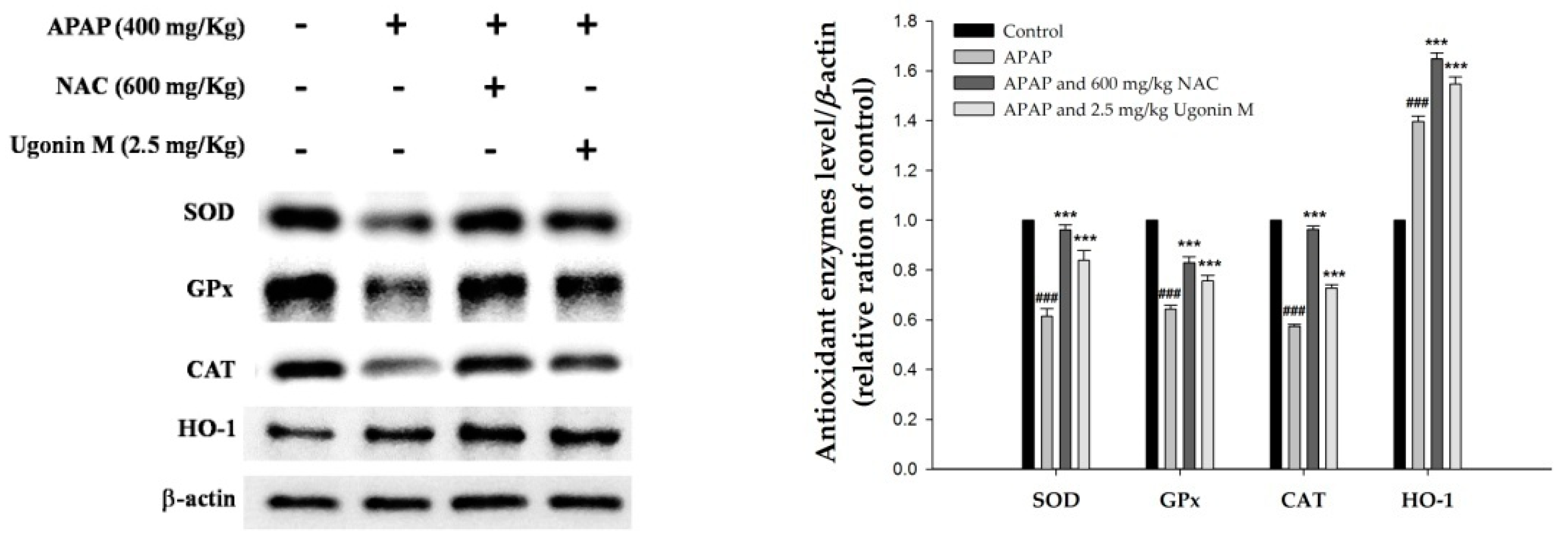
2.5. Effects of Ugonin M on the Activity of Antioxidant Enzymes in Liver Tissue
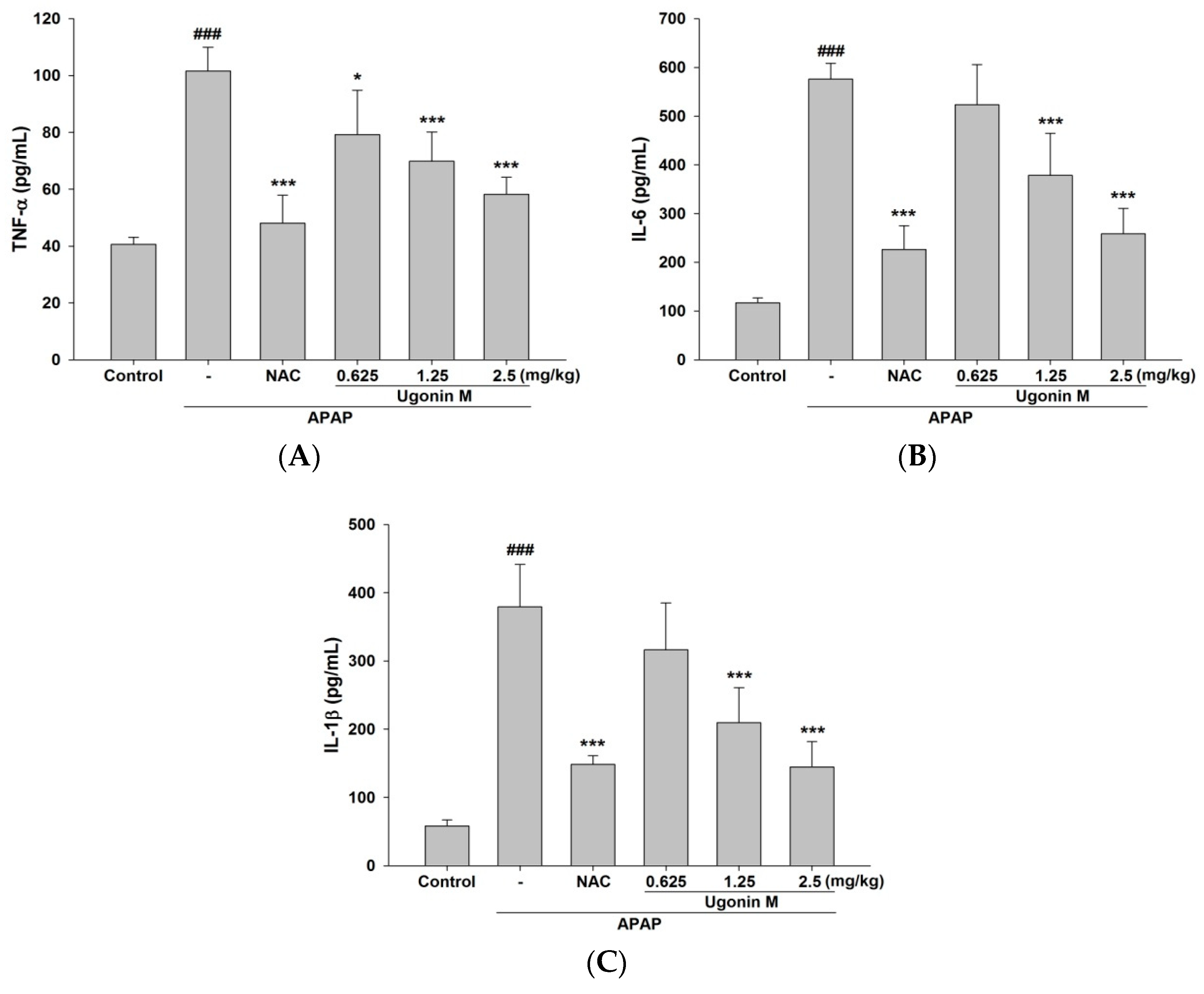
2.6. Effects of Ugonin M on Serum tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β Levels
2.7. Effects of Ugonin M on Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase-2 (COX-2) Protein Expression in Liver Tissue
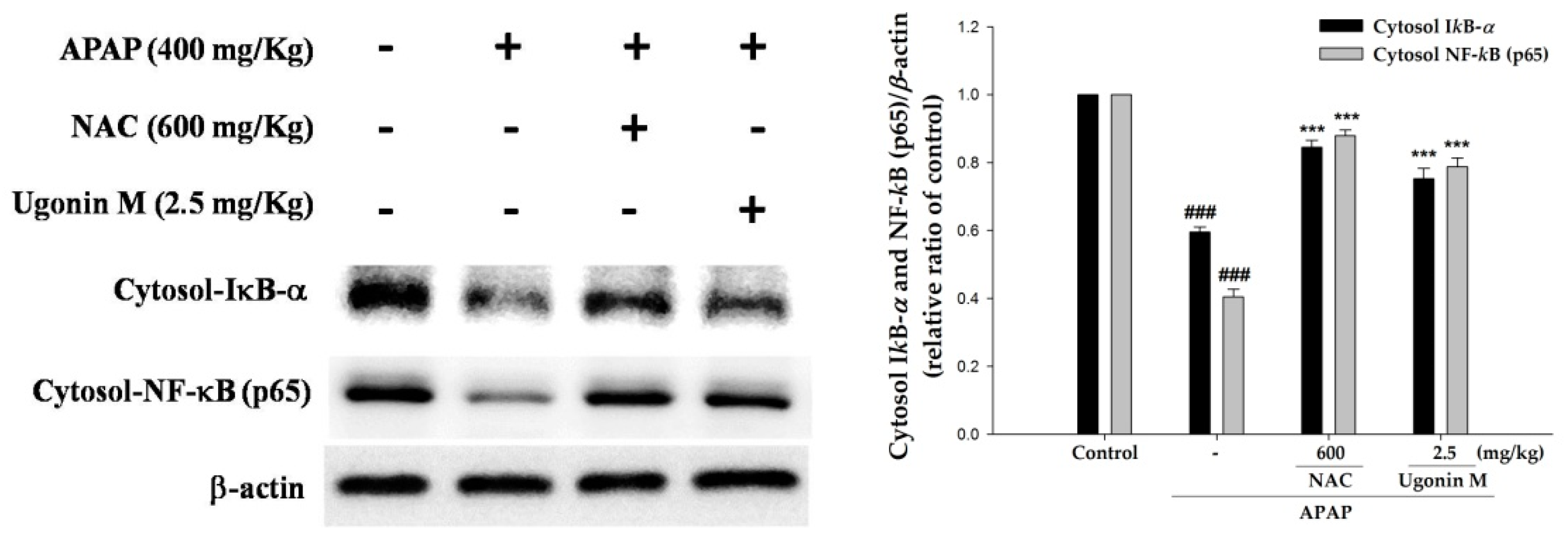
2.8. Effects of Ugonin M on Activities of Nuclear Factor-Kappa B (NF-κB) in Liver Tissue
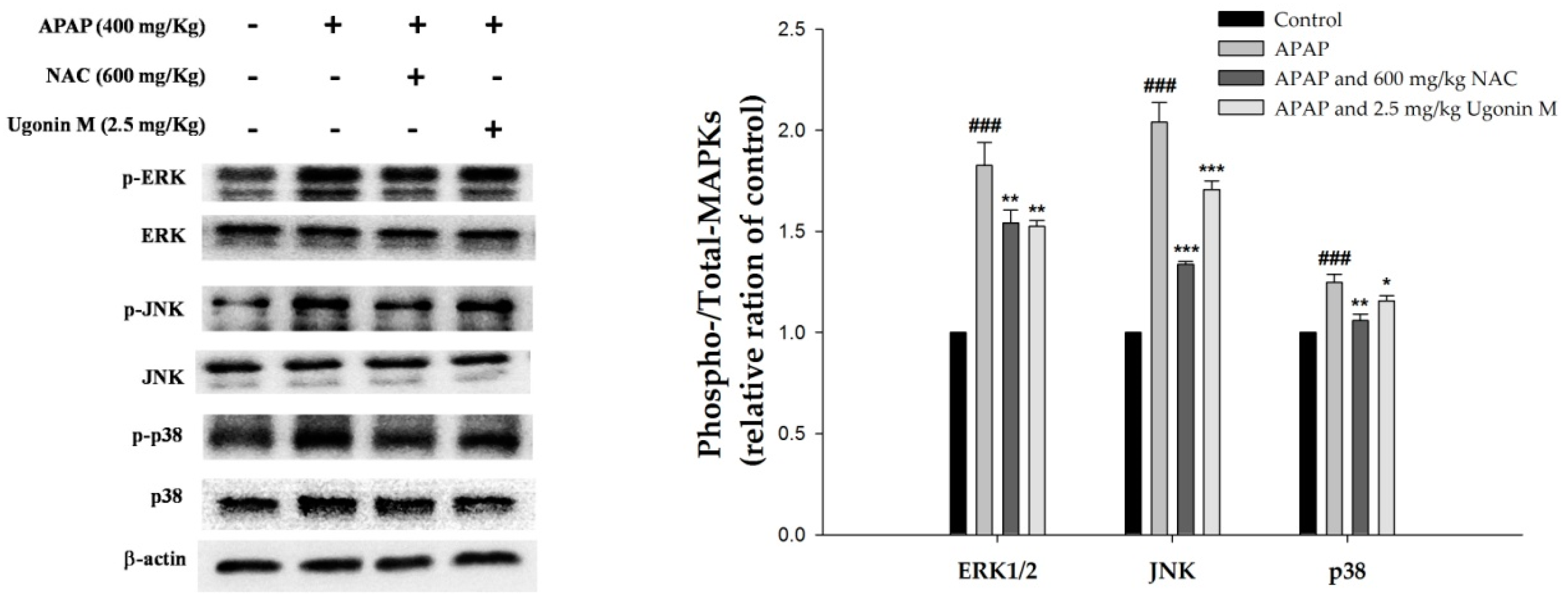
2.9. Effects of Ugonin M on Activities of MAPK in Liver Tissue
3. Discussion
4. Materials and Methods
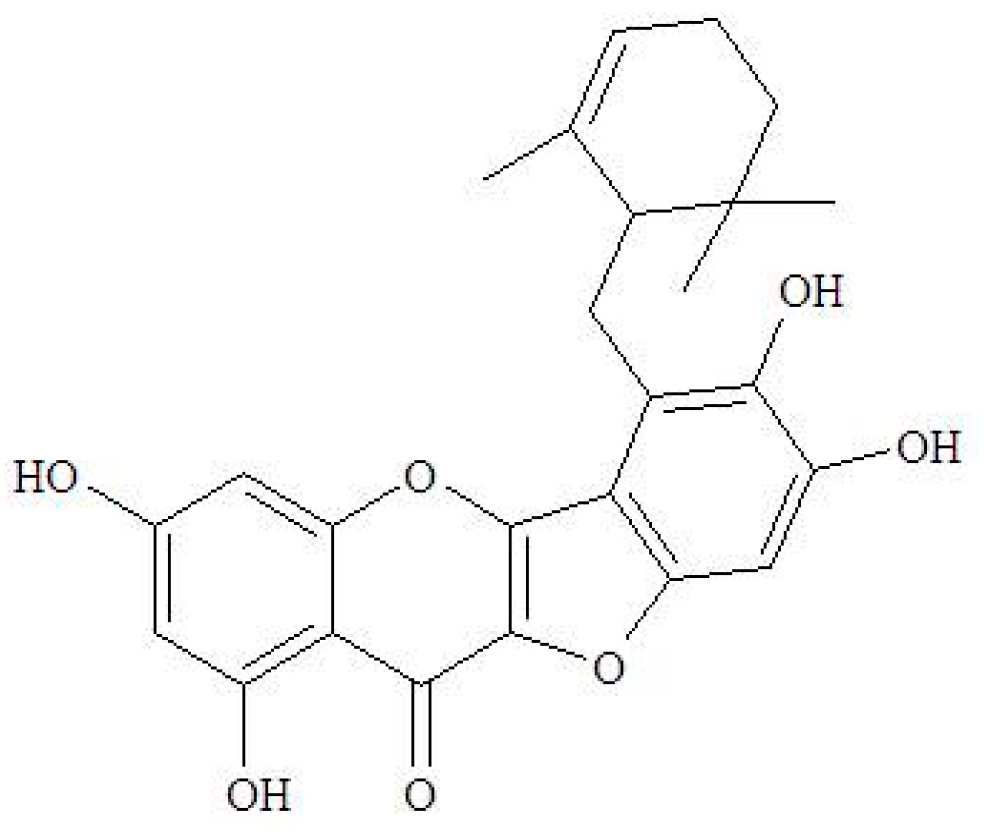
4.1. The Source of Ugonin M
4.2. Animal and Treatments
4.3. Histological Examination
4.4. Assessment of Liver Functions
4.5. The Measurement of Nitric Oxide and MDA
4.6. TNF-α, IL-6, and IL-1β Cytokines in Serum
4.7. Western Blot Analysis of the Liver Tissues
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vliegenthart, A.D.; Antoine, D.J.; Dear, J.W. Target biomarker profile for the clinical management of paracetamol overdose. Br. J. Clin. Pharmacol. 2015, 80, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Williams, C.D.; Ramachandran, A.; Bajt, M.L. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int. 2012, 32, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Thorgeirsson, S.S.; Potter, W.Z.; Jollow, D.J.; Keiser, H. Acetaminophen-induced hepatic injury: Protective role of glutathione in man and rationale for therapy. Clin. Pharmacol. Ther. 1974, 16, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Eugenio-Perez, D.; Montes de Oca-Solano, H.A.; Pedraza-Chaverri, J. Role of food-derived antioxidant agents against acetaminophen-induced hepatotoxicity. Pharm. Biol. 2016, 54, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M. Acetaminophen (APAP) hepatotoxicity—Isn’t it time for APAP to go away? J. Hepatol. 2017, 67, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.; Abushouk, A.I.; Reggi, R.; Yarla, N.S.; Palmery, M.; Peluso, I. Association of antioxidant nutraceuticals and acetaminophen (paracetamol): Friend or foe? J. Food Drug Anal. 2017, 2, S78–S87. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Setty, S.; Quereshi, A.A.; Viswanath Swamy, A.H.; Patil, T.; Prakash, T.; Prabhu, K.; Veeran Gouda, A. Hepatoprotective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia 2007, 78, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, M.M.; Abd-Allah, G.M.; Mohamadin, A.M.; Harisa, G.I.; Mariee, A.D. Quercetin protects against acetaminophen-induced hepatorenal toxicity by reducing reactive oxygen and nitrogen species. Pathophysiology 2015, 22, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Jaeschke, H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepatol. 2017, 66, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Lauterburg, B.H.; Corcoran, G.B.; Mitchell, J.R. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J. Clin. Investig. 1983, 71, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Huang, G.J.; Ho, Y.L. The Illustration of Common Medicinal Plants in Taiwan, 2nd ed.; Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan: Taipei, Taiwan, 2011; Volume I, p. 41. [Google Scholar]
- Chiu, N.Y.; Chang, K.H. The Illustrated Medicinal Plants of Taiwan; SMC Publishing Inc.: Taipei, Taiwan, 1992; Volume 3. [Google Scholar]
- Suja, S.R.; Latha, P.G.; Pushpangadan, P.; Rajasekharan, S. Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L.) Hook against carbon tetrachloride-induced liver damage in Wistar rats. J. Ethnopharmacol. 2004, 92, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Suja, S.R.; Latha, P.G.; Pushpangadan, P.; Shine, V.J.; Anuja, G.I.; Bijukumar, B.S.; Rajasekharan, S. Evaluation of antihepatotoxic potential of Helminthostachys zeylanica (Linn.) Hook. f., a medicinal fern against ethanol induced liver damage: In vitro and in vivo studies. AJEB 2014, 1, 16–31. [Google Scholar]
- Liou, C.J.; Huang, Y.L.; Huang, W.C.; Yeh, K.W.; Huang, T.Y.; Lin, C.F. Water extract of Helminthostachys zeylanica attenuates LPS-induced acute lung injury in mice by modulating NF-kappaB and MAPK pathways. J. Ethnopharmacol. 2017, 199, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Huang, Y.L.; Yeh, P.Y.; Ou, J.C. Cyclized geranyl stilbenes from the rhizomes of Helminthostachys zeylanica. Planta Med. 2003, 6, 964–967. [Google Scholar]
- Huang, Y.L.; Yeh, P.Y.; Shen, C.C.; Chen, C.C. Antioxidant flavonoids from the rhizomes of Helminthostachys zeylanica. Phytochemistry 2003, 6, 1277–1283. [Google Scholar] [CrossRef]
- Huang, Y.C.; Hwang, T.L.; Chang, C.S.; Yang, Y.L.; Shen, C.N.; Liao, W.Y.; Chen, S.C.; Liaw, C.C. Anti-inflammatory flavonoids from the rhizomes of Helminthostachys zeylanica. J. Nat. Prod. 2009, 72, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C. Anti-Inflammatory Constituents from the Rhizomes of Helminthostachys zeylanica. Ph.D. Thesis, China Medical University, Taichung, Taiwan, 2010. [Google Scholar]
- Huang, Y.C.; Hwang, T.L.; Yang, Y.L.; Wu, S.H.; Hsu, M.H.; Wang, J.P.; Chen, S.C.; Huang, L.J.; Liaw, C.C. Acetogenin and prenylated flavonoids from Helminthostachys zeylanica with inhibitory activity on superoxide generation and elastase release by neutrophils. Planta Med. 2010, 76, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Li, Y.P.; Li, H.M.; Dai, W.F.; Liu, D.; Cao, L.; Li, R.T. Anti-inflammatory prenylated flavonoids from Helminthostachys zeylanica. Chem. Pharm. Bull. 2016, 64, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Shen, C.C.; Shen, Y.C.; Chiou, W.F.; Chen, C.C. Anti-inflammatory and antiosteoporosis flavonoids from the rhizomes of Helminthostachys zeylanica. J. Nat. Prod. 2017, 80, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Huang, S.S.; Kuo, Y.H.; Ho, Y.L.; Yang, C.S.; Chang, Y.S.; Huang, G.J. Ugonin M, a Helminthostachys zeylanica constituent, prevents LPS-induced acute lung injury through TLR4-mediated MAPK and NF-kappaB signaling pathways. Molecules 2017, 22, 573. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mitsunaga, T.; Batubara, I. Novel quercetin glucosides from Helminthostachys zeylanica root and acceleratory activity of melanin biosynthesis. J. Nat. Med. 2013, 67, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mitsunaga, T.; Itakura, Y.; Batubara, I. Extracellular melanogenesis inhibitory activity and the structure-activity relationships of ugonins from Helminthostachys zeylanica roots. Fitoterapia 2015, 104, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Huang, Y.C.; Chen, S.C.; Liaw, C.C.; Kuo, S.C.; Huang, L.J.; Gean, P.W. Neuroprotective effects of ugonin K on hydrogen peroxide-induced cell death in human neuroblastoma SH-SY5Y cells. Neurochem. Res. 2009, 34, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Huang, Y.L.; Liao, J.F.; Chiou, W.F. Ugonin K promotes osteoblastic differentiation and mineralization by activation of p38 MAPK- and ERK-mediated expression of Runx2 and osterix. Eur. J. Pharmacol. 2011, 668, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Huang, Y.L.; Liao, J.F.; Chiou, W.F. Ugonin K-stimulated osteogenesis involves estrogen receptor-dependent activation of non-classical Src signaling pathway and classical pathway. Eur. J. Pharmacol. 2012, 676, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.Y.; Liaw, C.C.; Huang, Y.C.; Han, H.Y.; Hsu, H.W.; Hwang, S.M.; Kuo, S.C.; Shen, C.N. Cyclohexylmethyl flavonoids suppress propagation of breast cancer stem cells via downregulation of NANOG. Evid. Based Complement. Alternat. Med. 2013, 2013, 170261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H. Synthesis and Anticancer Activity of Ugonstilbenes A, B and C. Master’s Thesis, Chinese Culture University, Taipei, Taiwan, 2014. [Google Scholar]
- Chen, C.Y.; Liaw, C.C.; Chen, Y.H.; Chang, W.Y.; Chung, P.J.; Hwang, T.L. A novel immunomodulatory effect of ugonin U in human neutrophils via stimulation of phospholipase C. Free Radic. Biol. Med. 2014, 72, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.T. The Inhibitory Mechanisms of Ugonin J on Balloon Injury-Induced Neointima Formation in the Rat Carotid Artery. Master’s Thesis, China Medical University, Taichung, Taiwan, 2012. [Google Scholar]
- Wu, K.C.; Kao, C.P.; Ho, Y.L.; Chang, Y.S. Quality control of the root and rhizome of Helminthostachys zeylanica (Daodi-Ugon) by HPLC using quercetin and ugonins as markers. Molecules 2017, 22, 1115. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.P.; Huang, S.S.; Matsuda, Y.; Saito, H.; Uramaru, N.; Ho, H.Y.; Wu, J.B.; Huang, G.J. Protective effects of tormentic acid, a major component of suspension cultures of Eriobotrya japonica cells, on acetaminophen-induced hepatotoxicity in mice. Molecules 2017, 22, 830. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.S.; Silva-Filho, S.E.; Aguiar, R.P.; Wiirzler, L.A.M.; Cardia, G.F.E.; Cavalcante, H.A.O.; Silva-Comar, F.M.S.; Becker, T.C.A.; Silva, E.L.; Bersani-Amado, C.A.; et al. Protective effect of Cymbopogon citratus essential oil in experimental model of acetaminophen-induced liver injury. Am. J. Chin. Med. 2017, 45, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Feng, Y.; Cui, R.; Qiu, M.; Zhang, J.; Liu, C. Simvastatin protects against acetaminophen-induced liver injury in mice. Biomed. Pharmacother. 2018, 98, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Zentella, M.L.; Hernández-Muñoz, R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid. Med. Cell. Longev. 2016, 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Casas-Grajales, S.; Muriel, P. Antioxidants in liver health. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J. Hepatol. 2009, 1, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.J.; Chou, S.C.; Chiu, C.S.; Kao, C.P.; Wu, K.C.; Chen, C.J.; Tsai, J.C.; Peng, W.H. Hepatoprotective effect of the ethanol extract of Polygonum orientale on carbon tetrachloride-induced acute liver injury in mice. J. Food Drug Anal. 2018, 26, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Dutra, F.F.; Bozza, M.T. Heme on innate immunity and inflammation. Front. Pharmacol. 2014, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, K.M.; Park, J.; Kwak, J.H.; Kim, Y.S.; Lee, S.M. Geniposidic acid protects against d-galactosamine and lipopolysaccharide-induced hepatic failure in mice. J. Ethnopharmacol. 2013, 146, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen-induced liver necrosis. In Adverse Drug Reactions; Uetrecht, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 369–405. [Google Scholar]
- Reilly, T.P.; Brady, J.N.; Marchick, M.R.; Bourdi, M.; George, J.W.; Radonovich, M.F.; Pise-Masison, C.A.; Pohl, L.R. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem. Res. Toxicol. 2001, 14, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Day, Y.J.; Lee, H.C.; Liou, J.T.; Chou, A.H.; Liu, F.C. ERK signaling pathway plays a key role in baicalin protection against acetaminophen-induced liver injury. Am. J. Chin. Med. 2017, 45, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, Q.; Xu, Y.; Chen, Y.; Deng, Y.; Zhi, F.; Qian, K. Attenuating oxidative stress by paeonol protected against acetaminophen-induced hepatotoxicity in mice. PLoS ONE 2016, 11, e0154375. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Lian, L.H.; Jiang, Y.Z.; Wu, Y.L.; Nan, J.X. Gentiana manshurica Kitagawa prevents acetaminophen-induced acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway. World J. Gastroenterol. 2010, 16, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Jiang, P.; Lu, B.; Sheng, Y.; Wang, X.; Wang, Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013, 24, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Lee, C.Y.; Hou, W.C.; Wang, S.Y.; Sung, P.J.; Kuo, Y.H. Hepatoprotective effects of eburicoic acid and dehydroeburicoic acid from Antrodia camphorata in a mouse model of acute hepatic injury. Food Chem. 2013, 141, 3020–3027. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-C.; Ho, Y.-L.; Kuo, Y.-H.; Huang, S.-S.; Huang, G.-J.; Chang, Y.-S. Hepatoprotective Effect of Ugonin M, A Helminthostachys zeylanica Constituent, on Acetaminophen-Induced Acute Liver Injury in Mice. Molecules 2018, 23, 2420. https://doi.org/10.3390/molecules23102420
Wu K-C, Ho Y-L, Kuo Y-H, Huang S-S, Huang G-J, Chang Y-S. Hepatoprotective Effect of Ugonin M, A Helminthostachys zeylanica Constituent, on Acetaminophen-Induced Acute Liver Injury in Mice. Molecules. 2018; 23(10):2420. https://doi.org/10.3390/molecules23102420
Chicago/Turabian StyleWu, Kun-Chang, Yu-Ling Ho, Yueh-Hsiung Kuo, Shyh-Shyun Huang, Guan-Jhong Huang, and Yuan-Shiun Chang. 2018. "Hepatoprotective Effect of Ugonin M, A Helminthostachys zeylanica Constituent, on Acetaminophen-Induced Acute Liver Injury in Mice" Molecules 23, no. 10: 2420. https://doi.org/10.3390/molecules23102420
APA StyleWu, K.-C., Ho, Y.-L., Kuo, Y.-H., Huang, S.-S., Huang, G.-J., & Chang, Y.-S. (2018). Hepatoprotective Effect of Ugonin M, A Helminthostachys zeylanica Constituent, on Acetaminophen-Induced Acute Liver Injury in Mice. Molecules, 23(10), 2420. https://doi.org/10.3390/molecules23102420