Abstract
Transition-metal-catalyzed amide-bond formation from alcohols and amines is an atom-economic and eco-friendly route. Herein, we identified a highly active in situ N-heterocyclic carbene (NHC)/ruthenium (Ru) catalytic system for this amide synthesis. Various substrates, including sterically hindered ones, could be directly transformed into the corresponding amides with the catalyst loading as low as 0.25 mol.%. In this system, we replaced the p-cymene ligand of the Ru source with a relatively labile cyclooctadiene (cod) ligand so as to more efficiently obtain the corresponding poly-carbene Ru species. Expectedly, the weaker cod ligand could be more easily substituted with multiple mono-NHC ligands. Further high-resolution mass spectrometry (HRMS) analyses revealed that two tetra-carbene complexes were probably generated from the in situ catalytic system.
1. Introduction
Amides are a series of fundamental functional structures in nature and biological systems, as well as crucial building blocks for organic synthesis [1,2,3,4,5,6]. As of late, numerous synthetic methods were reported for the construction of amide bonds. However, they generally suffer from the usage of various stoichiometric additives and the production of unfavorable equimolar byproducts [7,8,9,10,11,12,13,14]. Therefore, green and eco-friendly strategies are highly required for amide synthesis [15]. Recently, a methodology employing transition-metal-based catalytic systems for direct amide synthesis from alcohols and amines was proven to be far more atom-economic and environmentally friendly as the only byproduct is hydrogen [16,17,18,19,20,21,22]. Throughout this research, ruthenium (Ru) was most extensively studied [23]. Initially, the Murahashi [24] and Milstein [25] groups pioneered Ru-catalyzed amide synthesis in intramolecular and intermolecular manners, respectively. Notably, the Milstein catalyst, a Ru complex bearing a PNN-type pincer ligand, was highly active for this reaction. With a catalyst loading of 0.1 mol.%, various amides could be synthesized from alcohols and amines [25]. Later, great progress was achieved by the Milstein [26,27,28], Madsen [29,30,31], Williams [32,33], Hong [34,35,36,37,38,39,40,41,42,43], Crabtree [44,45], Albrecht [46], Guan [47,48], Glorius [49], Möller [50,51], Bera [52], Huynh [53], Viswanathamurthi [54,55,56], Mashima [57], Verpoort [58,59], and Kundu [60] groups. In particular, Ru combined with N-heterocyclic carbenes (NHCs) attracted more and more interest due to the flexible tunability of the electronic and steric properties of NHCs, which may easily access the optimal structures of the corresponding NHC/Ru complexes [61,62,63]. Accordingly, a multitude of efficient NHC/Ru catalytic systems were discovered for this reaction. Furthermore, considering the merits of the in situ catalytic systems, such as easy operation and convenient investigation of electronically and sterically distinct NHCs, a number of versatile and potent in situ NHC/Ru catalytic systems recently emerged. However, satisfactory yields could only be attained by these reported systems if relatively high Ru loadings of 2.0–5.0 mol.% were employed [29,34,36,37,49]. Therefore, the development of more efficient in situ NHC/Ru catalytic systems which can accomplish the formation of amide linkage are urgently required.
In our previous work, the development of various in situ generated (p-cymene)/Ru catalytic systems, which contain benzimidazole-based NHC precursors bearing different electronic and steric properties, was accomplished [58]. Further experiments revealed that two mono-NHC/Ru complexes were observed as major species and two poly-carbene complexes were detected as only minor species (as depicted in Figure 1a) [59]. Herein, we envisioned that replacing the p-cymene ligand of the Ru center with a relatively labile cyclooctadiene (cod) ligand could possibly give rise to poly-carbene complexes as a major species (as shown in Figure 1b). Expectedly, the weaker cod ligand could be more easily substituted with multiple mono-NHC ligands. Based on this, an efficient in situ NHC/Ru catalytic system was developed through extensive screening of various conditions. Notably, this system demonstrated excellent catalytic activity for amide synthesis with the applied catalyst loading as low as 0.25 mol.%. Various amides, including sterically congested ones, were directly synthesized from alcohols and amines in moderate to excellent yields. Furthermore, high-resolution mass spectrometry (HRMS) analyses suggested several Ru species bearing multiple NHC ligands as major species, which was in accordance with our prospection.
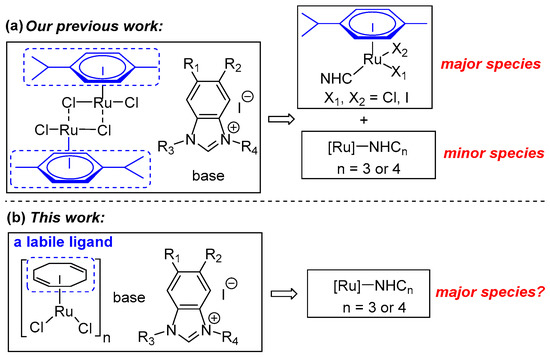
Figure 1.
The design strategy of this work.
2. Results and Discussion
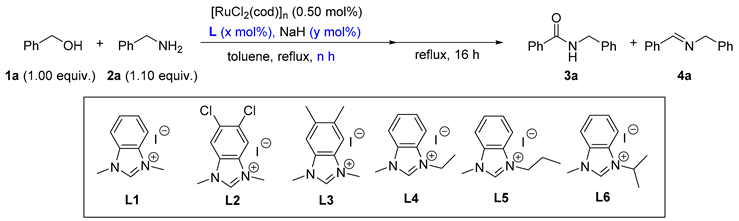
The reaction of benzyl alcohol (1a) and benzylamine (2a) was selected as a model reaction for the optimization of the reaction conditions. Based on our previous work [59], 0.5 mol.% of [RuCl2(cod)]n, 2.00 mol.% of an NHC precursor, 3.50 mol.% of NaH, 0.5 h of catalyst generation time, and 16 h of reaction time were originally applied (as listed in Table 1). In the beginning, NHC precursors L1–L6 with different backbone and wingtip substituents were prepared (entries 1–6, Table 1). The first and foremost, 62% of amide 3a and 15% of imine 4a, were obtained with 18% of 1a remaining if L1 was used (entry 1). Electron-deficient precursor L2 gave rise to lower amide content in the product distribution, demonstrating its disadvantage for amide formation (entry 2 vs. entry 1). In the case of an electron-rich NHC precursor (L3), a similar result was obtained compared with L1 (entry 3 vs. entry 1). Moreover, the substituents on the N-terminus of the NHC precursors were adjusted (entries 1, 4–6). With retaining Me as the substituent for one N-terminus, different groups including Et, nPr, and iPr were introduced for the other terminus. The result was indicative that Et was the optimized group for this reaction (entry 4 vs. entries 1, 5, and 6). After establishing the ideal NHC precursor (L4), we continued the optimization by screening other reaction conditions. It was found that the catalyst generation time was crucial for the catalysis (entries 4, 7–11); 57% of the amide product could be detected if every substance was added simultaneously (entry 5). As we elongated the period for the in situ catalyst generation from 0 h to 2.0 h, the yields of 3a gradually increased (entries 4, 7–10). A further increment of the time led to a similar yield (entry 11 vs. entry 10). Therefore, the ideal duration for the catalyst generation was finalized as 2 h. Next, the ratio of [Ru]: L4:NaH was varied (entries 12–17). It is worth emphasizing that the amount of both L4 and NaH changed so as to ascertain three additional equivalents of NaH to activate [RuCl2(cod)]n for all cases. Without L4, no amide was formed (entry 12). As the ratio increased from 1:0:3 to 1:5:8, gradually higher yields of 3a were observed (entries 10, 12–16). However, a higher ratio prompted a reduced yield of 3a (entry 17 vs. entry 16). Thus, the ratio of 1:5:8 was recognized as the best one (entry 16), and further increasing the reaction time from 16 h to 36 h produced 3a in 93% yield (entry 18).

Table 1.
Optimization of reaction conditions with a catalyst loading of 0.5 mol.% a.
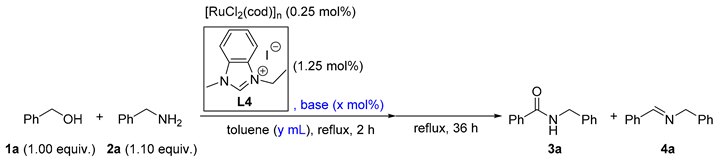
In order to identify a more active catalytic system, a reduced Ru loading of 0.25 mol.% was attempted (as listed in Table 2). At the outset, 65% of 3a was afforded if the loading of the above-optimized catalytic system was directly reduced to 0.25 mol.% (entry 1). In addition, different bases including potassium bis(trimethylsilyl)amide (KHMDS), KOtBu, and Cs2CO3 were exploited instead of NaH (entries 2–4). Interestingly, compared with NaH, the milder Cs2CO3 led to an increased yield of 3a (entry 4 vs. entry 1). It was also noticed that the volume of toluene was crucial for the reaction (entries 4–8). Either a more concentrated or diluted solution triggered a lower amide/imine selectivity (entry 5–8 vs. entry 4). Furthermore, the adjustment of the base amounts influenced the reaction (entries 4, 9–12), and 1.75 mol.% of Cs2CO3 was found to be optimal for the selective amide formation (entry 10). Therefore, the optimized reaction conditions were identified as 1 (5.00 mmol), 2 (5.50 mmol), [RuCl2(cod)]n (0.0125 mmol), L4 (0.0625 mmol), Cs2CO3 (0.075 mmol), toluene (1.50 mL), reflux, and 36 h unless otherwise noted.

Table 2.
Optimization of reaction conditions with a catalyst loading of 0.25 mol.% a.
With the optimized reaction conditions at hand, the substrate scope and limitations of this strategy were further investigated (as depicted in Figure 2). For the sterically non-hindered substrates (1a–1e), the corresponding amides could be obtained in good to excellent yields. If a secondary amine (1f) was employed, tertiary amide 3f was also given in 80% yield with 0.5 mol.% of [Ru]. Expectedly, lactam 3g was efficiently afforded from amino alcohol 1f in an intramolecular pattern. On the other hand, the reactions of benzyl alcohol with substituted benzylamines were evaluated. It seemed that these substituents had no obvious influence on the reactivity, and amides 3h–3k were synthesized in 75–85% yields. In the case of coupling benzylamine with various benzyl alcohols, a substituent at either the para or meta position resulted in good yields of amides 3l–3n. However, an ortho group gave amide 3o in a moderate yield. Apparently, aromatic amines were less reactive, and aniline (2p) produced amide 3p in only 25% yield. To our delight, this newly developed catalytic system was not as sensitive to steric bulks as our previous systems [58,59]. With an Ru loading of 0.5 mol.%, several sterically hindered substrates could be efficiently transformed into amides 3q–3t.
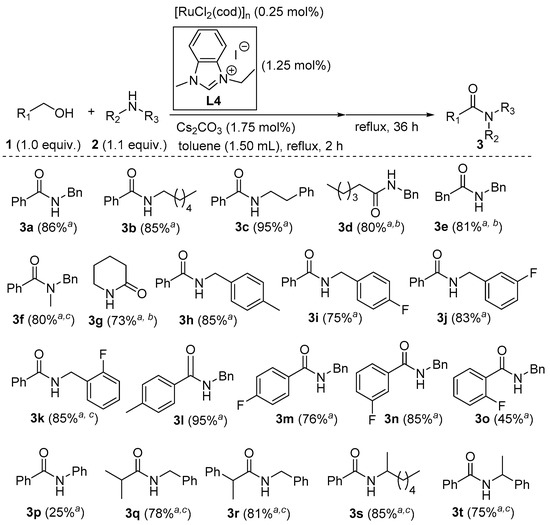
Figure 2.
Amide synthesis from various alcohols and amines; a isolated yields (averages of two consistent runs); b in m-xylene at reflux; c 0.5 mol.% of [Ru].
Concerning the in situ catalytic systems, it is crucial to explore the possible structures of the generated Ru species. As a result, HRMS analyses were performed to clarify this matter (as shown in Figure 3). In accordance with our speculation, no mono-carbene complexes were detected. Instead, two poly-carbene Ru species were observed from the spectrum. [Ru]-1 (corresponding to an isotopic peak at m/z = 812.24209), consistent with an Ru species comprising four-fold NHC ligands, was observed as a major species. Furthermore, another tetra-carbene Ru species, assigned as [Ru]-2 with the isotopic peak at m/z = 793.26709, was also found as a minor species. Presumably, during exposure to air and/or the HRMS measurements, the Ru centers in [Ru]-1 and [Ru]-2 were oxidized to +3 and +4, respectively. Unfortunately, attempts to isolate these tetra-carbene complexes were unsuccessful, probably due to the complexity of the in situ catalyst generation. Therefore, it was still unclear whether the high activity of the current catalytic system was attributed to the observed tetra-carbene Ru species or other species.
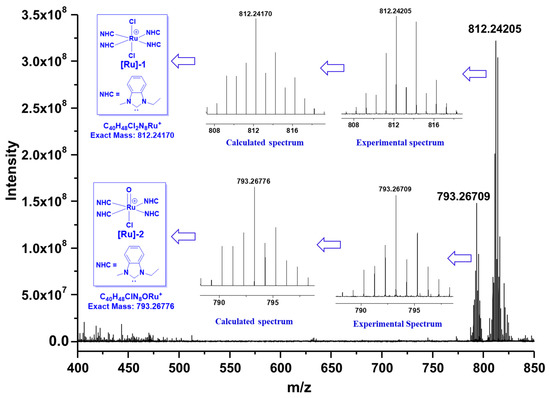
Figure 3.
The high-resolution mass spectrometry (HRMS) analyses for the identification of the possible Ru species.
3. Experimental
3.1. General Considerations
All reactions were carried out using standard Schlenk techniques or in an argon-filled glove box unless otherwise mentioned. All the substrates and solvents were obtained from commercial suppliers and used as received without further purification. 1H-NMR spectra were recorded on a Bruker Avance 500 spectrometer (Billerica, MA, USA) in CDCl3 or DMSO-d6 with TMS as the internal reference, and 13C-NMR spectra were recorded in CDCl3 or DMSO-d6 on a Bruker Avance 500 (126 MHz) spectrometer. The following abbreviations were used to designate multiplicities: s = singlet, brs = broad singlet, d = doublet, t = triplet, dd = doublet of doublets, dq = doublet of quartets, td = triplet of doublets, ddd = doublet of doublets of doublets, and m = multiplet. Melting points were taken on a Buchi M-560 melting point apparatus (Flawil, Switzerland) and were uncorrected. HRMS analyses were done with a Bruker Daltonics microTOF-QII instrument (Billerica, MA, USA). NHC precursors L1–L6 were prepared according to a previous publication [58,59], and all the amide products were identified by spectral comparison with the literature data [58,59]. 1H-NMR, 13C-NMR data and original spectra of amides 3a–3t could be found in the Supplementary Materials.
3.2. General Procedure for the Amide Synthesis
Inside an argon-filled glove box, [Ru(cod)Cl2]n (3.5 mg, 0.0125 mmol), L4 (18.0 mg, 0.0625 mmol), Cs2CO3 (28.6 mg, 0.0875 mmol), and dry toluene (1.50 mL) were added to an oven-dried 25-mL Schlenk flask. The tube was taken out of the glove box and heated to reflux under argon for 2 h. Then, an alcohol (5.00 mmol) and an amine (5.50 mmol) were added, and the mixture was stirred at a refluxing temperature for 36 h. The procedures for calculating the NMR yields were as follows: when the reaction was complete, 1,3,5-trimethoxybenzene (0.5 mmol, 84.0 mg) and CHCl3 (1.0 mL) were added to the reaction mixture. Afterward, to an NMR tube was added 0.1 mL of the above solution and 0.4 mL of CDCl3. The NMR yields were obtained based on the exact amount of 1,3,5-trimethoxybenzene. In order to obtain the isolated yields of the amides, the reaction mixture was cooled down to room temperature, and the solvent was removed under reduced pressure. Finally, the residue was purified by silica-gel flash column chromatography to afford the amides.
4. Conclusions
In summary, based on the assumption that the relatively labile cod ligand could be replaced by multiple NHC ligands to obtain versatile and active catalytic systems, we prepared several NHC precursors with distinct electronic and steric properties, then combined them with [RuCl2(cod)]n and a mild Cs2CO3 to obtain a series of in situ NHC/Ru catalytic systems. Through extensive screening of these systems and other conditions, the L4-based NHC/Ru catalytic system exhibited optimal activity for the dehydrogenative amidation of alcohols and amines. Various amides, especially sterically hindered ones, could be afforded in an efficient manner. Notably, the applied catalyst loading was as low as 0.25 mol.%. Further experiments revealed that the higher amount of L4 compared to Ru probably facilitated the formation of two tetra-carbene species ([Ru]-1 and [Ru]-2), which were observed from HRMS analyses. However, since the in situ catalytic system was relatively complicated, it is still uncertain whether these tetra-carbene Ru species or other species were key catalytic intermediates for this reaction.
Supplementary Materials
Supplementary materials, which contain 1H-NMR and 13C-NMR data, as well as spectra of amides 3a–3t, are available online.
Author Contributions
C.C., Y.Y and F.V. discussed and designed the whole project together. C.C., Y.M., K.D.W., and H.W. performed the experiments. C.C. and Y.M. wrote the manuscript. Y.Y., F.V. and P.D. revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 21502062). F.V. acknowledges the support from the Russian Foundation for Basic Research (No. 18-29-04047) and the Tomsk Polytechnic University Competitiveness Enhancement Program grant (VIU-195/2018).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Humphrey, J.M.; Chamberlin, A.R. Chemical synthesis of natural product peptides: Coupling methods for the incorporation of noncoded amino acids into peptides. Chem. Rev. 1997, 97, 2243–2266. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.W. Emerging methods in amide- and peptide-bond formation. Curr. Opin. Drug Discov. Dev. 2006, 9, 765–775. [Google Scholar] [CrossRef]
- Cupido, T.; Tulla-Puche, J.; Spengler, J.; Albericio, F. The synthesis of naturally occurring peptides and their analogs. Curr. Opin. Drug Discov. Dev. 2007, 10, 768–783. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Punniyamurthy, T. Palladium-catalyzed one-pot conversion of aldehydes to amides. Adv. Synth. Catal. 2010, 352, 288–292. [Google Scholar] [CrossRef]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kim, Y.A. Recent development of peptide coupling reagents in organic synthesis. Tetrahedron 2004, 60, 2447–2467. [Google Scholar] [CrossRef]
- Kohn, M.; Breinbauer, R. The Staudinger ligation-A gift to chemical biology. Angew. Chem. Int. Ed. 2004, 43, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Kolakowski, R.V.; Shangguan, N.; Sauers, R.R.; Williams, L.J. Mechanism of thio acid/azide amidation. J. Am. Chem. Soc. 2006, 128, 5695–5702. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Murphy, J.A. Azide rearrangements in electron-deficient systems. Chem. Soc. Rev. 2006, 35, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, J.R.; Clark, T.P.; Watson, D.A.; Munday, R.H.; Buchwald, S.L. Palladium-catalyzed aminocarbonylation of aryl chlorides at atmospheric pressure: The dual role of sodium phenoxide. Angew. Chem. Int. Ed. 2007, 46, 8460–8463. [Google Scholar] [CrossRef] [PubMed]
- Owston, N.A.; Parker, A.J.; Williams, J.M.J. Iridium-catalyzed conversion of alcohols into amides via oximes. Org. Lett. 2007, 9, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.W.; Chan, P.W.H. Highly efficient ruthenium (II) porphyrin catalyzed amidation of aldehydes. Angew. Chem. Int. Ed. 2008, 47, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Constable, D.J.C.; Dunn, P.J.; Hayler, J.D.; Humphrey, G.R.; Leazer, J.L., Jr.; Linderman, R.J.; Lorenz, K.; Manley, J.; Pearlman, B.A.; Wells, A.; et al. Key green chemistry research areas-a perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411–420. [Google Scholar] [CrossRef]
- Allen, C.L.; Williams, J.M.J. Metal-catalysed approaches to amide bond formation. Chem. Soc. Rev. 2011, 40, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hong, S.H. Oxidative amide synthesis directly from alcohols with amines. Org. Biomol. Chem. 2011, 9, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Applications of acceptorless dehydrogenation and related transformations in chemical synthesis. Science 2013. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Bond activation and catalysis by ruthenium pincer complexes. Chem. Rev. 2014, 114, 12024–12087. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, R.M.; Suppo, J.S.; Campagne, J.M. Nonclassical routes for amide bond formation. Chem. Rev. 2016, 116, 12029–12122. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Q.; Fan, G.M.; Zhu, R.J.; Shi, L.; Xiao, S.Y.; Bi, C. Highly efficient synthesis of amides. Prog. Chem. 2016, 28, 497–506. [Google Scholar]
- Gusey, D.G. Rethinking the dehydrogenative amide synthesis. ACS Catal. 2017, 7, 6656–6662. [Google Scholar]
- Chen, C.; Verpoort, F.; Wu, Q.Y. Atom-economic dehydrogenative amide synthesis via ruthenium catalysis. RSC Adv. 2016, 6, 55599–55607. [Google Scholar] [CrossRef]
- Naota, T.; Murahashi, S.I. Ruthenium-catalyzed transformations of amino-alcohols to lactams. Synlett 1991, 10, 693–694. [Google Scholar] [CrossRef]
- Gunanathan, C.; Ben-David, Y.; Milstein, D. Direct synthesis of amides from alcohols and amines with liberation of H-2. Science 2007, 317, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, B.; Balaraman, E.; Ben-David, Y.; Milstein, D. Synthesis of peptides and pyrazines from β-Amino alcohols through extrusion of H2 catalyzed by ruthenium pincer complexes: Ligand-controlled selectivity. Angew. Chem. Int. Ed. 2011, 50, 12240–12244. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, B.; Balaraman, E.; Gunanathan, C.; Milstein, D. Synthesis of polyamides from diols and diamines with liberation of H2. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1755–1765. [Google Scholar] [CrossRef]
- Srimani, D.; Balaraman, E.; Hu, P.; Ben-David, Y.; Milstein, D. Formation of tertiary amides and dihydrogen by dehydrogenative coupling of primary alcohols with secondary amines catalyzed by ruthenium bipyridine-based pincer complexes. Adv. Synth. Catal. 2013, 355, 2525–2530. [Google Scholar] [CrossRef]
- Nordstrøm, L.U.; Vogt, H.; Madsen, R. Amide synthesis from alcohols and amines by the extrusion of dihydrogen. J. Am. Chem. Soc. 2008, 130, 17672–17673. [Google Scholar] [CrossRef] [PubMed]
- Dam, J.H.; Osztrovszky, G.; Nordstrøm, L.U.; Madsen, R. Amide synthesis from alcohols and amines catalyzed by ruthenium N-heterocyclic carbene complexes. Chem. Eur. J. 2010, 16, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Makarov, I.S.; Fristrup, P.; Madsen, R. Mechanistic investigation of the ruthenium-N-heterocyclic-carbene-catalyzed amidation of amines with alcohols. Chem. Eur. J. 2012, 18, 15683–15692. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Maxwell, A.C.; Williams, J.M.J. Ruthenium-catalyzed oxidation of alcohols into amides. Org. Lett. 2009, 11, 2667–2670. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Wakeham, R.J.; Maxwell, A.C.; Williams, J.M.J. Ruthenium-catalysed oxidation of alcohols to amides using a hydrogen acceptor. Tetrahedron 2014, 70, 3683–3690. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Muthaiah, S.; Zhang, Y.; Xu, X.Y.; Hong, S.H. Direct amide synthesis from alcohols and amines by phosphine-free ruthenium catalyst systems. Adv. Synth. Catal. 2009, 351, 2643–2649. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Ghosh, S.C.; Li, Y.X.; Hong, S.H. Well-defined N-heterocyclic carbene based ruthenium catalysts for direct amide synthesis from alcohols and amines. Organometallics 2010, 29, 1374–1378. [Google Scholar] [CrossRef]
- Muthaiah, S.; Ghosh, S.C.; Jee, J.E.; Chen, C.; Zhang, J.; Hong, S.H. Direct amide synthesis from either alcohols or aldehydes with amines: Activity of Ru (II) hydride and Ru (0) complexes. J. Org. Chem. 2010, 75, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.C.; Hong, S.H. Simple RuCl3-catalyzed amide synthesis from alcohols and amines. Eur. J. Org. Chem. 2010, 4266–4270. [Google Scholar] [CrossRef]
- Zhang, J.; Senthilkumar, M.; Ghosh, S.C.; Hong, S.H. Synthesis of cyclic imides from simple diols. Angew. Chem. Int. Ed. 2010, 49, 6391–6395. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Hong, S.H. N-heterocyclic carbene based ruthenium-catalyzed direct amide synthesis from alcohols and secondary amines: Involvement of esters. J. Org. Chem. 2011, 76, 10005–10010. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hong, S.H. Selective catalytic sp3 C-O bond cleavage with C-N bond formation in 3-alkoxy-1-propanols. Org. Lett. 2012, 14, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kang, B.; Hong, S.H. N-Heterocyclic carbene-based well-defined ruthenium hydride complexes for direct amide synthesis from alcohols and amines under base-free conditions. Tetrahedron 2015, 71, 4565–4569. [Google Scholar] [CrossRef]
- Kang, B.; Hong, S.H. Hydrogen acceptor- and base-free N-formylation of nitriles and amines using methanol as C-1 Source. Adv. Synth. Catal. 2015, 357, 834–840. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, S.H. Ruthenium-catalyzed urea synthesis using methanol as the C1 source. Org. Lett. 2016, 18, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Nova, A.; Balcells, D.; Schley, N.D.; Dobereiner, G.E.; Crabtree, R.H.; Eisenstein, O. An experimental-theoretical study of the factors that affect the switch between ruthenium-catalyzed dehydrogenative amide formation versus amine alkylation. Organometallics 2010, 29, 6548–6558. [Google Scholar] [CrossRef]
- Schley, N.D.; Dobereiner, G.E.; Crabtree, R.H. Oxidative synthesis of amides and pyrroles via dehydrogenative alcohol oxidation by ruthenium diphosphine diamine complexes. Organometallics 2011, 30, 4174–4179. [Google Scholar] [CrossRef]
- Prades, A.; Peris, E.; Albrecht, M. Oxidations and oxidative couplings catalyzed by triazolylidene ruthenium complexes. Organometallics 2011, 30, 1162–1167. [Google Scholar] [CrossRef]
- Zeng, H.; Guan, Z. Direct synthesis of polyamides via catalytic dehydrogenation of diols and diamines. J. Am. Chem. Soc. 2011, 133, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Oldenhuis, N.J.; Dong, V.M.; Guan, Z. Catalytic acceptorless dehydrogenations: Ru-Macho catalyzed construction of amides and imines. Tetrahedron 2014, 70, 4213–4218. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Richter, C.; Glorius, F. N-formylation of amines by methanol activation. Org. Lett. 2013, 15, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Malineni, J.; Merkens, C.; Keul, H.; Möller, M. An efficient N-heterocyclic carbene based ruthenium-catalyst: Application towards the synthesis of esters and amides. Catal. Commun. 2013, 40, 80–83. [Google Scholar] [CrossRef]
- Malineni, J.; Keul, H.; Möller, M. An efficient N-heterocyclic carbene-ruthenium complex: Application towards the synthesis of polyesters and polyamides. Macromol. Rapid Commun. 2015, 36, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Sengupta, G.; Sarbajna, A.; Dutta, I.; Bera, J.K. Amide synthesis from alcohols and amines catalyzed by a Ru-II-N-heterocyclic carbene (NHC)-carbonyl complex. J. Organomet. Chem. 2014, 771, 124–130. [Google Scholar] [CrossRef]
- Xie, X.K.; Huynh, H.V. Tunable dehydrogenative amidation versus amination using a single ruthenium-NHC catalyst. ACS Catal. 2015, 5, 4143–4151. [Google Scholar] [CrossRef]
- Nirmala, M.; Viswanathamurthi, P. Design and synthesis of ruthenium (II) OCO pincer type NHC complexes and their catalytic role towards the synthesis of amides. J. Chem. Sci. 2016, 128, 9–21. [Google Scholar] [CrossRef]
- Selvamurugan, S.; Ramachandran, R.; Prakash, G.; Viswanathamurthi, P.; Malecki, J.G.; Endo, A. Ruthenium (II) carbonyl complexes containing bidentate 2-oxo-1,2-dihydroquinoline-3-carbaldehyde hydrazone ligands as efficient catalysts for catalytic amidation reaction. J. Organomet. Chem. 2016, 803, 119–127. [Google Scholar] [CrossRef]
- Selvamurugan, S.; Ramachandran, R.; Prakash, G.; Nirmala, M.; Viswanathamurthi, P.; Fujiwara, S.; Endo, A. Ruthenium (II) complexes encompassing 2-oxo-1,2-dihydroquinoline-3-carbaldehyde thiosemicarbazone hybrid ligand: A new versatile potential catalyst for dehydrogenative amide synthesis. Inorg. Chim. Acta 2017, 454, 46–53. [Google Scholar] [CrossRef]
- Higuchi, T.; Tagawa, R.; Iimuro, A.; Akiyama, S.; Nagae, H.; Mashima, K. Tunable ligand effects on ruthenium catalyst activity for selectively preparing imines or amides by dehydrogenative coupling reactions of alcohols and amines. Chem. Eur. J. 2017, 23, 12795–12804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, M.Q.; Cheng, C.X.; Wang, H.J.; Lu, Q.; Liu, H.F.; Yao, F.B.; Chen, C.; Verpoort, F. In situ generated ruthenium catalyst systems bearing diverse N-heterocyclic carbene precursors for atom-economic amide synthesis from alcohols and amines. Chem. Asian J. 2018, 13, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, M.Q.; Zhang, N.; Wang, H.J.; Miao, Y.; Su, W.; Yuan, Y.; Chen, C.; Verpoort, F. Efficient N-heterocyclic carbene/ruthenium catalytic systems for the alcohol amidation with amines: Involvement of poly-carbene complexes? ChemCatChem 2018. [Google Scholar] [CrossRef]
- Maji, M.; Chakrabarti, K.; Paul, B.; Roy, B.C.; Kundu, S. Ruthenium(II)-NNN-pincer-complex-catalyzed reactions between various alcohols and amines for sustainable C-N and C-C bond formation. Adv. Synth. Catal. 2018, 360, 722–729. [Google Scholar] [CrossRef]
- Huynh, H.V.; Han, Y.; Jothibasu, R.; Yang, J.A. 13C-NMR spectroscopic determination of ligand donor strengths using N-heterocyclic carbene complexes of palladium (II). Organometallics 2009, 28, 5395–5404. [Google Scholar] [CrossRef]
- Chen, C.; Kim, M.H.; Hong, S.H. N-heterocyclic carbene-based ruthenium-catalyzed direct amidation of aldehydes with amines. Org. Chem. Front. 2015, 2, 241–247. [Google Scholar] [CrossRef]
- Kaufhold, S.; Petermann, L.; Staehle, R.; Rau, S. Transition metal complexes with N-heterocyclic carbene ligands: From organometallic hydrogenation reactions toward water splitting. Coord. Chem. Rev. 2015, 304, 73–87. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 3a–3t are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).