Beneficial Effects of Monascus sp. KCCM 10093 Pigments and Derivatives: A Mini Review
Abstract
:1. Introduction
2. Antimicrobial and Antiviral Effects of Monascus Pigment Derivatives
3. Regulation of Cholesterol Synthesis and Anti-Obesity Effects
4. Conclusions and Research Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviation
| FDA | Food and Drug Administration |
References
- Burchi, F.; Fanzo, J.; Frison, E. The role of food and nutrition system approaches in tackling hidden hunger. Int. J. Environ. Res. Public Health 2011, 8, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Younesi, E.; Ayseli, M.T. An integrated systems-based model for substantiation of health claims in functional food development. Trends Food Sci. Technol. 2015, 41, 95–100. [Google Scholar] [CrossRef]
- Ku, S. Finding and Producing Probiotic Glycosylases for the Biocatalysis of Ginsenosides: A Mini Review. Molecules 2016, 21, 645. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.; Park, M.S.; Ji, G.E.; You, H.J. Review on bifidobacterium bifidum bgn4: Functionality and nutraceutical applications as a probiotic microorganism. Int. J. Mol. Sci. 2016, 17, 1544. [Google Scholar] [CrossRef] [PubMed]
- Functional Foods Market is Expected to Drive Growth due to Awareness towards Changing Lifestyle, Health and Proper Diet Till 2024: Grand View Research, Inc. Available online: http://www.abnewswire.com/pressreleases/functional-foods-market-is-expected-to-drive-growth-due-to-awareness-towards-changing-lifestyle-health-and-proper-diet-till-2024-grand-view-research-inc_133119.html (accessed on 26 September 2017).
- Functional Foods Market is Expected to Reach $255.10 Billion by 2024. Available online: http://www.grandviewresearch.com/press-release/global-functional-foods-market (accessed on 26 September 2017).
- Li, Y.; Ku, S.; Park, M.S.; Li, Z.; Ji, G.E. Acceleration of Aglycone isoflavones and γ-Aminobutyric Acid Production from Doenjang using Whole cell Biocatalysis Accompanied by Protease Treatment. J. Microbiol. Biotechnol. 2017, 27, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Carels, M.; Shepherd, D. The effect of different nitrogen sources on pigment production and sporulation of Monascus species in submerged, shaken culture. Can. J. Microbiol. 1977, 23, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Iizuka, H. Production of Extracellular Pigment by a Mutant of Monascus kaoliang sp. nov. Appl. Environ. Microbiol. 1982, 43, 671–676. [Google Scholar] [PubMed]
- Nout, M.J.R.; Aidoo, K.E. Asian Fungal Fermented Food. Ind. Appl. 2002, 10, 23–47. [Google Scholar]
- Shi, Y.C.; Pan, T.M. Beneficial effects of Monascus purpureus NTU 568-fermented products: A review. Appl. Microbiol. Biotechnol. 2011, 90, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, Y.; Zhou, Y.; Shao, Y.; Feng, Y.; Li, M.; Chen, F. Edible Filamentous Fungi from the Species Monascus: Early Traditional Fermentations, Modern Molecular Biology, and Future Genomics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 555–567. [Google Scholar] [CrossRef]
- Blanc, P.J.; Loret, M.O.; Goma, G.; Rangueil, C.S. De Production of citrinin by various species of Monascus. Biotechnol. Lett. 1995, 17, 291–294. [Google Scholar] [CrossRef]
- Sabater-Vilar, M.; Maas, R.F.M.; Fink-Gremmels, J. Mutagenicity of commercial Monascus fermentation products and the role of citrinin contamination. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 444, 7–16. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Bühler, R.M.M.; De Carvalho, J.C.; De Oliveira, D.; Moritz, D.E.; Schmidell, W.; Ninow, J.L. Monascus: A Reality on the production and application of microbial pigments. Appl. Biochem. Biotechnol. 2016, 178, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Yongsmith, B.; Kitprechavanich, V.; Chitradon, L.; Chaisrisook, C.; Budda, N. Color mutants of Monascus sp. KB9 and their comparative glucoamylases on rice solid culture. J. Mol. Catal. B Enzym. 2000, 10, 263–272. [Google Scholar] [CrossRef]
- Yongsmith, B.; Krairak, S.; Bavavoda, R. Production of yellow pigments in submerged culture of a mutant of Monascus spp. J. Ferment. Bioeng. 1994, 78, 223–228. [Google Scholar] [CrossRef]
- Jongrungruangchok, S.; Kittakoop, P.; Yongsmith, B.; Bavovada, R.; Tanasupawat, S.; Lartpornmatulee, N.; Thebtaranonth, Y. Azaphilone pigments from a yellow mutant of the fungus Monascus kaoliang. Phytochemistry 2004, 65, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, Red, Yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Jeun, J.; Jung, H.; Kim, J.H.; Kim, Y.O.; Youn, S.H.; Shin, C.S. Effect of the Monascus pigment threonine derivative on regulation of the cholesterol level in mice. Food Chem. 2008, 107, 1078–1085. [Google Scholar] [CrossRef]
- Hajjaj, H.; Blanc, P.; Groussac, E. Kinetic analysis of red pigment and citrinin production by Monascus ruber as a function of organic acid accumulation. Enzyme Microb. Technol. 2000, 27, 619–625. [Google Scholar] [CrossRef]
- Jung, H.; Kim, C.; Kim, K.; Shin, C.S. Color characteristics of Monascus pigments derived by fermentation with various amino acids. J. Agric. Food Chem. 2003, 51, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, C.; Shin, C.S. Enhanced photostability of Monascus pigments derived with various amino acids via fermentation. J. Agric. Food Chem. 2005, 53, 7108–7114. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.S.; Kim, H.J.; Kim, M.J.; Ju, J.Y. Morphological change and enhanced pigment production of Monascus when cocultured with Saccharomyces cerevisiae or Aspergillus oryzae. Biotechnol. Bioeng. 1998, 59, 576–581. [Google Scholar] [CrossRef]
- Ju, J.Y.; Kim, D.Y.; Suh, J.H.; Shin, C.S. Optimization of Monascus red pigment fermentation by regulating chitinase activity level in fermentor. Bioprocess. Eng. 1999, 21, 25–29. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Oh, H.J.; Shin, C.S. Morphology control of Monascus cells and scale-up of pigment fermentation. Process Biochem. 2002, 38, 649–655. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Oishi, B.O.; Pandey, A.; Soccol, C.R. Biopigments from Monascus: Strains selection, citrinin production and color stability. Braz. Arch. Biol. Technol. 2005, 48, 885–894. [Google Scholar] [CrossRef]
- Meinicke, R.M.; Vendruscolo, F.; Esteves Moritz, D.; de Oliveira, D.; Schmidell, W.; Samohyl, R.W.; Ninow, J.L. Potential use of glycerol as substrate for the production of red pigments by Monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2012, 1, 238–242. [Google Scholar] [CrossRef]
- Babitha, S.; Soccol, C.R.; Pandey, A. Jackfruit seed for production of Monascus pigments. Food Technol. Biotechnol. 2006, 44, 465–471. [Google Scholar]
- Babitha, S.; Soccol, C.R.; Pandey, A. Solid-state fermentation for the production of Monascus pigments from jackfruit seed. Bioresour. Technol. 2007, 98, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, L.; Goma, G. Estimation of bioprocess variables from Monascus ruber cultures by means of stoichiometric models. Process Biochem. 1995, 30, 607–613. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Rossi, M.J.; Schmidell, W.; Ninow, J.L. Determination of oxygen solubility in liquid media. ISRN Chem. Eng. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Chen, M.H.; Johns, M.R. Effect of pH and nitrogen source on pigment production by Monascus purpureus. Appl. Microbiol. Biotechnol. 1993, 40, 132–138. [Google Scholar] [CrossRef]
- Orozco, S.F.B.; Kilikian, B.V. Effect of pH on citrinin and red pigments production by Monascus purpureus CCT3802. World J. Microbiol. Biotechnol. 2008, 24, 263–268. [Google Scholar] [CrossRef]
- Ahn, J.; Jung, J.; Hyung, W.; Haam, S.; Shin, C. Enhancement of Monascus pigment production by the culture of Monascus sp. J101 at low temperature. Biotechnol. Prog. 2006, 22, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; Pitol, L.O.; Carciofi, B.A.M.; Moritz, D.E.; Laurindo, J.B.; Schmidell, W.; Ninow, J.L. Construction and application a vane system in a rotational rheometer for determination of the rheological properties of Monascus ruber CCT 3802. J. Biorheol. 2010, 24, 29–35. [Google Scholar] [CrossRef]
- Jung, H.; Choe, D.; Nam, K.Y.; Cho, K.H.; Shin, C.S. Degradation patterns and stability predictions of the original reds and amino acid derivatives of Monascus pigments. Eur. Food Res. Technol. 2011, 232, 621–629. [Google Scholar] [CrossRef]
- Krairak, S.; Yamamura, K.; Irie, R.; Nakajima, M.; Shimizu, H.; Chim-Anage, P.; Yongsmith, B.; Shioya, S. Maximizing yellow pigment production in fed-batch culture of Monascus sp. J. Biosci. Bioeng. 2000, 90, 363–367. [Google Scholar] [CrossRef]
- Carels, M.; Shepherd, D. Sexual reproductive cycle of Monascus in submerged shaken culture. J. Bacteriol. 1975, 122, 288–294. [Google Scholar] [PubMed]
- Ju, J.Y.; Shin, C.S.; Whitcombe, M.J.; Vulfson, E.N. Imprinted polymers as tools for the recovery of secondary metabolites produced by fermentation. Biotechnol. Bioeng. 1999, 64, 232–239. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Chen, D.-C.; Chauvatcharin, S.; Seki, T.; Yoshida, T. Monascus of pigments by a solid-liquid culture method. J. Ferment. Bioeng. 1995, 79, 516–518. [Google Scholar] [CrossRef]
- Lin, T.F.; Yakushijin, K.; Büchi, G.H.; Demain, A.L. Formation of water-soluble Monascus red pigments by biological and semi-synthetic processes. J. Ind. Microbiol. 1992, 9, 173–179. [Google Scholar] [CrossRef]
- Stchigel, A.M.; Cano, J.F.; Abdullah, S.K.; Guarro, J. New and interesting species of Monascus from soil, with a key to the known species. Stud. Mycol. 2004, 50, 299–306. [Google Scholar]
- Hawksworth, D.L.; Pitt, J.I. A new taxonomy for Monascus species based on cultural and microscopical characters. Aust. J. Bot. 1983, 31, 51–61. [Google Scholar] [CrossRef]
- Hocking, A.D.; Pitt, J.I.; Mycologia, S.; Feb, N.J. Two new species of xerophilic fungi and a further record of eurotium halophilicum. Mycologia 2017, 80, 82–88. [Google Scholar] [CrossRef]
- Cannon, P.F.; Abdullah, S.K.; Abbas, B.A. Two new species of Monascus from Iraq, with a key to known species of the genus. Mycol. Res. 1995, 99, 659–662. [Google Scholar] [CrossRef]
- Lian, X.; Wang, C.; Guo, K. Identification of new red pigments produced by Monascus ruber. Dyes Pigments 2007, 73, 121–125. [Google Scholar] [CrossRef]
- Hamano, P.S.; Orozco, S.F.B.; Kilikian, B.V. Concentration determination of extracellular and intracellular red pigments produced by Monascus sp. Braz. Arch. Biol. Technol. 2005, 48, 43–49. [Google Scholar] [CrossRef]
- Dominguez-Espinosa, R.M.; Webb, C. Submerged fermentation in wheat substrates for production of Monascus pigments. World J. Microbiol. Biotechnol. 2003, 19, 329–336. [Google Scholar] [CrossRef]
- Lopes, F.C.; Tichota, D.M.; Pereira, J.Q.; Segalin, J.; De Oliveira Rios, A.; Brandelli, A. Pigment production by filamentous fungi on agro-industrial byproducts: An eco-friendly alternative. Appl. Biochem. Biotechnol. 2013, 171, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Zlova, P.J.I.; Ezanka, T.I.; Martfnkov, L.; Krent, V. Long-chain fatty acids from Monascus purpureus. Phytochemistry 1996, 43, 151–153. [Google Scholar]
- Jůlová, P.; Martínkková, L.; Lozinski, J.; Machek, F. Ethanol as substrate for pigment production by the fungus Monascus purpureus. Enzyme Microb. Technol. 1994, 16, 996–1001. [Google Scholar] [CrossRef]
- Park, H.G.; Jong, S.C. Molecular characterization of Monascus strains based on the D1/D2 regions of LSU rRNA genes. Mycoscience 2003, 44, 25–32. [Google Scholar] [CrossRef]
- New Dietary Ingredients in Dietary Supplements—Backgroud for Industry. Available online: https://www.fda.gov/Food/DietarySupplements/ucm109764.htm (accessed on 29 November 2017).
- Kuhbacher, T. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut 2006, 55, 833–841. [Google Scholar] [PubMed]
- Velmurugan, P.; Hur, H.; Balachandar, V.; Kamala-Kannan, S.; Lee, K.J.; Lee, S.M.; Chae, J.C.; Shea, P.J.; Oh, B.T. Monascus pigment production by solid-state fermentation with corn cob substrate. J. Biosci. Bioeng. 2011, 112, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.; Lee, J.; Woo, S.; Shin, C.S. Evaluation of the amine derivatives of Monascus pigment with anti-obesity activities. Food Chem. 2012, 134, 315–323. [Google Scholar] [CrossRef]
- Jang, H.; Choe, D.; Shin, C.S. Novel derivatives of Monascus pigment having a high CETP inhibitory activity. Nat. Prod. Res. 2014, 28, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Kim, S.J.; Kim, G.W.; Rhee, J.K.; Kim, N.D.; Jung, H.; Jeun, J.; Lee, S.H.; Han, S.H.; Shin, C.S.; et al. Inhibition of hepatitis C virus replication by Monascus pigment derivatives that interfere with viral RNA polymerase activity and the mevalonate biosynthesis pathway. J. Antimicrob. Chemother. 2012, 67, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.J.; Park, H.W.; Youn, S.H.; Choi, D.Y.; Shin, C.S. Development of inhibitors against lipase and α-glucosidase from derivatives of Monascus pigment. FEMS Microbiol. Lett. 2007, 276, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jung, H.; Kim, Y.O.; Shin, C.S. Antimicrobial activities of amino acid derivatives of Monascus pigments. FEMS Microbiol. Lett. 2006, 264, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jung, H.; Kim, J.H.; Shin, C.S. Effect of Monascus pigment derivatives on the electrophoretic mobility of bacteria, and the cell adsorption and antibacterial activities of pigments. Colloids Surf. B Biointerfaces 2006, 47, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.; Pandey, A.; Babitha, S.; Soccol, C.R. Production of Monascus biopigments: An overview. Agro Food Ind. Hi-Tech 2003, 14, 37–42. [Google Scholar]
- Fang, H.H.P.; Li, C.; Zhang, T. Acidophilic biohydrogen production from rice slurry. Int. J. Hydrogen Energy 2006, 31, 683–692. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Ninow, J.L. Apple pomace as a substrate for fungal chitosan production in an airlift bioreactor. Biocatal. Agric. Biotechnol. 2014, 3, 338–342. [Google Scholar] [CrossRef]
- Francis, F.J. Less common natural colorants. Nat. Food Color. 1996, 2, 310–342. [Google Scholar]
- Yongsmith, B.; Tabloka, W.; Yongmanitchai, W.; Bavavoda, R. Culture conditions for yellow pigment formation by Monascus sp. KB 10 grown on cassava medium. World J. Microbiol. Biotechnol. 1993, 9, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hintz, T.; Matthews, K.K.; Di, R. Review: The use of plant antimicrobial compounds for food preservation. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zink, D.L. The impact of consumer demands and trends on food processing. Emerg. Infect. Dis. 1997, 3, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Grunert, K.G. Future trends and consumer lifestyles with regard to meat consumption. Meat Sci. 2006, 74, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Jůzlová, P.; Martínková, L.; Křen, V. Secondary metabolites of the fungus Monascus: A review. J. Ind. Microbiol. 1996, 16, 163–170. [Google Scholar] [CrossRef]
- Wong, H.-C.; Koehler, P.E. Production and Isolation of an Antibiotic from Monascus purpureus and its Relationship to Pigment Production. J. Food Sci. 1981, 46, 589–592. [Google Scholar] [CrossRef]
- Xu, W. Study on the liquid fermentation to produce Monascus pigment with corn starch and antibacteria. Adv. Mater. Res. 2011, 183–185, 1336–1340. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Antimicrobial and antioxidant properties of pigments synthesized from microorganisms. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015; pp. 27–32. [Google Scholar]
- Fabre, C.E.; Santerre, A.L.; Baberian, R.; Pareilleux, A.; Goma, G.; Blanc, P.J. Production and Food Applications of the Red Pigments of Monascus ruber. J. Food Sci. 1993, 58, 1099–1102. [Google Scholar] [CrossRef]
- Erdoğrul, Ö.; Sebile, A. Review of the studies on the red yeast rice (Monascus purpureus). Turk. Electron. J. Biotechnol. 2004, 2, 37–49. [Google Scholar]
- Yu, X.; Wu, H.; Zhang, J. Effect of Monascus as a nitrite substitute on color, lipid oxidation, and proteolysis of fermented meat mince. Food Sci. Biotechnol. 2015, 24, 575–581. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martínková, L.; Jzlová, P.; Veselý, D. Biological activity of polyketide pigments produced by the fungus Monascus. J. Appl. Microbiol. 1995, 79, 609–616. [Google Scholar]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Martinkova, L.; Patakova-Juzlova, P.; Krent, V.; Kucerova, Z.; Havlicek, V.; Olsovsky, P.; Hovorka, O.; Rihova, B.; Vesely, D.; Vesela, D.; et al. Biological activities of oligoketide pigments of Monascus purpureus. Food Addit. Contam. 1999, 16, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S. Hepatitis C and liver transplantation. Nature 2005, 436, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; López-Labrador, F.X.; Wright, T.L. Hepatitis C and liver transplantation. J. Hepatol. 2001, 35, 666–678. [Google Scholar] [CrossRef]
- Appel, N.; Schaller, T.; Penin, F.; Bartenschlager, R. From structure to function: New insights into hepatitis C virus RNA replication. J. Biol. Chem. 2005, 281, 9833–9836. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart disease and stroke statistics-2010 update: A report from the american heart association. Circulation 2010, 121, 948–954. [Google Scholar] [PubMed]
- Kiernan, T.J.; Yan, B.P.; Jaff, M.R. Antiplatelet therapy for the primary and secondary prevention of cerebrovascular events in patients with extracranial carotid artery disease. J. Vasc. Surg. 2009, 50, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Chemistry, biochemistry, and pharmacology of HMG-CoA reductase inhibitors. Klin. Wochenschr. 1988, 66, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D., Jr.; Bangdiwala, S.; Tyroler, H.A. High-Density Lipoprotein Cholesterol and Cardiovascular Disease Four Prospective American Studies. Circulation 1988, 79, 8–15. [Google Scholar] [CrossRef]
- Boden, W.E. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: Assessing the data from framingham to the veterans affairs high-density lipoprotein intervention trial. Am. J. Cardiol. 2000, 86, 19–22. [Google Scholar] [CrossRef]
- Barter, P.J.; Brewer, H.B.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R. Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K. Inhibition of CETP as a novel therapeutic strategy for reducing the risk of atherosclerotic disease. Eur. Heart J. 2007, 28, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ranalletta, M.; Bierilo, K.K.; Chen, Y.; Milot, D.; Chen, Q.; Tung, E.; Houde, C.; Elowe, N.H.; Garcia-Calvo, M.; Porter, G.; et al. Biochemical characterization of cholesteryl ester transfer protein inhibitors. J. Lipid Res. 2010, 51, 2739–2752. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.H.; Akhlaghi, F. Future of cholesteryl ester transfer protein (CETP) inhibitors: A pharmacological perspective. Clin. Pharmacokinet. 2013, 52, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Degoma, E.M. Future of cholesteryl ester transfer protein inhibitors. Annu. Rev. Med. 2014, 65, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; West, K.L.; Fernandez, M.L. Grape polyphenols decrease plasma triglycerides and cholesterol accumulation in the aorta of ovariectomized guinea pigs. J. Nutr. 2003, 133, 2268–2272. [Google Scholar] [PubMed]
- Cheuk, K.L.; Zhang, Z.; Yu, H.; Tsang, S.Y.; Huang, Y.; Zhen, Y.C. Apple polyphenols inhibit plasma CETP activity and reduce the ratio of non-HDL to HDL cholesterol. Mol. Nutr. Food Res. 2008, 52, 950–958. [Google Scholar]
- Hirata, H.; Takazumi, K.; Segawa, S.; Okada, Y.; Kobayashi, N.; Shigyo, T.; Chiba, H. Xanthohumol, a prenylated chalcone from Humulus lupulus L.; Inhibits cholesteryl ester transfer protein. Food Chem. 2012, 134, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, H. Cholesteryl ester transfer-protein modulator and inhibitors and their potential for the treatment of cardiovascular diseases. Vasc. Health Risk Manag. 2012, 8, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Tint, G.S.; Irons, M.; Elias, E.R.; Batta, A.K.; Frieden, R.; Chen, T.S.; Salen, G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Engl. J. Med. 1994, 330, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, N.M.F.S.A.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Vidyarthi, A.S.; Bhunia, B.; Mandal, T. A review on lovastatin and its production. J. Biochem. Technol. 2012, 4, 581–587. [Google Scholar]
- Klimek, M.; Wang, S.; Ogunkanmi, A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. P T Peer Rev. J. Formul. Manag. 2009, 34, 313–327. [Google Scholar]
- Tobert, J.A. Case history: Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff. 2009, 28, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Trogdon, J.G.; Finkelstein, E.A.; Hylands, T.; Dellea, P.S.; Kamal-Bahl, S.J. Indirect costs of obesity: A review of the current literature. Obes. Rev. 2008, 9, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.W.; Harp, J.B. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 2001, 280, C807–C813. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Park, Y.; Choi, H.; Lee, E.H. Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet. Biofactors 2006, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Kim, C.-T.; Kim, Y. Green tea (–)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann. Nutr. Metab. 2009, 54, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.; Brasel, J. Toxicity, metabolism and impact of mycotoxins on human and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Comerio, R.; Fernandez Pinto, V.E.; Vaamonde, G. Influence of water activity on Penicillium citrinum growth and kinetics of citrinin accumulation in wheat. Int. J. Food Microbiol. 1998, 42, 219–223. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yres, J.C.; Koehler, P.E. Effect of temperature cycling on the production of patulin and citrinin. J. Food Sci. 1981, 46, 974–975. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X. Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. Int. J. Food Microbiol. 2005, 103, 331–337. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10, 1–82. [Google Scholar]
- Lee, C.L.; Wang, J.J.; Pan, T.M. Red mold rice extract represses amyloid beta peptide-induced neurotoxicity via potent synergism of anti-inflammatory and antioxidative effect. Appl. Microbiol. Biotechnol. 2008, 79, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Kiyota, A.; Yasukawa, K.; Sakamoto, N.; Kimura, Y.; Suzuki, T.; Takayasu, J.; Nishino, H. Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers. 2005, 2, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Su, N.W.; Lin, Y.L.; Lee, M.H.; Ho, C.Y. Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J. Agric. Food Chem. 2005, 53, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Seeram, N.P.; Zhang, Y.; Heber, D. Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J. Nutr. Biochem. 2008, 19, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Hsu, W.-Y.; Hish, C.-H.; Pan, T.-M. Proteome changes in Caco-2 cells treated with Monascus—Fermented red mold rice extract. J. Agric. Food Chem. 2007, 55, 8987–8994. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.-Y.; Pan, T.-M. The Monascus metabolite monacolin K reduces tumor progression and metastasis of Lewis lung carcinoma cells. J. Agric. Food Chem. 2009, 57, 8258–8265. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-J.; Chen, J.-J.; Wu, M.-D.; Yang, P.-S.; Yuan, G.-F. Isolation and structure determination of one new metabolite isolated from the red fermented rice of Monascus purpureus. Nat. Prod. Res. 2010, 24, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.M.; Tsai, R.L.; Ho, B.Y. Red mold rice mitigates oral carcinogenesis in 7,12-dimethyl-1,2-benz[a] anthracene-induced oral carcinogenesis in hamster. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Protection of Monascus-fermented dioscorea against DMBA-induced oral injury in hamster by anti-inflammatory and antioxidative potentials. J. Agric. Food Chem. 2010, 58, 6715–6720. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Effects of red mold dioscorea on oral carcinogenesis in DMBA-induced hamster animal model. Food Chem. Toxicol. 2011, 49, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Lakrod, K.; Chaisrisook, C.; Yongsmith, B.; Skinner, D.Z. RAPD analysis of genetic variation within a collection of Monascus spp. isolated from red rice (ang-kak) and sofu. Mycol. Res. 2000, 104, 403–408. [Google Scholar] [CrossRef]
- Kim, J.G.; Choi, Y.D.; Chang, Y.J.; Kim, S.U. Genetic transformation of Monascus purpureus DSM1379. Biotechnol. Lett. 2003, 25, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Campoy, S.; Pérez, F.; Martín, J.F.; Gutiérrez, S.; Liras, P. Stable transformants of the azaphilone pigment-producing Monascus purpureus obtained by protoplast transformation and Agrobacterium-mediated DNA transfer. Curr. Genet. 2003, 43, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Xu, G. Promotion of Monacolin K production by Agrobacterium tumefaciens-mediated transformation in Monascus albidus 9901. Curr. Microbiol. 2011, 62, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kinoshita, H.; Nihira, T. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2007, 73, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tseng, C.; Liaw, L.; Wang, C.; Chen, I.; Wu, W.; Wu, M.; Yuan, G. Cloning and characterization of monacolin K biosynthetic gene cluster from Monascus pilosus. J. Agric. Food Chem. 2008, 56, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.I.P.; Yuan, G.F.; Hsieh, S.Y.; Lin, Y.U.S.; Wang, W.Y.I.; Liaw, L.I.L.; Tseng, C.P. Identification of the mokh gene encoding transcription factor for the upregulation of monacolin k biosynthesis in Monascus pilosus. J. Agric. Food Chem. 2010, 58, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Li, X.; Shao, Y.; Chen, F. MrflbA, encoding a putative FlbA, is involved in aerial hyphal development and secondary metabolite production in Monascus ruber M-7. Fungal Biol. 2012, 116, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Karki, S.; Chiu, S.H.; Kim, H.J.; Suh, J.W.; Nam, B.; Yoon, Y.M.; Chen, C.C.; Kwon, H.J. Genetic localization and in vivo characterization of a Monascus azaphilone pigment biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 2013, 97, 6337–6345. [Google Scholar] [CrossRef] [PubMed]
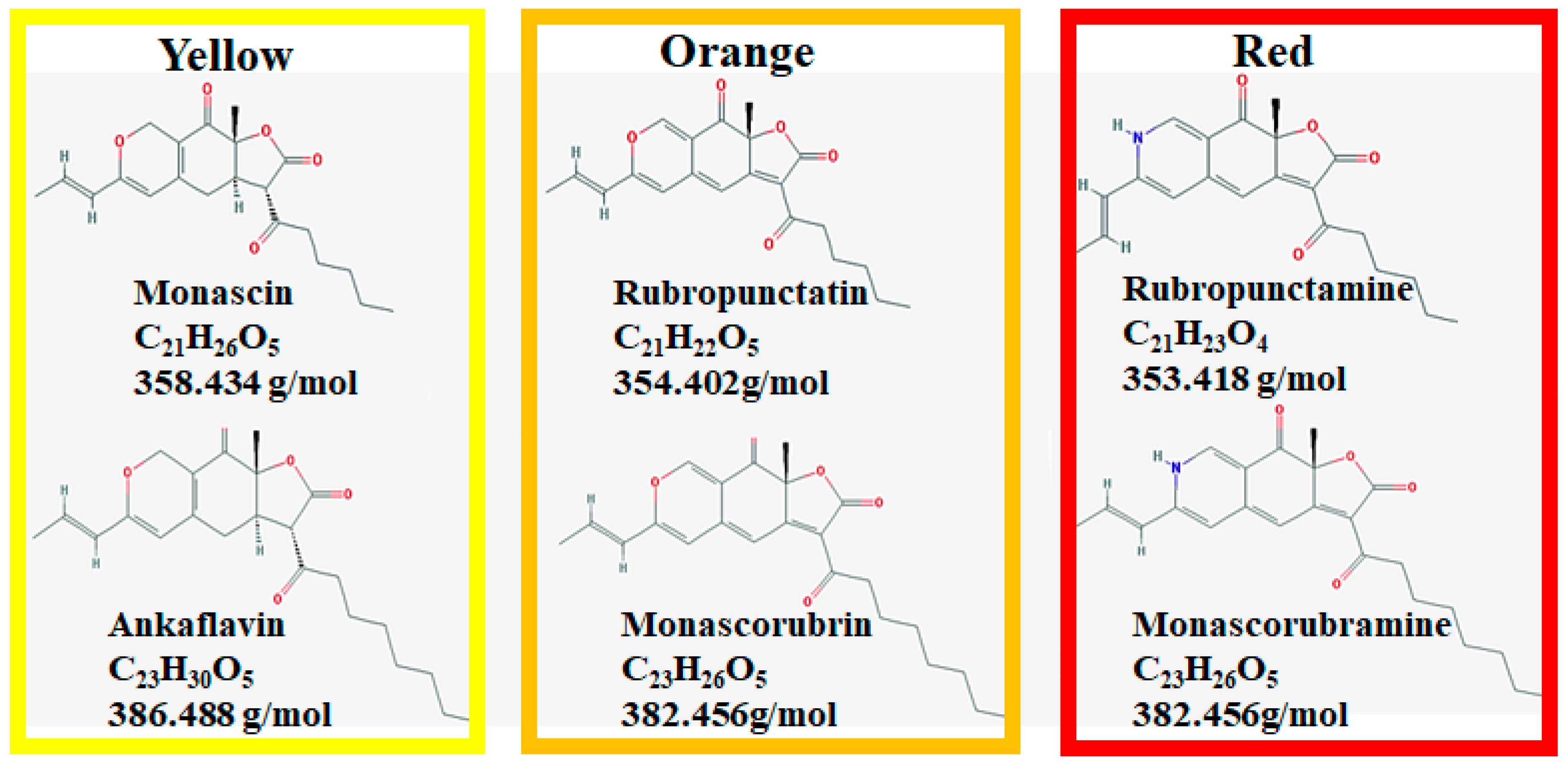
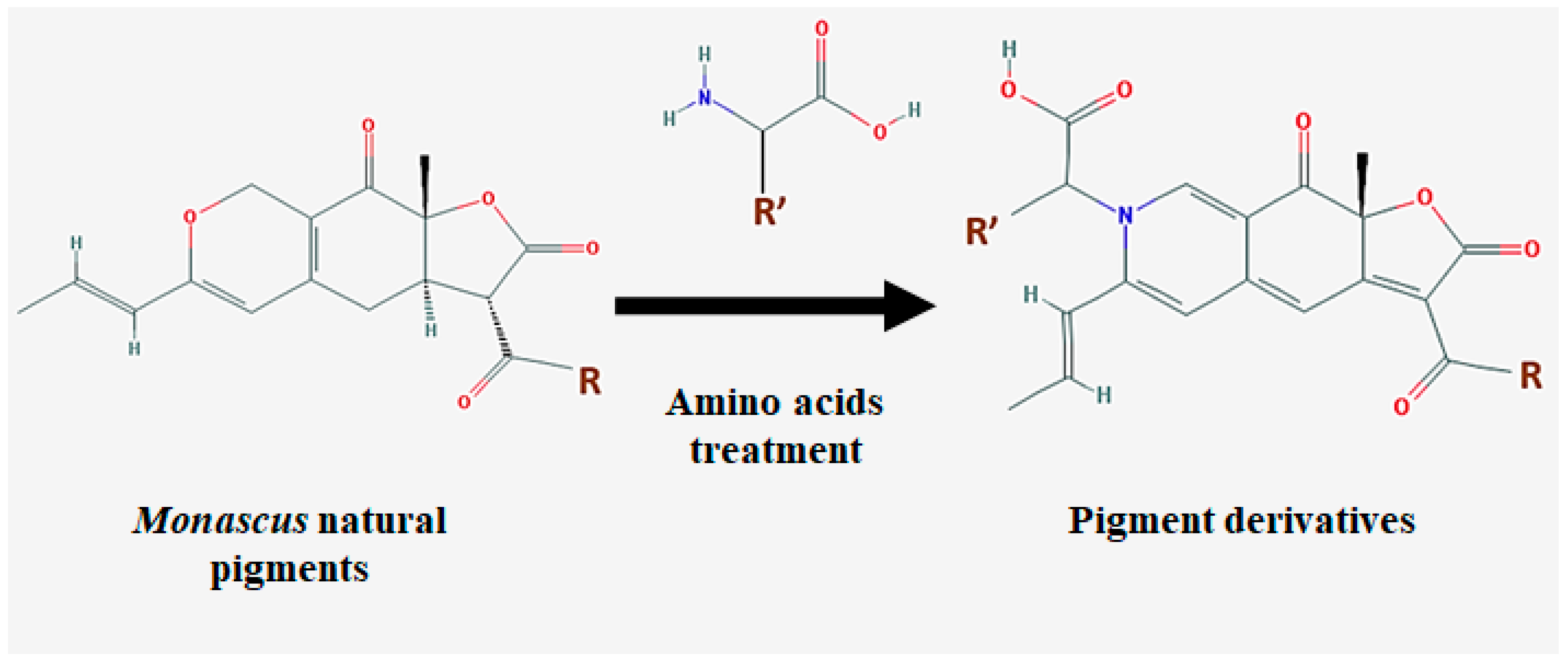
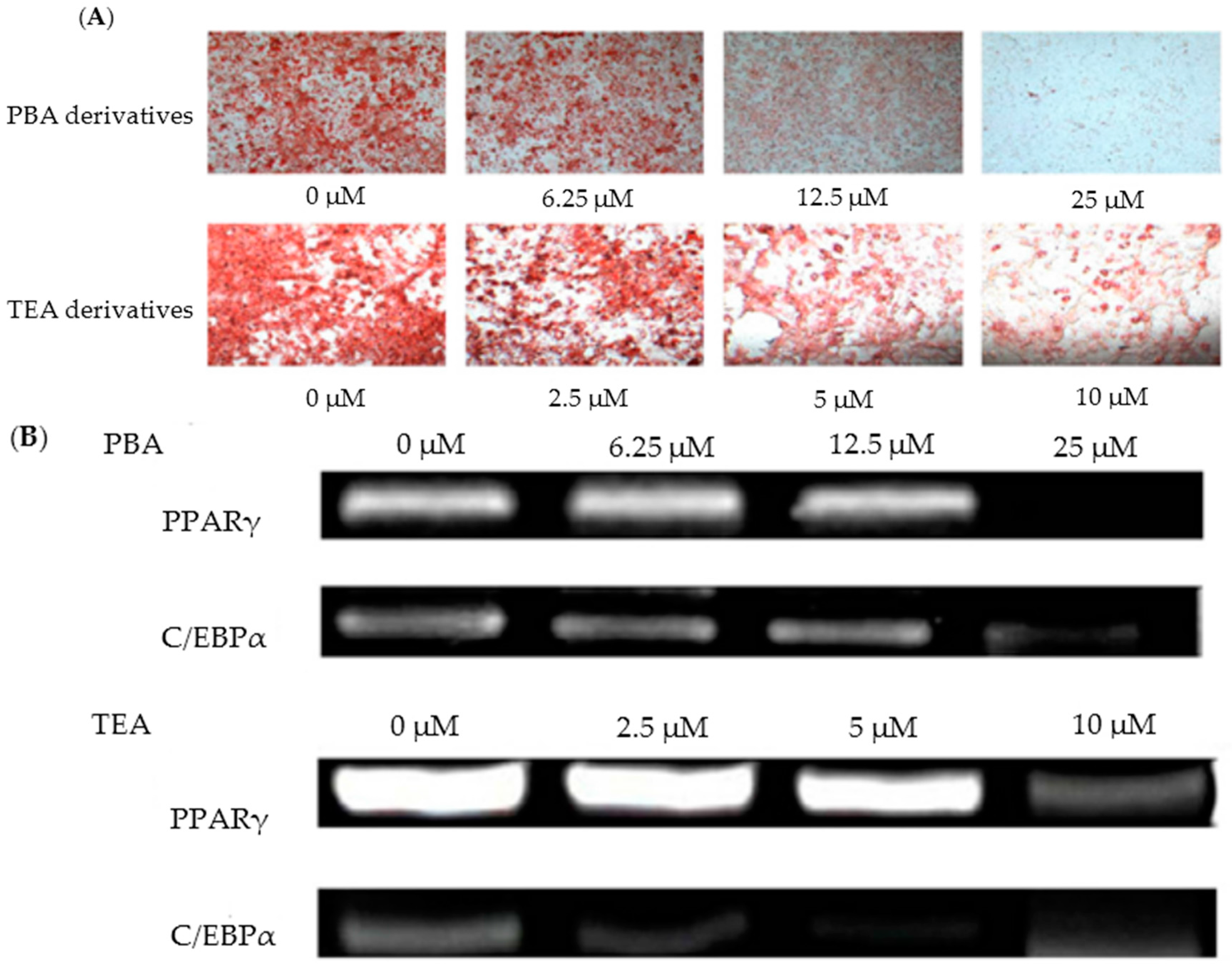
| Species | Strain | Medium | Color | Reference |
|---|---|---|---|---|
| Monascus sp. | KCCM 10093 | Chemically defined medium | Red, orange | [22,23,37] |
| Monascus sp. | KB20M10.2 | GPMY (chemically defined medium) | Yellow | [17,38] |
| Monascus sp. | ATCC 16436 | MP I, II and III (chemically defined medium) | Orange, yellow | [8,39] |
| Monascus sp. | J101 | Chemically defined medium | Red, yellow | [24,25,40] |
| Monascus sp. | B683 | Chemically defined medium | Red, yellow | [41] |
| Monascus sp. | TTWMB 6093 | Chemically defined medium | Red | [42] |
| Monascus kaoliang | ATCC 26264 | Solid culture medium | Orange, yellow | [9] |
| Monascus bisporus | ATCC 36964 | 319 1 | Yellow | [43] |
| Monascus eremophilus | ATCC 62925 | 319 1 | Orange | [44] |
| Monascus floridanus | ATCC 64205 | 336 1 | Orange | [44] |
| Monascus lunisporas | ATCC 204397 | 319 1 | Orange | [45] |
| Monascus sanguineus | ATCC 200613 | 325 1 | Red | [46] |
| Monascus pilosus | ATCC 16363 | 325 1 | Orange | [44] |
| Monascus ruber | ATCC 15670 | 336 1 | Orange | [31] |
| Monascus ruber | ATCC 96218 | Chemically-defined medium | Red | [21] |
| Monascus ruber | CCT 3802 | Chemically-defined medium | Red, orange, yellow | [28,32,36] |
| Monascus ruber | 102w | Chemically-defined medium | Red | [47] |
| Monascus ruber | LEB A 1-3 | PDA 2 | Red | [48] |
| Monascus purpureus | ATCC 16365 | 325 1 | Orange | [44] |
| Monascus purpureus | IMI 210765 | PDA 2 | Red, yellow | [49] |
| Monascus purpureus | NRRL 1992 | PDA 2 | Yellow | [50] |
| Monascus purpureus | CCM8152 | Chemically-defined medium | Red | [51,52] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Ku, S. Beneficial Effects of Monascus sp. KCCM 10093 Pigments and Derivatives: A Mini Review. Molecules 2018, 23, 98. https://doi.org/10.3390/molecules23010098
Kim D, Ku S. Beneficial Effects of Monascus sp. KCCM 10093 Pigments and Derivatives: A Mini Review. Molecules. 2018; 23(1):98. https://doi.org/10.3390/molecules23010098
Chicago/Turabian StyleKim, Daehwan, and Seockmo Ku. 2018. "Beneficial Effects of Monascus sp. KCCM 10093 Pigments and Derivatives: A Mini Review" Molecules 23, no. 1: 98. https://doi.org/10.3390/molecules23010098
APA StyleKim, D., & Ku, S. (2018). Beneficial Effects of Monascus sp. KCCM 10093 Pigments and Derivatives: A Mini Review. Molecules, 23(1), 98. https://doi.org/10.3390/molecules23010098






