Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits
Abstract
:1. Introduction
2. Results and Discussion
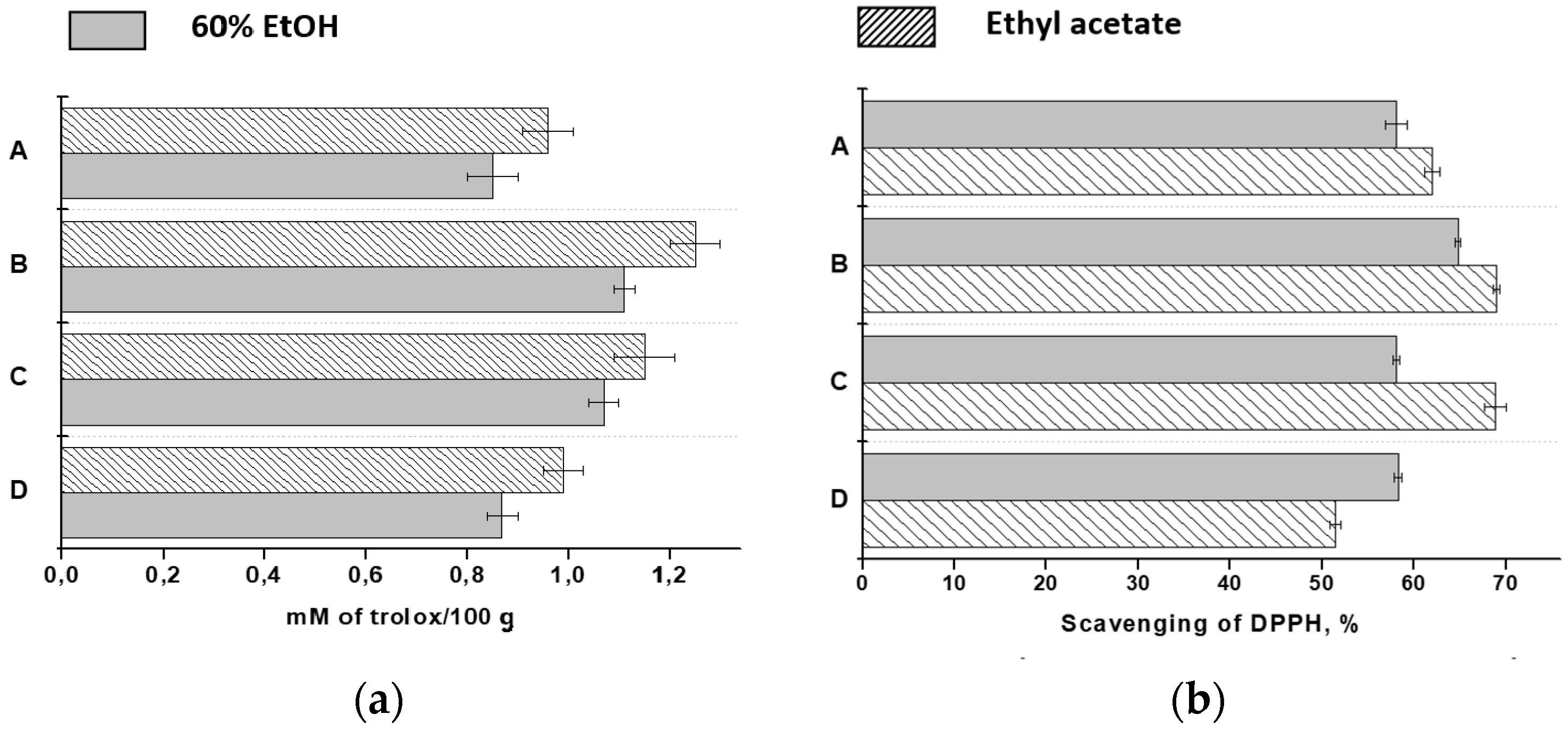
2.1. Total Phenols, Flavonoids and Monomeric Anthocyanins
2.2. Metals
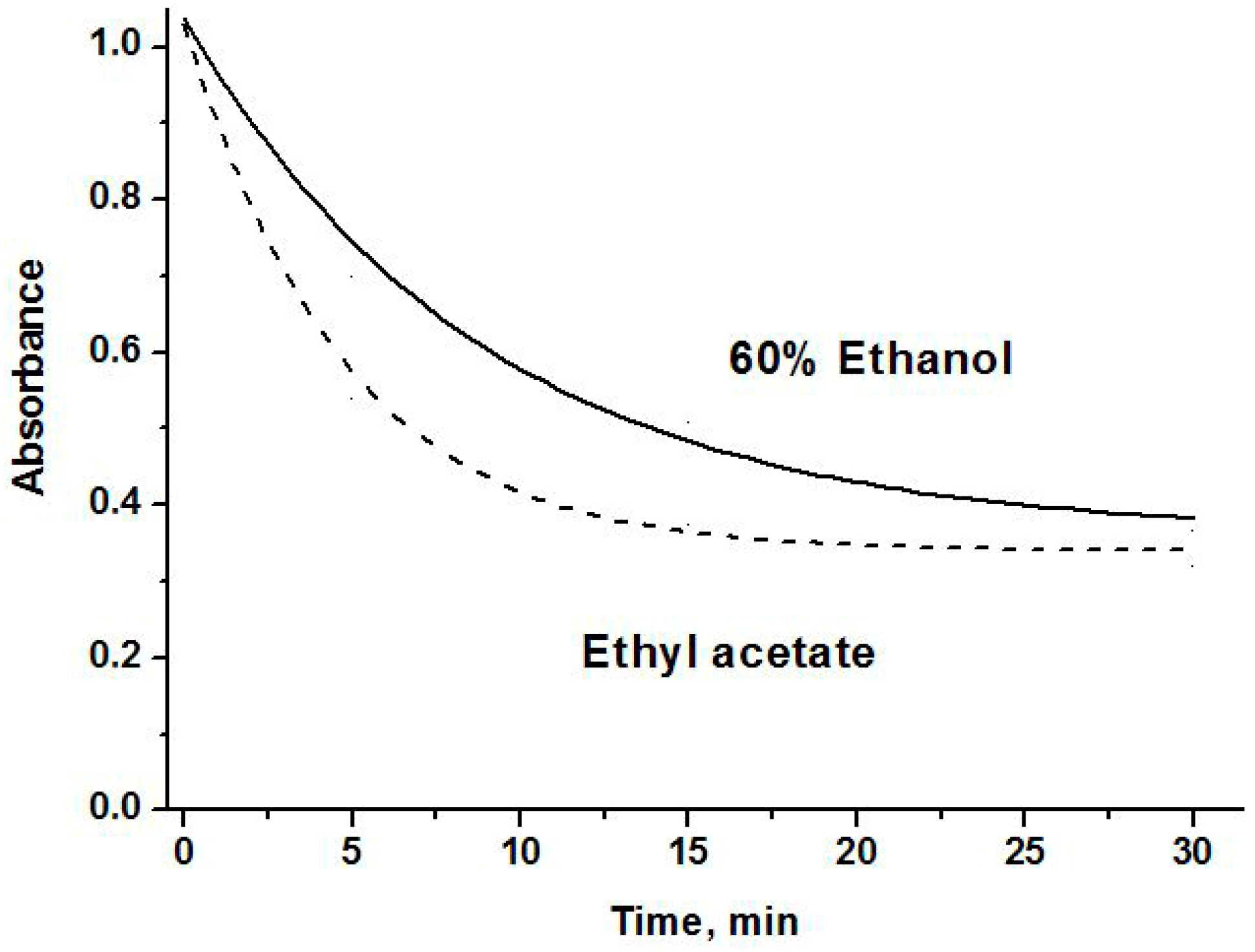
2.3. The Antioxidant Activity
3. Materials and Methods
3.1. Fruit Samples and Their Extraction
3.2. Total Phenolic Content
3.3. Total Flavonoids
3.4. Total Monomeric Anthocyanins
3.5. Determination of Metals
3.6. Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nile, S.H. Edible berries: Bioactive compounds and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Feng, R.; Bowman, L.; Penhallegon, R.; Ding, M.; Lu, Y. Antioxidant activity in lingonberries (Vaccinium vitis-idaea L.) and its inhibitory effect on activator protein-1, nuclear factor-κB, and mitogen-activated protein kinases activation. J. Agric. Food Chem. 2005, 53, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Puišo, J.; Jonkuviené, D.; Mačioniené, I.; Šalomskiené, J.; Jasutiené, I.; Kondrotas, R. Biosynthesis of silver nanoparticles using lingonberry and cranberry juices and their antimicrobial activity. Colloids Surf. B 2014, 121, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, A.S.; Siltari, A.; Ehlers, P.I.; Korpel, R.; Vapaatalo, H. Lingonberry juice negates the effects of a high salt diet on vascular function and low-grade inflammation. J. Funct. Foods 2014, 7, 238–245. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P.V. Antioxidant and cytoprotective properties of partridgeberry polyphenols. Food Chem. 2015, 169, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.L.; Wang, Z.Y.; Liu, J.R. Cold-field fruit extracts exert different antioxidant and antiproliferative activities in vitro. Food Chem. 2011, 129, 402–407. [Google Scholar] [CrossRef]
- Su, Z. Anthocyanins and flavonoids of Vaccinium L. Pharm. Crops 2012, 3, 7–37. [Google Scholar] [CrossRef]
- Paredes-López, O.; Cervantes-Ceja, M.; Vigna-Pérez, M.; Hérnàndez-Pérez, T. Berries: Improving human health and healthy aging, and promoting quality life—A review. Plant Food Hum. Nutr. 2010, 65, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and humans. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Lätti, K.; Riihinen, K.R.; Jaakola, L. Phenolic compounds in berries and flowers of a natural hybrid between bilberry and lingonberry (Vaccinium × intermedium Ruthe). Phytochemistry 2011, 72, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinum vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Finn, C.E. Lingonberry (Vaccinium vitis-idaea L.) grown in the Pacific Northwest of North America: Anthocyanin and free amino acid composition. J. Funct. Foods 2012, 4, 213–218. [Google Scholar] [CrossRef]
- Hajazimi, E.; Landberg, R.; Zamaratskaia, G. Simultaneous determination of flavonols and phenolic acids by HPLC-CoulArray in berries common in the Nordic diet. LWT—Food Sci. Technol. 2016, 74, 128–134. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berries plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Czyżowska, A.; Sakač, M.; Mišsan, A.; Duragič, O.; Kregiel, D. Phenolic compounds contained in little-known wild fruits as antiadhesive agents against the beverage-spoiling bacteria Asaia spp. Molecules 2017, 22, 1256–1274. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and analysis of polyphenols: Recent trends. Crit. Rev. Anal. Chem. 2011, 31, 227–249. [Google Scholar]
- Michiels, J.M.; Kevers, C.; Pincemai, J.; Defraigne, J.O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Hwang, S.J.; Yoon, W.B.; Lee, O.H.; Cha, S.J.; Kim, J.D. Radical-scavenging-linked antioxidant activities of extracts from black chokeberry and blueberry cultivated in Korea. Food Chem. 2014, 146, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Comp. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 154, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic distribution in liquid preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2014, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Luginina, E.A.; Egoshina, T.L. The peculiarities of heavy metals accumulation by wild medicinal and fruit plants. Ann. Warsaw Univ. Life Sci.–SGGW, Ser.: Agric. 2013, 61, 97–103. [Google Scholar]
- Levula, T.; Saarsalmi, A.; Rantavaara, A. Effects of ash fertilization and prescribed burning on macronutrients, heavy metal, sulphur and 137Cs concentrations in lingonberries (Vaccinium vitis-idaea). For. Ecol. Manag. 2000, 126, 269–279. [Google Scholar] [CrossRef]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Aspects Med. 2005, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 629/2008 amending Regulation (EC) No 188/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union L 2008, 364, 5–24.
- Nowakowska, M.; Ochmian, I.; Mijowska, K. The influence of street conditions on sea buckthorn fruit quality and content of micro- and macronutrients in berries and in soil. J. Elem. 2017, 22, 235–244. [Google Scholar]
- He, Z.; Yang, X.E.; Stoffellab, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Őzyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orhofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidanta by means Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Meth. 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [PubMed]
- Pękal, A.; Pyrzynska, K. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Meth. 2013, 5, 4288–4295. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Sample | Ethanol–Water (60:40) | Ethyl Acetate |
|---|---|---|
| Total Phenolics (mg GAE) | ||
| A | 521.0 ± 24.9 a | 465.8 ± 29.7 a |
| B | 661.1 ± 22.4 b | 594.0 ± 15.1 b |
| C | 654.9 ± 23.0 b | 579.8 ± 14.7 b |
| D | 468.0 ± 10.1 a | 458.2 ± 22.3 a |
| Total flavonoids (μg CE) | ||
| A | 1.40 ± 0.08 a | 1.28 ± 0.08 a |
| B | 1.79 ± 0.09 b | 1.49 ± 0.07 b |
| C | 1.12 ± 0.09 a | 1.43 ± 0.09 c |
| D | 1.33 ± 0.08 c | 1.36 ± 0.09 d |
| Total monomeric anthocyanins (mg CGE) | ||
| A | 43.0 ± 2.87 a | 38.1 ± 3.44 a |
| B | 32.1 ± 2.91 b | 33.0 ± 2.65 a,b |
| C | 39.2 ± 1.40 a | 38.1 ± 2.47 a |
| D | 26.1 ± 1.03 b | 26.2 ± 2.19 b |
| Sample | Cd | Cr | Cu | Fe | Pb | Zn |
|---|---|---|---|---|---|---|
| A | 0.009 ± 0.0004 a | 0.065 ± 0.003 a | 0.30 ± 0.015 a | 12.4 ± 0.05 a | 0.48 ± 0.013 a | 4.37 ± 0.17 a |
| B | 0.001 ± 0.00004 b | 0.083 ± 0.003 b | 0.29 ± 0.012 a | 8.25 ± 0.33 b | 0.17 ± 0.006 b | 5.42 ± 0.22 b |
| C | 0.003 ± 0.0001 c | 0.150 ± 0.005 c | 0.44 ± 0.021 a | 9.93 ± 0.40 a | 0.15 ± 0.005 b | 4.61 ± 0.16 a |
| D | 0.004 ± 0.0001 d | 0.029 ± 0.001 d | 1.14 ± 0.046 b | 13.4 ± 0.62 c | 0.14 ± 0.004 b | 4.55 ± 0.14 a |
| Sample | Cd | Cr | Cu | Fe | Pb | Zn |
|---|---|---|---|---|---|---|
| A | 0.009 ± 0.0004 a | 0.065 ± 0.003 a | 0.30 ± 0.015 a | 12.4 ± 0.05 a | 0.48 ± 0.013 a | 4.37 ± 0.17 a |
| B | 0.001 ± 0.00004 b | 0.083 ± 0.003 b | 0.29 ± 0.012 a | 8.25 ± 0.33 b | 0.17 ± 0.006 b | 5.42 ± 0.22 b |
| C | 0.003 ± 0.0001 c | 0.150 ± 0.005 c | 0.44 ± 0.021 a | 9.93 ± 0.40 a | 0.15 ± 0.005 b | 4.61 ± 0.16 a |
| D | 0.004 ± 0.0001 d | 0.029 ± 0.001 d | 1.14 ± 0.046 b | 13.4 ± 0.62 c | 0.14 ± 0.004 b | 4.55 ± 0.14 a |
| Element | Mixed Polish Herbs INCT-MPH-2 | Trace Metals—Silt Clay 1 CRM 045 | ||
|---|---|---|---|---|
| Certified | Determined | Certified | Determined | |
| Cd | 0.199 ± 0.015 | 0.203 ± 0.040 | 7.01 ± 0.177 | 7.00 ± 0.071 |
| Cr | 1.68 ± 0.12 | 1.67 ± 0.03 | 45.7 ± 5.38 | 47.2 ± 1.87 |
| Cu | 7.77 ± 0.53 | 7.97 ± 0.41 | 62.2 ± 1.54 | 62.4 ± 1.09 |
| Fe | 460 * | 465 ± 7.11 | nd | nd |
| Pb | 2.16 ± 0.23 | 2.16 ± 0.05 | 45.3 ± 1.92 | 116 ± 2.17 |
| Zn | 33.5 ± 2.1 | 33.1 ± 0.08 | 114 ± 6.77 | 119 ± 1.97 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dróżdż, P.; Šėžienė, V.; Wójcik, J.; Pyrzyńska, K. Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits. Molecules 2018, 23, 53. https://doi.org/10.3390/molecules23010053
Dróżdż P, Šėžienė V, Wójcik J, Pyrzyńska K. Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits. Molecules. 2018; 23(1):53. https://doi.org/10.3390/molecules23010053
Chicago/Turabian StyleDróżdż, Paulina, Vaida Šėžienė, Józef Wójcik, and Krystyna Pyrzyńska. 2018. "Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits" Molecules 23, no. 1: 53. https://doi.org/10.3390/molecules23010053
APA StyleDróżdż, P., Šėžienė, V., Wójcik, J., & Pyrzyńska, K. (2018). Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits. Molecules, 23(1), 53. https://doi.org/10.3390/molecules23010053





