Synthesis and Evaluation of Biological Activities of Aziridine Derivatives of Urea and Thiourea
Abstract
1. Introduction
2. Results and Discussion
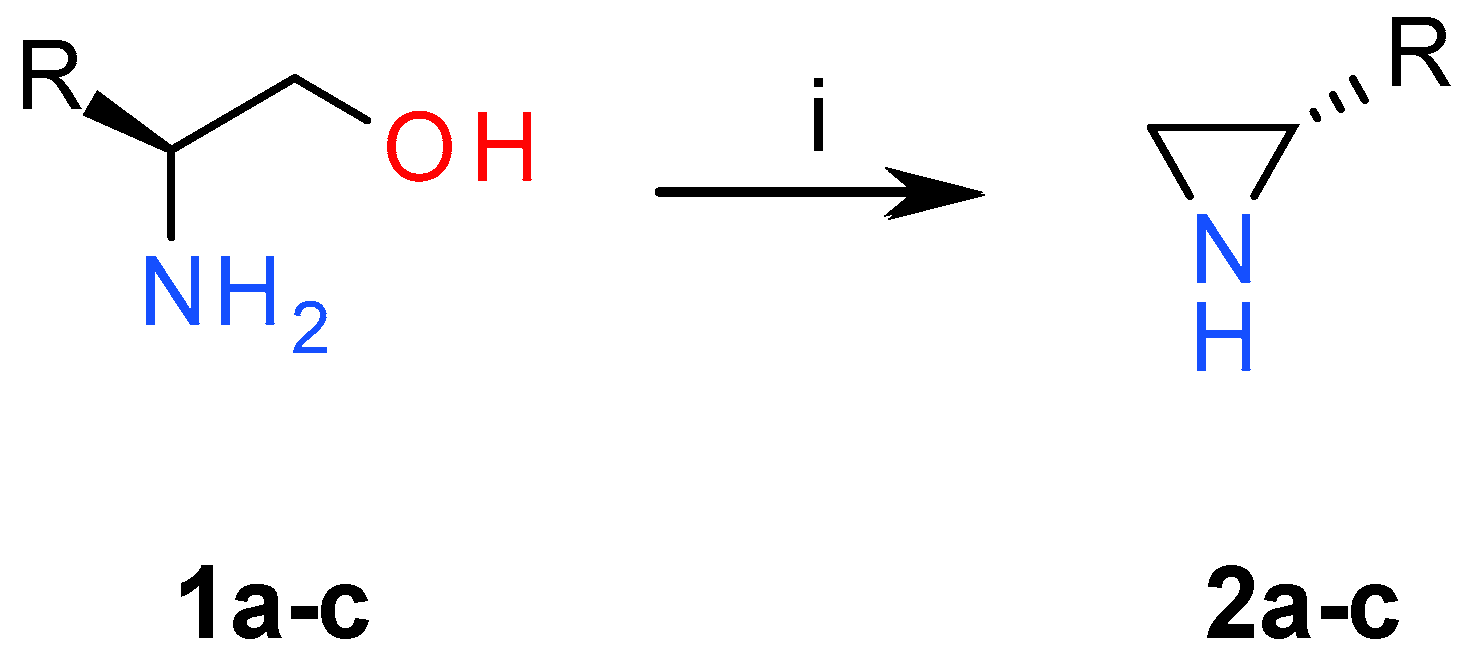
2.1. Chemistry
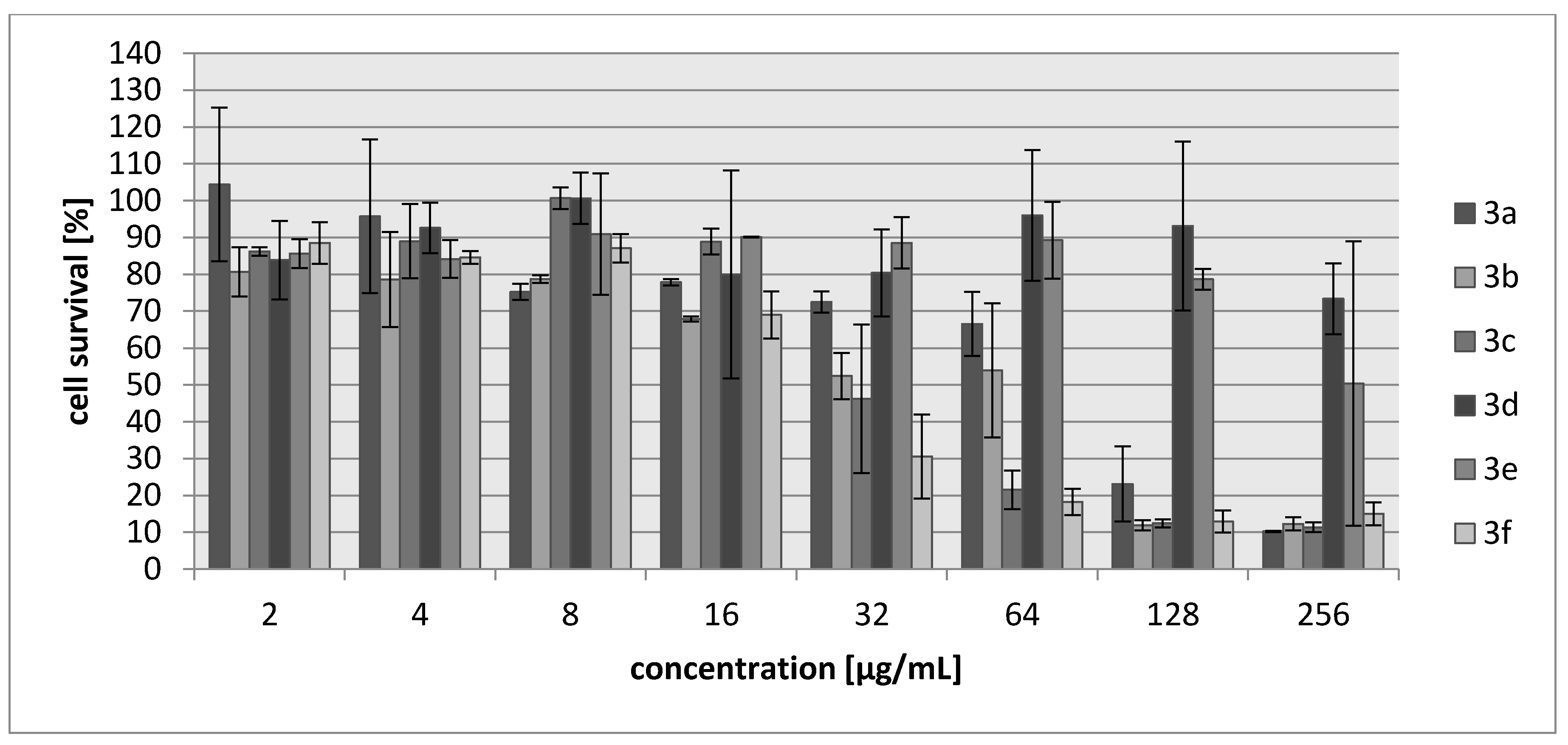
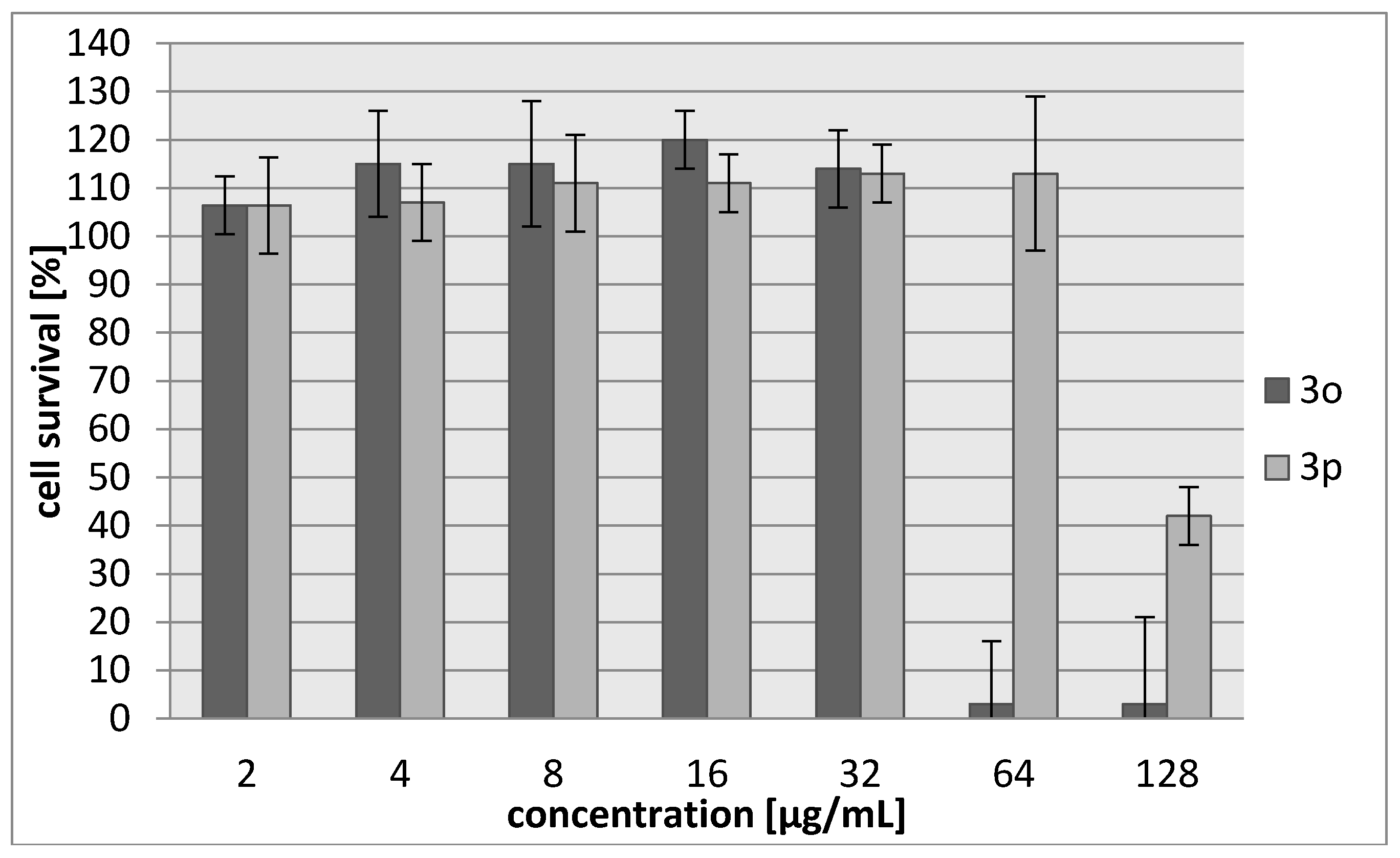
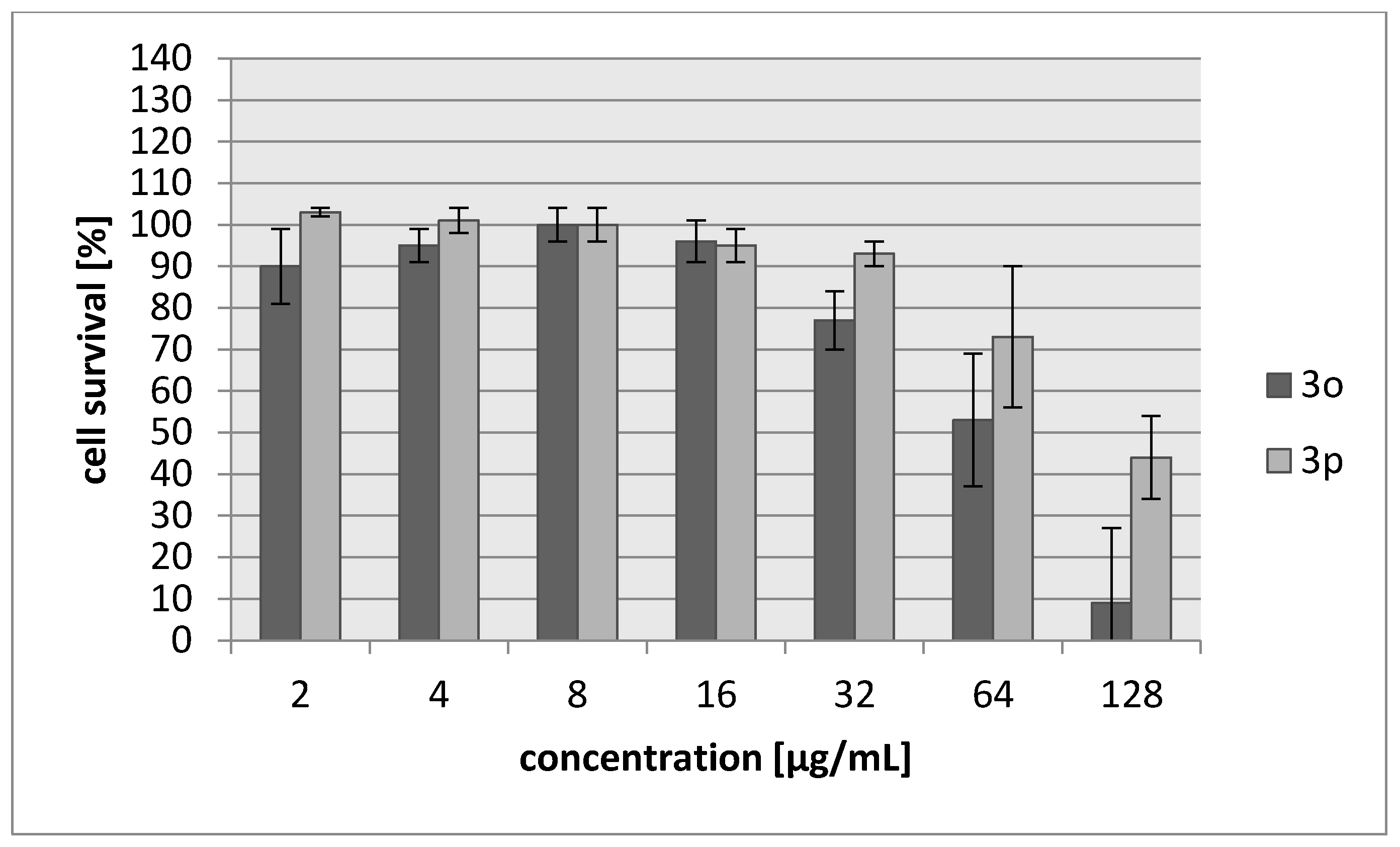
2.2. Biology
3. Experimental Section
3.1. Chemistry
3.2. General Procedure for Synthesis of Urea and Thiourea Derivatives 3
3.3. Biology
3.3.1. Antibacterial Assay
3.3.2. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yudin, A.K. Aziridines and Epoxides in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006; p. 494. [Google Scholar]
- Morieux, P.; Stables, J.P.; Kohn, H. Synthesis and anticonvulsant activities of N-benzyl (2R)-2-acetamido-3-oxysubstituted propionamide derivatives. Bioorg. Med. Chem. 2006, 16, 8968–8975. [Google Scholar] [CrossRef] [PubMed]
- Cimarelli, C.; Fratoni, D.; Palmieri, G. A Convenient Synthesis of New Diamine, Amino Alcohol and Aminophosphines Chiral Auxiliaries Based on Limonene Oxide. Tetrahedron Asymmetry 2009, 20, 2234–2239. [Google Scholar] [CrossRef]
- Leśniak, S.; Pieczonka, A.M.; Jarzyński, S.; Justyna, K.; Rachwalski, M. ChemInform Abstract: Synthesis and Evaluation of the Catalytic Properties of Semicarbazides Derived from N-Triphenylmethyl-aziridine-2-carbohydrazides. Tetrahedron Asymmetry 2013, 24, 1341–1344. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Leśniak, S.; Rachwalski, M. Direct asymmetric aldol condensation catalyzed by aziridine semicarbazide zinc(II) complexes. Tetrahedron Lett. 2014, 55, 2373–2375. [Google Scholar] [CrossRef]
- Jarzyński, S.; Leśniak, S.; Pieczonka, A.M.; Rachwalski, M. N-Trityl-aziridinyl alcohols as highly efficient chiral catalysts in asymmetric additions of organozinc species to aldehydes. Tetrahedron Asymmetry 2015, 26, 35–40. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Leśniak, S.; Jarzyński, S.; Rachwalski, M. Aziridinylethers as highly enantioselective ligands for the asymmetric addition of organozinc species to carbonyl compounds. Tetrahedron Asymmetry 2015, 26, 148–151. [Google Scholar] [CrossRef]
- Lesniak, S.; Rachwalski, M.; Pieczonka, A.M. Optically Pure Aziridinyl Ligands as Useful Catalysts in the Stereocontrolled Synthesis. Curr. Org. Chem. 2014, 18, 3045–3065. [Google Scholar] [CrossRef]
- Cheung, L.L.W.; Zhi, H.; Decker, S.M.; Yudin, A.K. Skeletal Fusion of Small Heterocycles with Amphoteric Molecules. Angew. Chem. Int. Ed. 2011, 50, 11798–11802. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.M.D.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Wakaki, S.; Marumo, H.; Tomioka, K.; Shimizu, G.; Kato, E.; Kamada, H.; Kudo, S.; Fujimoto, Y. Isolation of new fractions of antitumor mitomycins. Antibiot. Chemother. 1958, 8, 228–235. [Google Scholar]
- Herr, R.R.; Bergy, M.E.; Eble, T.E.; Jahnke, H.K. Porfiromycin, a new antibiotic. Antimicrob. Agents Ann. 1960, 8, 23–31. [Google Scholar]
- Zang, H.; Gates, K.S. DNA Binding and Alkylation by the “Left Half” of Azinomycin B. Biochemistry 2000, 39, 14968–14975. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, K.; Payne, C.M.; Tome, M.E.; Briehl, M.M.; McClure, T.; Dorr, R.T. Induction of oxidative stress and apoptosis in myeloma cells by the aziridine-containing agent imexon. Biochem. Pharm. 2000, 60, 749–758. [Google Scholar] [CrossRef]
- Dvorakova, K.; Waltmire, C.N.; Payne, C.M.; Tome, M.E.; Briehl, M.M.; Dorr, R.T. Induction of mitochondrial changes in myeloma cells by imexon. Blood 2001, 97, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- Sheveleva, E.V.; Landowski, T.H.; Samulitis, B.K.; Bartholomeusz, G.; Powis, G.; Dorr, R.T. Imexon Induces an Oxidative Endoplasmic Reticulum Stress Response in Pancreatic Cancer Cells. Mol. Cancer Res. 2012, 10, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Moulder, S.; Dhillon, N.; Ng, C.; Hong, D.; Wheler, J.; Naing, A.; Tse, S.; La Paglia, A.; Dorr, R.; Hersh, E.; et al. A phase I trial of imexon, a pro-oxidant, in combination with docetaxel for the treatment of patients with advanced breast, non-small cell lung and prostate cancer. Investig. New Drugs 2010, 28, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Barr, P.M.; Miller, T.P.; Friedberg, J.W.; Peterson, D.R.; Baran, A.M.; Herr, M.; Spier, C.M.; Cui, H.; Roe, D.J.; Persky, D.O.; et al. Phase 2 study of imexon, a prooxidant molecule, in relapsed and refractory B-cell non-Hodgkin lymphoma. Blood 2014, 124, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, D.; Haag, R.; Bosies, E.; Bicker, U.; Kampe, W. Use of Imexone as an Immunosuppressive Agent. Patent EP 352652 A2, 31 January 1990. [Google Scholar]
- Kinoshita, S.; Uzu, K.; Nakano, M.; Shimizu, A.; Takahashi, T. Mitomycin derivatives. 1. Preparation of mitosane and mitosene compounds and their biological activities. J. Med. Chem. 1971, 14, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Stapley, E.O.; Hendlin, D.; Jackson, M.; Miller, A.K.; Hernandez, S.; Martinez, M.; Martinez, M. Azirinomycin. I. Microbial production and biological characteristics. J. Antibiot. 1971, 24, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Tristram, E.W.; Wolf, F.J. Azirinomycin. II. Isolation and chemical characterization as 3-methyl-2(2H) azirinecarboxylic acid. J. Antibiot. 1971, 24, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Argoudelis, A.D.; Reusser, F.; Whaley, H.A. The Antibiotic U-47,929 and Its Preparation. U.S. Patent 542,226, 24 February 1976. [Google Scholar]
- Harada, K.I.; Tomita, K.; Fujii, K.; Masuda, K.; Mikami, Y.; Yazawa, K.; Komaki, H. Isolation and Structural Characterization of Siderophores, Madurastatins, Produced by a Pathogenic Actinomadura madurae. J. Antibiot. 2004, 57, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Iinuma, H.; Kinoshita, N.; Ikeda, T.; Sawa, T.; Hamada, M.; Takeuchi, T. Azicemicins A and B, a New Antimicrobial Agent Produced by Amycolatopsis. J. Antibiot. 1995, 48, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.R.; Colson, K.L.; Klohr, S.E.; Zein, N.; Langley, D.R.; Lee, M.S.; Matson, J.A.; Doyle, T.W. Isolation, Structure Determination, and Proposed Mechanism of Action for Artifacts of Maduropeptin Chromophore. J. Am. Chem. Soc. 1994, 116, 9351–9352. [Google Scholar] [CrossRef]
- Budzisz, E.; Bobka, R.; Hauss, A.; Roedel, J.N.; Wirth, S.; Lorenz, I.-P.; Rozalska, B.; Więckowska-Szakiel, M.; Krajewska, U.; Rozalski, M. Synthesis, structural characterization, antimicrobial and cytotoxic effects of aziridine, 2-aminoethylaziridine and azirine complexes of copper(II) and palladium(II). Dalton Trans. 2012, 41, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Swapnaja, K.J.M.; Yennam, S.; Chavali, M.; Poornachandra, Y.; Kumar, C.G.; Muthusamy, K.; Jayaraman, V.B.; Arumugam, P.; Balasubramanian, S.; Sriram, K.K. Design, synthesis and biological evaluation of diaziridinyl quinone isoxazole hybrids. Eur. J. Med. Chem. 2016, 117, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Moonen, K.; Laureyn, I.; Stevens, C.V. Synthetic methods for azaheterocyclic phosphonates and their biological activity. Chem. Rev. 2004, 104, 6177–6215. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Ö.; Babiz, H.; Gözen, A.G.; Budak, S. Synthesis of 2-aziridinyl phosphonates by modified Gabriel–Cromwell reaction and their antibacterial activities. Eur. J. Med. Chem. 2011, 46, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Chebanov, V.A.; Zbruyev, A.I.; Desenko, S.M.; Orlov, V.D.; Yaremenko, F.G. Three-Membered Azaheterocycles Based on α, β-Unsaturated Ketones. Curr. Org. Chem. 2008, 12, 792–812. [Google Scholar] [CrossRef]
- Singh, G.S.; D’hooghe, M.; De Kimpe, N. Synthesis and Reactivity of C-Heteroatom-Substituted Aziridines. Chem. Rev. 2007, 107, 2080–2135. [Google Scholar] [CrossRef] [PubMed]
- Callebaut, G.; Meiresonne, T.; De Kimpe, N.; Mangelinckx, S. Synthesis and Reactivity of 2-(Carboxymethyl)aziridine Derivatives. Chem. Rev. 2014, 114, 7954–8015. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, N.; Xu, J. An improved and mild Wenker synthesis of aziridines. Synthesis 2010, 20, 3423–3428. [Google Scholar]
- Fishbein, P.L.; Kohn, H. Synthesis and antineoplastic activity of 1a-formyl and 1a-thioformyl derivatives of mitomycin C and 2-methylaziridine. J. Med. Chem. 1987, 30, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Kwan, B.W.; Chowdhury, N.; Wood, T.K. Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 2015, 17, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Tokushige, M.; Umezawa, H. Specific inhibition of aspartase by S-2,3-dicarboxyaziridine. Biochem. Int. 1988, 16, 449–452. [Google Scholar] [PubMed]
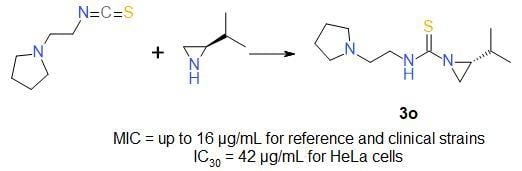
Sample Availability: Samples of the compounds 3a–3s are available from the authors. |
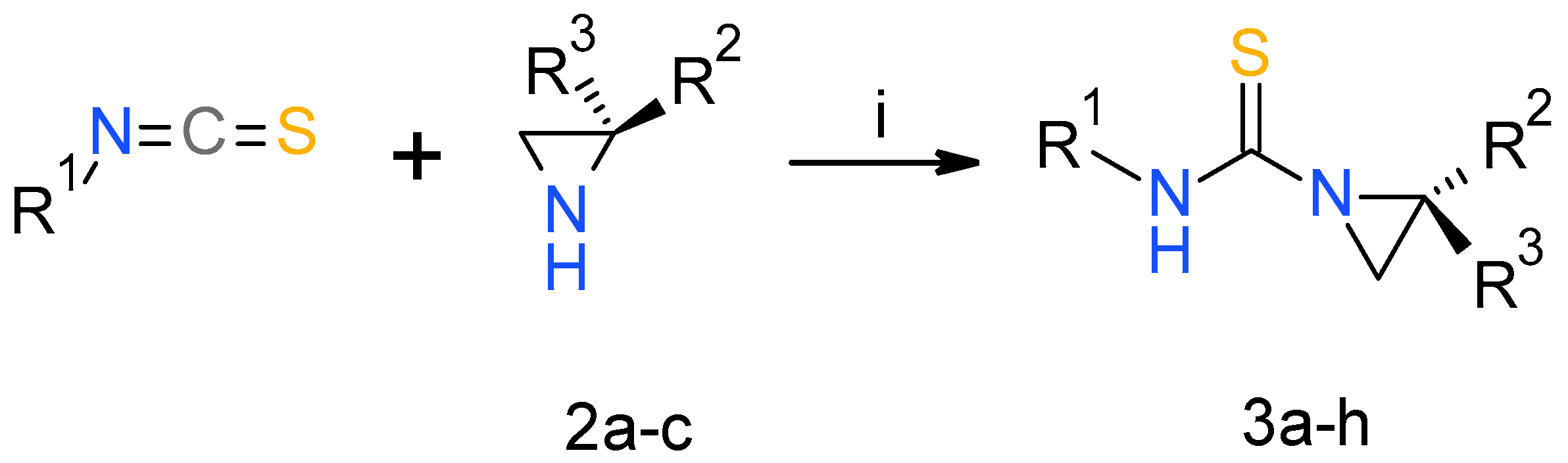
| Compound | R1 | R2 | R3 | Yield (%) |
|---|---|---|---|---|
| 3a | Bu | iPr | H | 92 |
| 3b | Me | iPr | H | 90 |
| 3c | cHex | iPr | H | 89 |
| 3d | Me | Me | H | 89 |
| 3e | Me | Me | Me | 94 |
| 3f | cHex | Me | H | 97 |
| 3g | cHex | Me | Me | 96 |
| 3h | Allyl | iPr | H | 76 |
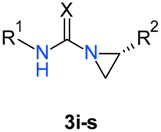
| Compound | R1 | R2 | X | Yield (%) |
|---|---|---|---|---|
| 3i | CH2Ph | iPr | S | 90 |
| 3j | CH2Ph | Me | S | 89 |
| 3k | CHPh2 | iPr | S | 88 |
| 3l | CHPh2 | Me | S | 85 |
| 3m | 2-(4-morpholino)ethyl | iPr | S | 92 |
| 3n | 2-(4-morpholino)ethyl | Me | S | 93 |
| 3o | 2-piperidinoethyl | iPr | S | 95 |
| (R)-3o | 2-piperidinoethyl | iPr | S | 90 |
| 3p | 2-piperidinoethyl | Me | S | 91 |
| 3r | Bu | iPr | O | 96 |
| 3s | cHex | iPr | O | 98 |
| Tested Strain | E. coli NCTC 8196 NCTC 8196 | S. aureus ATCC 6538 ATCC 6538 | S. aureus ATCC 29213 ATCC 29213 | S. epidermidis ATCC 12228 ATCC 12228 | ||||
|---|---|---|---|---|---|---|---|---|
| Compound | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| 3a | >512 | nd | 32 | 128 | 32 | 128 | 32 | 64 |
| 3b | 32 | 32 | 32 | 64 | 32 | 64 | 16 | 16 |
| 3c | >512 | nd | 32 | 64 | 32 | 64 | 32 | 64 |
| 3d | 128 | 128 | 256 | 512 | 256 | 512 | 256 | 512 |
| 3e | >512 | nd | 128 | 128 | 128 | 128 | 128 | 128 |
| 3f | 256 | 512 | 16 | 128 | 16 | 128 | 16 | 16 |
| NTF | 8 | 8 | 16 | 32 | 16 | 32 | 8 | 8 |
| AMP | 4 | 4 | 1 | 1 | 2 | 4 | 1 | 1 |
| STR | 1 | 2 | 1 | 1 | 1 | 2 | >512 | >512 |
| Compound | 3a | 3b | 3c | 3f | OX | AMP | NTF | STR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested Strain | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| naso-pharynx isolates | ||||||||||||||||
| S. aureus | ||||||||||||||||
| C4 | 32 | 64 | 32 | 64 | 32 | 64 | 16 | 16 | 0.25 | 0.25 | 512 | 512 | 16 | 16 | 8 | 8 |
| C7 | 32 | 64 | 32 | 32 | 32 | 64 | 16 | 16 | 0.25 | 0.25 | 64 | 128 | 16 | 16 | 8 | 16 |
| C8 | 32 | 64 | 32 | 32 | 32 | 64 | 16 | 16 | 0.25 | 0.25 | 512 | >512 | 16 | 16 | 8 | 16 |
| C19 | 32 | 64 | 32 | 32 | 32 | 64 | 16 | 16 | 0.5 | 0.5 | 64 | 128 | 32 | 32 | 8 | 8 |
| ulcers/furuncles isolates | ||||||||||||||||
| D12 | 32 | 64 | 16 | 32 | 32 | 128 | 8 | 16 | 0.5 | 0.5 | 256 | 256 | 32 | 32 | 8 | 16 |
| F1 | 32 | 64 | 32 | 128 | 64 | 128 | 16 | 64 | 0.25 | 0.25 | 128 | 128 | 32 | 32 | 8 | 8 |
| F7 | 32 | 64 | 32 | 128 | 32 | 64 | 16 | 64 | 0.25 | 0.25 | 4 | 4 | 16 | 16 | 8 | 16 |
| F12 | 32 | 64 | 16 | 32 | 32 | 64 | 8 | 16 | 0.25 | 0.25 | 64 | 128 | 16 | 32 | 8 | 32 |
| bone isolates | ||||||||||||||||
| D14 | 32 | 64 | 32 | 32 | 32 | 64 | 16 | 16 | 0.5 | 0.5 | 256 | 512 | 32 | 32 | 8 | 16 |
| D15 (MRSA) | 32 | 64 | 32 | 32 | 32 | 64 | 16 | 16 | >512 | >512 | >512 | >512 | 32 | 32 | 256 | >512 |
| D17 (MRSA) | 32 | 64 | 16 | 16 | 32 | 64 | 8 | 32 | >512 | >512 | >512 | >512 | 32 | 32 | 256 | >512 |
| D20 | 32 | 64 | 32 | 32 | 64 | 128 | 16 | 64 | 0.5 | 0.5 | 128 | 256 | 16 | 32 | 8 | 8 |
| Compound | IC30 (µg/mL)/(µM) L929 Cells | HeLa Cells |
|---|---|---|
| 3a | 68/340 | 83/388 |
| 3b | 27/171 | 28/177 |
| 3c | 34/147 | 69/304 |
| 3d | >250/>1922 | >250/1922 |
| 3e | 214/1485 | 250/1735 |
| 3f | 20/102 | 22/111 |
| Cisplatin | 7.24/24 | 6.53/22 |
| Tested Strain | E. coli NCTC 8196 NCTC 8196 | S. aureus ATCC 6538 ATCC 6538 | S. aureus ATCC 29213 ATCC 29213 | S. epidermidis ATCC 12228 ATCC 12228 |
|---|---|---|---|---|
| Compound | MIC (µg/mL) | |||
| 3j | 256 | 128 | 128 | 128 |
| 3k | 256 | na* | Na | 256 |
| 3l | 256 | 256 | 256 | 256 |
| 3m | na | 128 | 256 | 128 |
| 3o | 64 | 8 | 8 | 4 |
| 3p | 256 | 64 | 64 | 64 |
| NTF | 8 | 16 | 16 | 8 |
| AMP | 4 | 1 | 2 | 1 |
| STR | 1 | 1 | 1 | >512 |
| Tested Strain | E. coli NCTC 8196 NCTC 8196 | S. aureus ATCC 6538 ATCC 6538 | S. aureus ATCC 29213 ATCC 29213 | S. epidermidis ATCC 12228 ATCC 12228 |
|---|---|---|---|---|
| Compound | MBC (µg/mL) | |||
| 3o | 128 | 16 | 16 | 8 |
| 3p | 256 | 128 | 128 | 128 |
| Compound | 3o | 3p | OX | AMP | NTF | STR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested Strain | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| naso-pharynx isolates | ||||||||||||
| S. aureus | ||||||||||||
| C4 | 32 | 64 | 256 | 256 | 0.25 | 0.25 | 512 | 512 | 16 | 16 | 8 | 8 |
| C7 | 32 | 64 | 256 | 256 | 0.25 | 0.25 | 64 | 128 | 16 | 16 | 8 | 16 |
| C8 | 32 | 64 | 256 | 256 | 0.25 | 0.25 | 512 | >512 | 16 | 16 | 8 | 16 |
| C19 | 32 | 64 | 256 | 256 | 0.5 | 0.25 | 64 | 128 | 32 | 32 | 8 | 8 |
| ulcers/furuncles isolates | ||||||||||||
| D12 | 32 | 64 | 256 | 256 | 0.5 | 0.5 | 256 | 256 | 32 | 32 | 8 | 16 |
| F1 | 32 | 64 | 256 | 256 | 0.25 | 0.25 | 128 | 128 | 32 | 32 | 8 | 8 |
| F7 | 32 | 64 | 256 | 256 | 0.25 | 0.25 | 4 | 4 | 16 | 16 | 8 | 16 |
| F12 | 32 | 64 | 256 | 256 | 0.25 | 0.25 | 64 | 128 | 16 | 32 | 8 | 32 |
| bone isolates | ||||||||||||
| D14 | 32 | 64 | 256 | 256 | 0.5 | 0.5 | 256 | 512 | 32 | 32 | 8 | 16 |
| D15 (MRSA) | 32 | 64 | 256 | 512 | >512 | >512 | >512 | >512 | 32 | 32 | 256 | >512 |
| D17 (MRSA) | 32 | 64 | 256 | 512 | >512 | >512 | >512 | >512 | 32 | 32 | 256 | >512 |
| D20 | 32 | 64 | 256 | 512 | 0.5 | 0.5 | 128 | 256 | 16 | 32 | 8 | 8 |
| Compound | IC30 (µg/mL)/(µM) L929 Cells | HeLa Cells |
|---|---|---|
| 3o | 45/187 | 42/174 |
| 3p | 103/483 | 71/333 |
| Cisplatin | 7.24/24 | 6.53/22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Pieczonka, A.M.; Rachwalski, M.; Leśniak, S.; Stączek, P. Synthesis and Evaluation of Biological Activities of Aziridine Derivatives of Urea and Thiourea. Molecules 2018, 23, 45. https://doi.org/10.3390/molecules23010045
Kowalczyk A, Pieczonka AM, Rachwalski M, Leśniak S, Stączek P. Synthesis and Evaluation of Biological Activities of Aziridine Derivatives of Urea and Thiourea. Molecules. 2018; 23(1):45. https://doi.org/10.3390/molecules23010045
Chicago/Turabian StyleKowalczyk, Aleksandra, Adam M. Pieczonka, Michał Rachwalski, Stanisław Leśniak, and Paweł Stączek. 2018. "Synthesis and Evaluation of Biological Activities of Aziridine Derivatives of Urea and Thiourea" Molecules 23, no. 1: 45. https://doi.org/10.3390/molecules23010045
APA StyleKowalczyk, A., Pieczonka, A. M., Rachwalski, M., Leśniak, S., & Stączek, P. (2018). Synthesis and Evaluation of Biological Activities of Aziridine Derivatives of Urea and Thiourea. Molecules, 23(1), 45. https://doi.org/10.3390/molecules23010045