Characterization of Odors of Wood by Gas Chromatography-Olfactometry with Removal of Extractives as Attempt to Control Indoor Air Quality
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Components
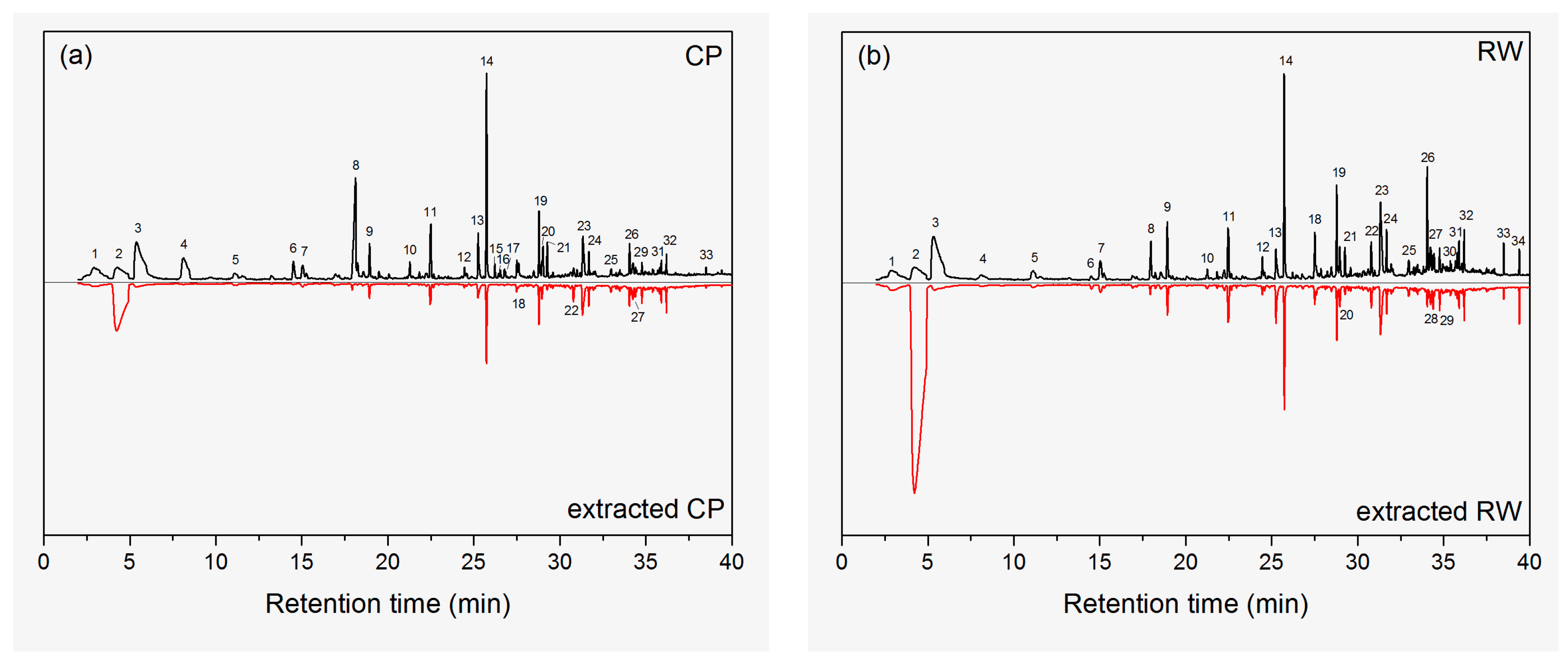
2.2. Identification of VOCs
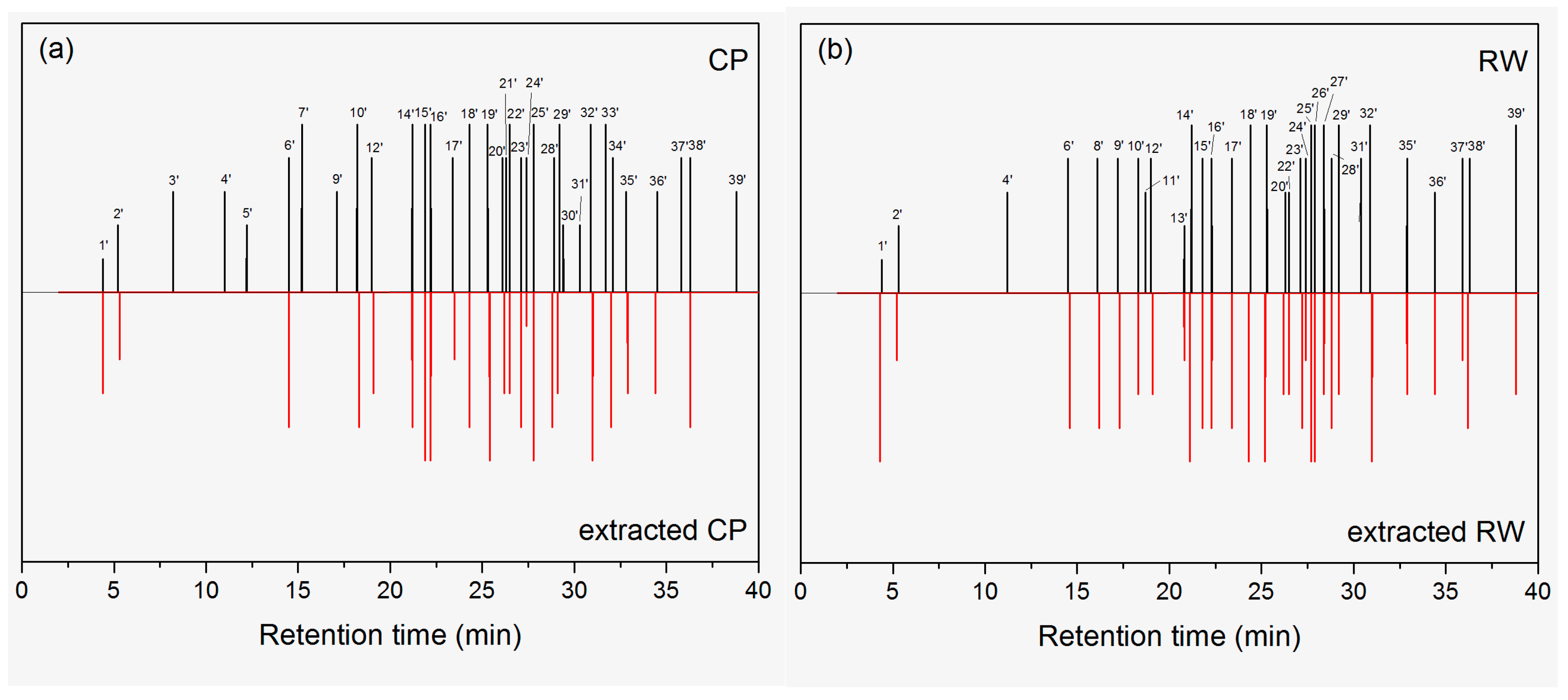
2.3. Characterization of Odors
3. Materials and Methods
3.1. Materials
3.2. Analyisis of Chemical Components
3.3. Odor Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kasper, P.L.; Mannebeck, D.; Oxbøl, A.; Nygaard, J.V.; Hansen, M.J.; Feilberg, A. Effects of dilution systems in olfactometry on the recovery of typical livestock odorants determined by PTR-MS. Sensors 2017, 17, 1859. [Google Scholar] [CrossRef] [PubMed]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Lan, C.; Wu, T. Investigation of indoor chemical pollutants and perceived odor in an area with complaints of unpleasant odors. Build. Environ. 2009, 44, 2106–2113. [Google Scholar] [CrossRef]
- Bitter, F.; Müller, B.; Müller, D. Estimation of odour intensity of indoor air pollutants from building materials with a multi-gas sensor system. Build. Environ. 2010, 45, 197–204. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Yao, Y.; Li, J. Indoor decorating and refurbishing materials and furniture volatile organic compounds emission labeling systems: A review. Chin. Sci. Bull. 2012, 57, 2533–2543. [Google Scholar] [CrossRef]
- Rosenkranz, H.S.; Cunningham, A.R. Environmental odors and health hazards. Sci. Total Environ. 2003, 313, 15–24. [Google Scholar] [CrossRef]
- Ka, M.H.; Choi, E.H.; Chun, H.S.; Lee, K.G. Antioxidative activity of volatile extracts isolated from Angelica tenuissimae roots, peppermint leaves, pine needles, and sweet flag leaves. J. Agric. Food Chem. 2005, 53, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Uçar, G.; Balaban, M. Volatile wood extractives of black pine (Pinus nigra Arnold) grown in Eastern Thrace. Eur. J. Wood Prod. 2001, 59, 301–305. [Google Scholar] [CrossRef]
- Xu, K.; Feng, J.; Zhong, T.; Zheng, Z.; Chen, T. Effects of volatile chemical components of wood species on mould growth susceptibility and termite attack resistance of wood plastic composites. Int. Biodeterior. Biodegrad. 2015, 100, 106–115. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Hanninen, T.; Kyyhkynen, A.; Ohlmeyer, M.; Siitonen, A.; Rautkari, L. Effect of volatile organic compounds from Pinus sylvestris and Picea abies on Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae and Salmonella enterica serovar Typhimurium. Holzforschung 2017, 71, 905–912. [Google Scholar] [CrossRef]
- Schreiner, L.; Beauchamp, J.; Buettner, A. Characterisation of odorants in wood and related products: Strategies, methodologies, and achievements. In Proceedings of the International Conference “Wood Science and Engineering in the Third Millennium”—ICWSE 2017, Brasov, Romania, 2–4 November 2017. [Google Scholar]
- Risholm-Sundman, M.; Lundgren, M.; Vestin, E.; Herder, P. Emissions of acetic acid and other volatile organic compounds from different species of solid wood. Holz Roh Werkst. 1998, 56, 125–129. [Google Scholar] [CrossRef]
- Manninen, A.M.; Pasanen, P.; Holopainen, J.K. Comparing the VOC emissions between air-dried and heat-treated Scots pine wood. Atmos. Environ. 2002, 36, 1763–1768. [Google Scholar] [CrossRef]
- Ekeberg, D.; Flæte, P.O.; Eikenes, M.; Fongen, M.; Naess-Andresen, C.F. Qualitative and quantitative determination of extractives in heartwood of Scots pine (Pinussylvestris L.) by gas chromatography. J. Chromatogr. A 2006, 1109, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, B.; Ters, T.; Srebotnik, E.; Rieder-Gradinger, C. Reduction of aldehydes and terpenes within pine wood by microbial activity. Holzforschung 2016, 70, 895–900. [Google Scholar] [CrossRef]
- Sassoli, M.; Taiti, C.; Nissim, W.G.; Costa, C.; Mancuso, S.; Menesatti, P.; Fioravanti, M. Characterization of VOC emission profile of different wood species during moisture cycles. iForest 2017, 10, 576–584. [Google Scholar] [CrossRef]
- Culleré, L.; de Simón, B.F.; Cadahía, E.; Ferreira, V.; Hernández-Orte, P.; Cacho, J. Characterization by gas chromatography–olfactometry of the most odor-active compounds in extracts prepared from acacia, chestnut, cherry, ash and oak woods. LWT-Food Sci. Technol. 2013, 53, 240–248. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Aroma-active compounds of American, French, Hungarian and Russian oak woods, studied by GC-MS and GC-O. Flavour Fragr. J. 2008, 23, 93–98. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Cao, T.; Shen, J. Identification of key odor compounds from three kinds of wood species. In Proceedings of the 2017 International Conference on Environmental Science and Sustainable Energy (ESSE2017), Suzhou, China, 23–25 June 2017. [Google Scholar]
- Schreiner, L.; Loos, H.M.; Buettner, A. Identification of odorants in wood of Calocedrus decurrens (Torr.) Florin by aroma extract dilution analysis and two-dimensional gas chromatography–mass spectrometry/olfactometry. Anal. Bioanal. Chem. 2017, 409, 3719–3729. [Google Scholar] [CrossRef] [PubMed]
- Ai, N.; Liu, H.; Wang, J.; Zhang, X.; Zhang, H.; Chen, H.; Huang, M.; Liu, Y.; Zheng, F.; Sun, B. Triple-channel comparative analysis of volatile flavour composition in raw whole and skim milk via electronic nose, GC-MS and GC-O. Anal. Methods 2015, 7, 4278–4284. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Khummueng, W.; Park, S.K. Identification of odor-active components of agarwood essential oils from Thailand by solid phase microextraction-GC/MS and GC-O. J. Essent. Oil Res. 2011, 23, 46–53. [Google Scholar] [CrossRef]
- Gao, W.; Fan, W.; Xu, Y. Characterization of the key odorants in light aroma type Chinese liquor by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2014, 62, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Soso, S.B.; Koziel, J.A. Analysis of odorants in marking fluid of siberian tiger (Panthera tigris altaica) using simultaneous sensory and chemical analysis with headspace solid-phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. Molecules 2016, 21, 834. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Fan, B.; Niu, Y.; Wu, M.; Liu, J.; Ma, S. Characterization of odor-active compounds of various Chrysanthemum essential oils by gas chromatography–olfactometry, gas chromatography–mass spectrometry and their correlation with sensory attributes. J. Chromatogr. B 2016, 1009–1010, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Denk, P.; Buettner, A. Sensory characterization and identification of odorous constituents in acrylic adhesives. Int. J. Adhes. Adhes. 2017, 78, 182–188. [Google Scholar] [CrossRef]
- Elsharif, S.A.; Buettner, A. Influence of the chemical structure on the odor characters of β-citronellol and its oxygenated derivatives. Food Chem. 2017, 232, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cao, J.; Cui, F.; Wang, X. Effect of pH on chemical components and mechanical properties of thermally modified wood. Wood Fiber Sci. 2012, 44, 46–53. [Google Scholar]
- Pelaez-Samaniego, M.R.; Yadama, V.; Lowell, E.; Amidon, T.E.; Chaffee, T.L. Hot water extracted wood fiber for production of wood plastic composites (WPCs). Holzforschung 2012, 67, 193–200. [Google Scholar] [CrossRef]
- Félix, J.S.; Domeño, C.; Nerín, C. Characterization of wood plastic composites made from landfill-derived plastic and sawdust: Volatile compounds and olfactometric analysis. Waste Manag. 2013, 33, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ezquerro, O.; Pons, B.; Tena, M.T. Development of a headspace solid-phase microextraction–gas chromatography–mass spectrometry method for the identification of odour-causing volatile compounds in packaging materials. J. Chromatogr. A 2002, 963, 381–392. [Google Scholar] [CrossRef]
- Ou, R.; Xie, Y.; Wolcott, M.P.; Sui, S.; Wang, Q. Morphology, mechanical properties, and dimensional stability of wood particle/high density polyethylene composites: Effect of removal of wood cell wall composition. Mater. Des. 2014, 58, 339–345. [Google Scholar] [CrossRef]
Sample Availability: All the Samples of the compounds are available from the authors. |

| Labels | Extractives (%) * | Holocelluloses (%) | α-Cellulose (%) | Lignin (%) |
|---|---|---|---|---|
| CP | 2.87 (0.02) | 61.33 (0.01) | 44.24 (0.01) | 23.26 (0.03) |
| RW | 4.67 (0.03) | 67.11 (0.02) | 43.78 (0.02) | 26.07 (0.01) |
| Peak Number | Retention Time (min) | Compounds | Classification | Percentage (%) * | |||
|---|---|---|---|---|---|---|---|
| CP | Extracted CP | RW | Extracted RW | ||||
| 1 | 2.92 | Ethanol | Alcohol | 2.3 (0.2) | 1.4 | 1.9 (0.1) | 1.0 |
| 2 | 4.23 | Benzene | Aromatic | 5.6 (0.6) | 32.8 (0.5) | 5.9 (0.2) | 68.5 (0.9) |
| 3 | 5.41 | Acetic acid | Carboxylic acid | 14.8 (0.2) | 1.0 | 16.3 (0.2) | 1.0 |
| 4 | 8.10 | Hexanal | Aldehyde | 6.6 (0.2) | - | 1.2 | 0.3 |
| 5 | 11.13 | Furfural | Aldehyde | 1.0 | - | 1.5 (0.1) | 0.3 |
| 6 | 14.50 | Pentanoic acid | Carboxylic acid | 1.8 | - | 0.4 | 0.3 |
| 7 | 15.06 | 1,3-Dichlorobenzene | Aromatic | 1.6 | - | 2.1 (0.2) | 0.5 |
| 8 | 18.12 | Hexanoic acid | Carboxylic acid | 10.8 (0.4) | 0.8 | 1.9 (0.1) | 0.3 |
| 9 | 18.94 | Nonanal | Aldehyde | 2.2 (0.2) | 1.3 | 2.8 (0.2) | 1.0 |
| 10 | 21.32 | Heptanoic acid | Carboxylic acid | 0.9 | - | 0.4 | 0.2 |
| 11 | 22.50 | Tridecane | Alkane | 3.5 (0.2) | 3.0 (0.2) | 3.2 (0.1) | 1.5 |
| 12 | 24.46 | Octanoic acid | Carboxylic acid | 0.5 | - | 0.9 | 0.2 |
| 13 | 25.26 | (Z)-2-Decenal | Aldehyde | 2.8 (0.2) | 2.1 (0.1) | 2.2 (0.1) | 1.6 (0.1) |
| 14 | 25.73 | Tetradecane | Alkane | 9.2 (0.5) | 9.0 (0.5) | 8.3 (0.1) | 3.2 (0.1) |
| 15 | 26.21 | Hexyltrimethoxysilane | Miscellaneous | 0.2 | - | - | - |
| 16 | 26.53 | 4-Oxononanal | Aldehyde | 0.2 | - | - | - |
| 17 | 26.78 | (E)-2-Decenal | Aldehyde | 0.7 | - | - | - |
| 18 | 27.51 | Nonanoic acid | Carboxylic acid | 0.9 | 0.4 | 2.3 (0.2) | 0.6 |
| 19 | 28.78 | Pentadecane | Alkane | 3.0 (0.2) | 4.0 (0.3) | 3.5 (0.2) | 1.3 |
| 20 | 29.02 | Dodecanal | Aldehyde | 2.2 (0.1) | 1.6 (0.1) | 1.4 | 0.6 |
| 21 | 29.27 | n-Octyltriethoxysilane | Miscellaneous | 1.5 | - | 1.2 | 0.2 |
| 22 | 30.79 | (E)-6,10-Dimethyl-5,9-undecadien-2-one | Ketone | 0.7 | 1.2 | 1.4 | 0.7 |
| 23 | 31.35 | 1-Dodecanol | Alcohol | 3.6 (0.1) | 3.1 (0.1) | 6.8 (0.3) | 3.0 (0.2) |
| 24 | 31.69 | Hexadecane | Alkane | 1.3 | 2.0 | 1.9 | 0.7 |
| 25 | 32.98 | 2,6,10-Trimethyl-pentadecane | Alkane | 0.5 | 1.0 | 1.0 | 0.4 |
| 26 | 34.05 | 2,4-di-tert-Butylphenol | Alcohol | 1.0 | 0.7 | 3.4 (0.1) | 0.4 |
| 27 | 34.25 | 2,6,10,14-Tetramethyl-pentadecane | Alkane | 1.1 | 2.3 | 1.3 | 0.8 |
| 28 | 34.45 | Tetradecanal | Aldehyde | - | - | 0.7 | 0.4 |
| 29 | 34.76 | Cedrol | Alcohol | 0.6 | 1.2 | 1.0 | 0.6 |
| 30 | 35.40 | 2-Methyl-hetadecane | Alkane | - | - | 0.5 | - |
| 31 | 35.90 | (4-Octyldodecyl)-cyclopentane | Alkane | 0.5 | 0.9 | 1.1 | 0.4 |
| 32 | 36.20 | Benzoic acid 2-ethylhexyl ester | Ester | 0.7 | 1.2 | 1.3 | 0.6 |
| 33 | 38.51 | 1,2-Benzenedicarboxylic acid bis(2-methylpropyl) ester | Ester | 0.2 | - | 0.7 | 0.2 |
| 34 | 39.40 | Dibutyl phthalate | Ester | - | - | 0.6 | 0.5 |
| Peak Number in GC-O/MS | Retention Time (min) | Odorant | Odor Descriptor | Intensity | |||
|---|---|---|---|---|---|---|---|
| CP | Extracted CP | RW | Extracted RW | ||||
| 1’/2 | 4.4 | Benzene | Gasoline, solvent | 1 | 3 | 1 | 5 |
| 2’/3 | 5.2 | Acetic acid | Irritant, sour | 2 | 2 | 2 | 2 |
| 3’/4 | 8.2 | Hexanal | Bitter, green | 3 | 0 | 0 | 0 |
| 4’/5 | 11.1 | Furfural | Nut | 3 | 0 | 3 | 0 |
| 5’/- | 12.2 | 2-Furan-carboxaldehyde | Special, grain | 2 | 0 | 0 | 0 |
| 6’/6 | 14.5 | Pentanoic acid | Cheese, paint | 4 | 4 | 4 | 4 |
| 7’/- | 15.2 | Octanal | Fruit, soap | 5 | 0 | 0 | 0 |
| 8’/- | 16.1 | 5-Methyl-2-furan-carboxaldehyde | Chocolate, coconut | 0 | 0 | 4 | 4 |
| 9’/- | 17.1 | Decamethyl-cyclopentasiloxane | Bread, nut | 3 | 0 | 4 | 4 |
| 10’/8 | 18.2 | Hexanoic acid | Cheese, fishy | 5 | 4 | 4 | 3 |
| 11’/- | 18.7 | 2-Octenal | Bitter | 0 | 0 | 3 | 0 |
| 12’/9 | 19.0 | Nonanal | Flower, fat | 4 | 3 | 4 | 3 |
| 13’/- | 20.8 | α-Methyl-α-2,5,7-octatrienyl-benzenemethanol | Bitter, nut | 0 | 0 | 2 | 2 |
| 14’/10 | 21.2 | Heptanoic acid | Stink, leather, bug | 5 | 4 | 5 | 5 |
| 15’/- | 21.9 | 2-Nonenal | Stink, fat, iris | 5 | 5 | 4 | 4 |
| 16’/- | 22.2 | 1-Methoxy-4-(2-propenyl)-benzene | Bitter, herb, burnt | 5 | 5 | 4 | 4 |
| 17’/- | 23.4 | 5,5,8-Trimethyl-3,6,7-nonatrien-2-one | Stink, chemical, bug | 4 | 2 | 4 | 4 |
| 18’/12 | 24.4 | Octanoic acid | Stink, fat, bug | 5 | 4 | 5 | 5 |
| 19’/13 | 25.3 | (Z)-2-Decenal | Stink, fat, herb | 5 | 5 | 5 | 5 |
| 20’/15 | 26.1 | Hexyltrimethoxysilane | Irritant, stink, herb | 4 | 3 | 3 | 3 |
| 21’/- | 26.3 | Hexadecanoic acid | Fruit, sour | 4 | 0 | 0 | 0 |
| 22’/16 | 26.5 | 4-Oxononanal | Herb | 5 | 3 | 3 | 3 |
| 23’/17 | 27.0 | (E)-2-Decenal | Kerosene, herb | 4 | 4 | 4 | 4 |
| 24’/18 | 27.4 | Nonanoic acid | Milk | 4 | 1 | 4 | 2 |
| 25’/- | 27.8 | (E,E)-2,4-Decadienal | Animal, burnt | 5 | 5 | 5 | 5 |
| 26’/- | 27.9 | 3,7-Dimethyl-2,6-octadien-1-ol, acetate | Stink, bug | 0 | 0 | 5 | 5 |
| 27’/- | 28.4 | 2-Undecenal | Citrus, fishy, herb | 0 | 0 | 5 | 3 |
| 28’/20 | 28.8 | Dodecanal | Mint, pungent | 4 | 4 | 4 | 4 |
| 29’/21 | 29.2 | n-Octyl-triethoxysilane | Burnt, bug | 5 | 3 | 5 | 3 |
| 30’/- | 29.4 | 2-Methylpropanoic acid, 3-hydroxy-2,4,4-trimethylpentyl ester | Sour, bitter, herb | 2 | 0 | 0 | 0 |
| 31’/- | 30.3 | Decanoic acid | Herb | 2 | 0 | 4 | 0 |
| 32’/- | 31.0 | 8-Methyl-1-undecene | Paint, dirt | 5 | 5 | 5 | 5 |
| 33’/- | 31.7 | 1-Tetradecanol | Dirt, bronze | 5 | 0 | 0 | 0 |
| 34’/- | 32.1 | 1-Pentadecanol | Bronze | 4 | 4 | 0 | 0 |
| 35’/25 | 32.8 | 2,6,10-Trimethyl-pentadecane | Gasoline, bronze | 3 | 3 | 4 | 3 |
| 36’/29 | 34.6 | Cedrol | Fat, wood | 3 | 3 | 3 | 3 |
| 37’/- | 35.9 | Octadecanal | Bronze, grease | 4 | 0 | 4 | 2 |
| 38’/32 | 36.3 | Benzoic acid, 2-ethylhexyl ester | Milk, fruit | 4 | 4 | 4 | 4 |
| 39’/33 | 38.8 | 1,2-Benzene dicarboxylic acid, bis(2-methylpropyl) ester | Cake, grass | 3 | 0 | 5 | 3 |
| Label | Wood | Scientific Name | Average Growth Ring Width (cm) | Density (g/cm3) * | Origin of Place |
|---|---|---|---|---|---|
| CP | Cathy poplar | Populus cathayana Rehd. | 0.6 | 0.49–0.52 | Hebei, China |
| RW | Rubberwood | Hevea brasiliensis | 0.2 | 0.64–0.67 | Hainan, China |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Wang, C.; Huang, A.; Lv, B. Characterization of Odors of Wood by Gas Chromatography-Olfactometry with Removal of Extractives as Attempt to Control Indoor Air Quality. Molecules 2018, 23, 203. https://doi.org/10.3390/molecules23010203
Liu R, Wang C, Huang A, Lv B. Characterization of Odors of Wood by Gas Chromatography-Olfactometry with Removal of Extractives as Attempt to Control Indoor Air Quality. Molecules. 2018; 23(1):203. https://doi.org/10.3390/molecules23010203
Chicago/Turabian StyleLiu, Ru, Chen Wang, Anmin Huang, and Bin Lv. 2018. "Characterization of Odors of Wood by Gas Chromatography-Olfactometry with Removal of Extractives as Attempt to Control Indoor Air Quality" Molecules 23, no. 1: 203. https://doi.org/10.3390/molecules23010203
APA StyleLiu, R., Wang, C., Huang, A., & Lv, B. (2018). Characterization of Odors of Wood by Gas Chromatography-Olfactometry with Removal of Extractives as Attempt to Control Indoor Air Quality. Molecules, 23(1), 203. https://doi.org/10.3390/molecules23010203