The Effect of Resveratrol on Cell Viability in the Burkitt’s Lymphoma Cell Line Ramos
Abstract
1. Introduction
2. Results
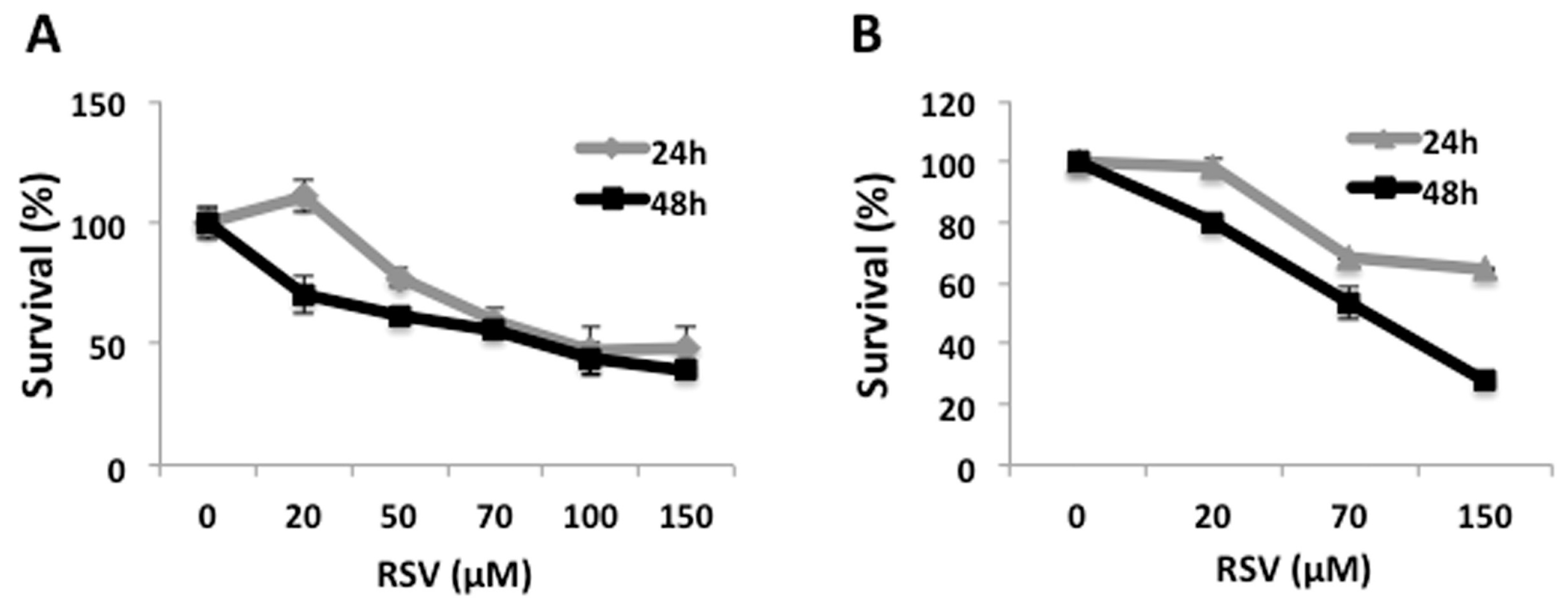
2.1. RSV Induces Decrease in Cell Viability in Ramos Cells
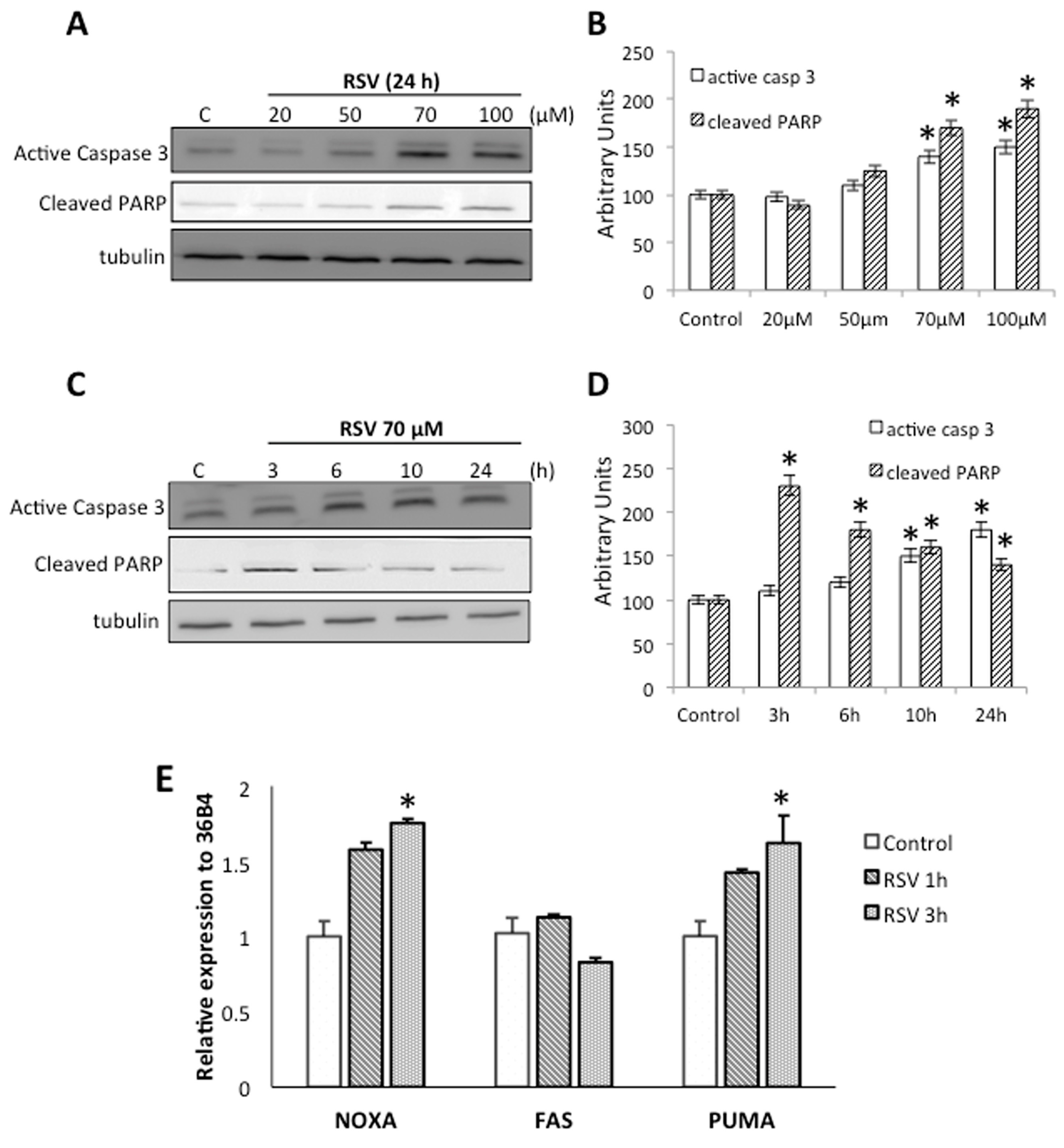
2.2. RSV Induce Apoptotic Cell Death in Ramos Cells
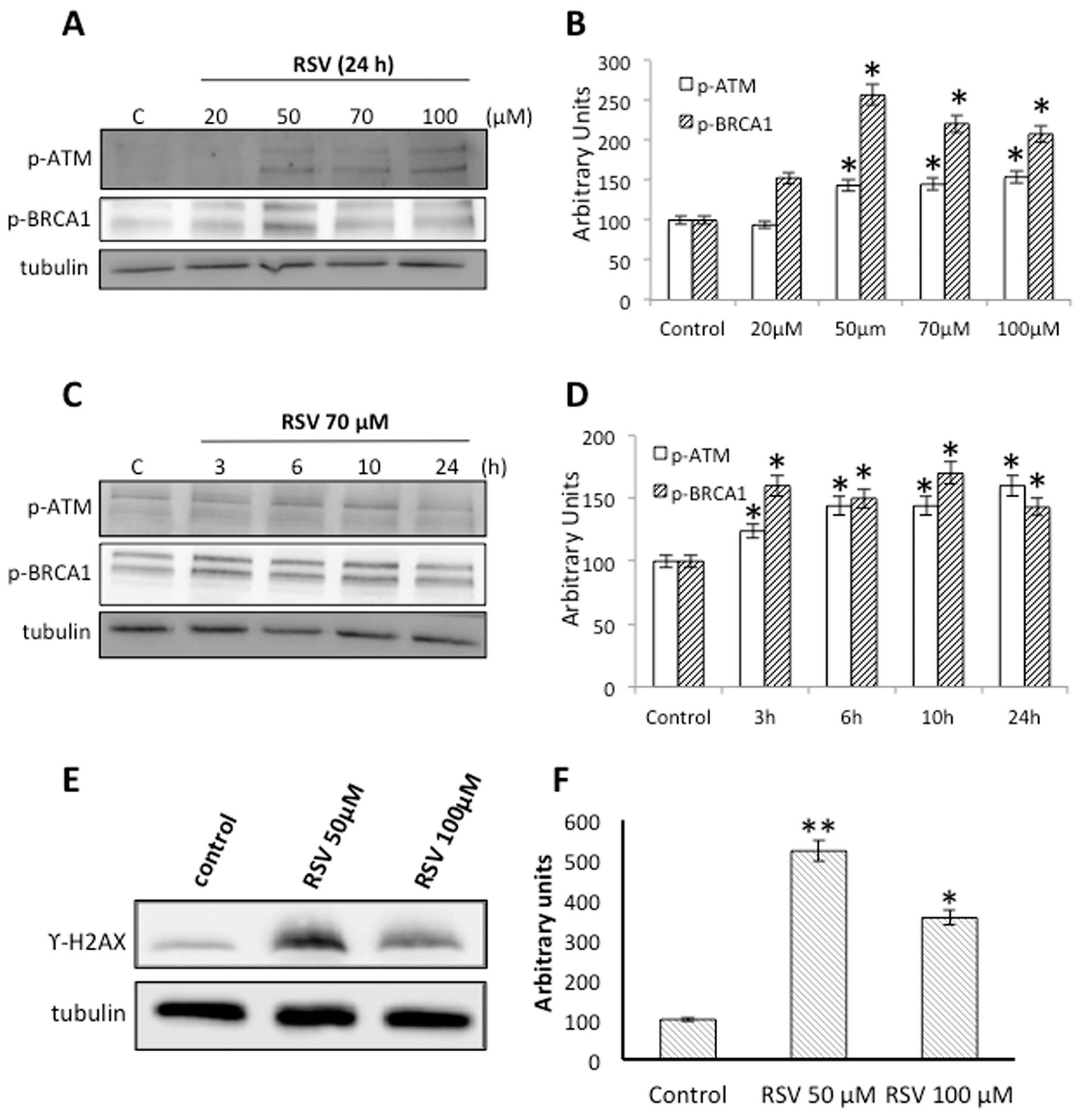
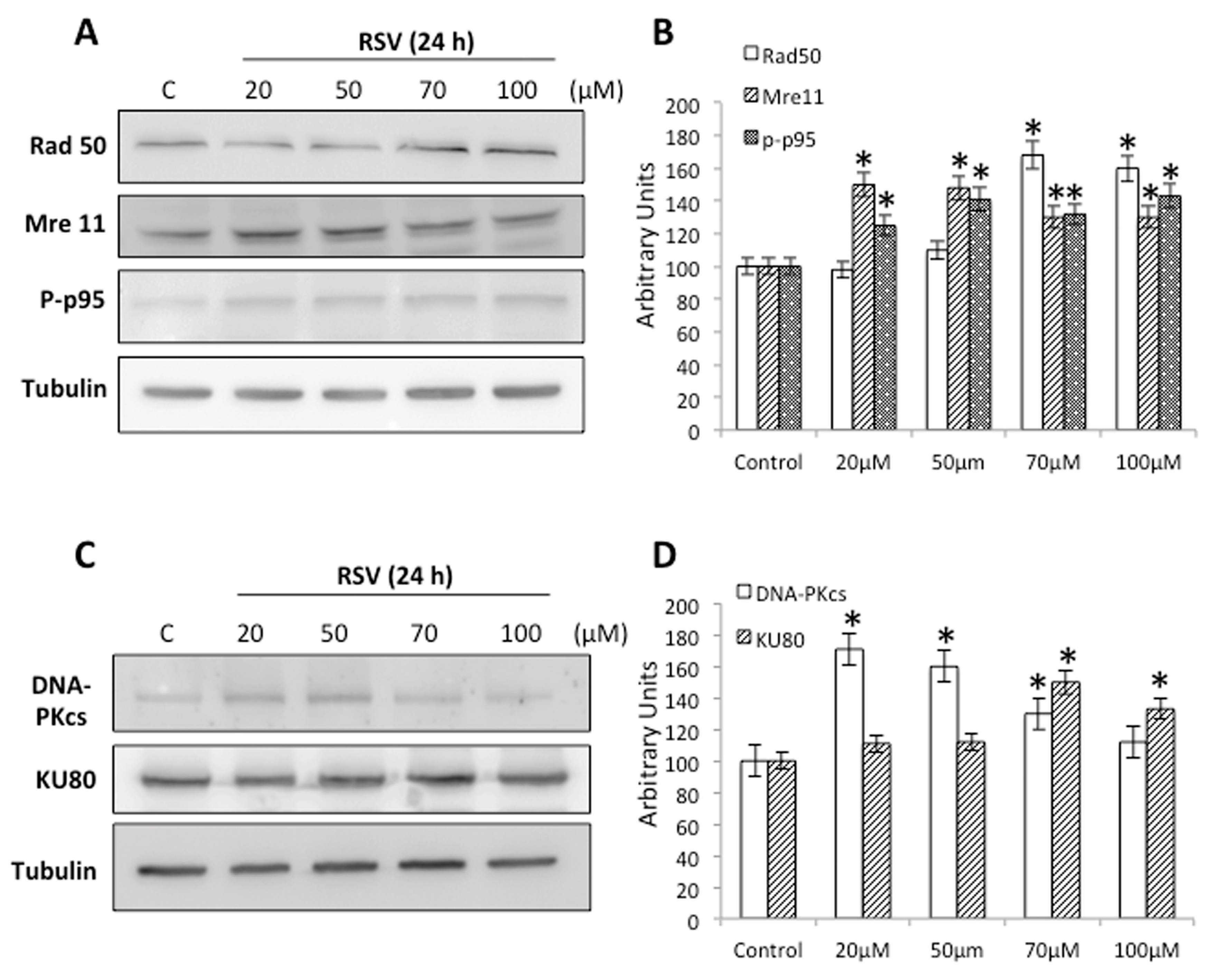
2.3. RSV Induces DNA Damage and DNA Repair in Ramos Cells
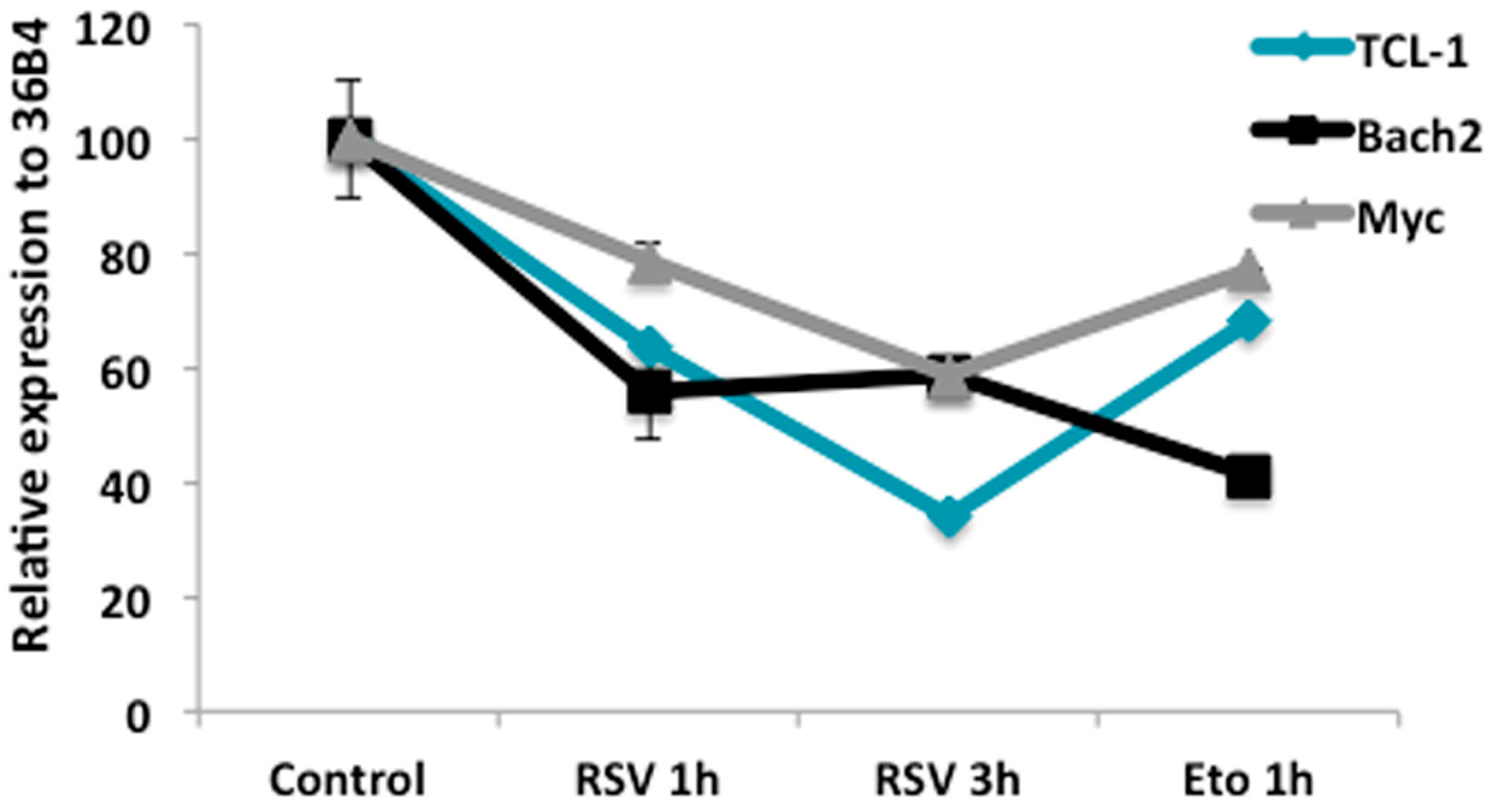
2.4. RSV Regulates Gene Expression Related to Proliferation and B Cell Differentiation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assays
4.3. Western Blot Analysis
4.4. Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-qPCR)
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeandet, P.; Bessis, R.; Maume, B.F.; Meunier, P.; Peyron, D.; Trollat, P. Effect of Enological Practices on the Resveratrol Isomer Content of Wine. J. Agric. Food Chem. 1995, 43, 316–319. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clement, C.; Courot, E. Biosynthesis, metabolism, molecular engineering and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Hebrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Baur, J.A. Resveratrol and life extension. Ann. N. Y. Acad. Sci. 2011, 1215, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 431. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Fauconneau, B.; WaffoTeguo, P.; Huguet, F.; Barrier, L.; Decendit, A.; Merillon, J.M. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997, 61, 2103–2110. [Google Scholar] [CrossRef]
- Juan, M.E.; Alfaras, I.; Planas, J.M. Colorectal cancer chemoprevention by trans-resveratrol. Pharmacol. Res. 2012, 65, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Su, Y.C.; Chen, N.C.; Hsieh, H.S.; Chen, K.S. Resveratrol inhibits migration and invasion of human breast-cancer cells. Mol. Nutr. Food Res. 2008, 52, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Kumar, R.; Ahmad, N. Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms. Int. J. Oncol. 2003, 23, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, Y.J.; Im, J.H.; Won, S.Y.; Kim, Y.B.; Cho, M.K.; Nam, H.S.; Choi, Y.J.; Lee, S.H. Synergistic anti-cancer effects of resveratrol and chemotherapeutic agent clofarabine against human malignant mesothelioma MSTO-211H cells. Food Chem. Toxicol. 2013, 52, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Can, G.; Cakir, Z.; Kartal, M.; Gunduz, U.; Baran, Y. Apoptotic Effects of Resveratrol, a Grape Polyphenol, on Imatinib-Sensitive and Resistant K562 Chronic Myeloid Leukemia Cells. Anticancer Res. 2012, 32, 2673–2678. [Google Scholar] [PubMed]
- Iguchi, K.; Toyama, T.; Ito, T.; Shakui, T.; Usui, S.; Oyama, M.; Iinuma, M.; Hirano, K. Antiandrogenic Activity of Resveratrol Analogs in Prostate Cancer LNCaP Cells. J. Androl. 2012, 33, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Uddin, S.; Bu, R.; Khan, O.S.; Ahmed, S.O.; Ahmed, M.; Al-Kuraya, K.S. Resveratrol suppresses constitutive activation of AKT via generation of ROS and induces apoptosis in diffuse large B cell lymphoma cell lines. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nakazato, T.; Xian, M.J.; Sayawa, M.; Ikeda, Y.; Kizaki, M. Resveratrol induces apoptosis of human malignant B cells by activation of caspase-3 and p38 MAP kinase pathways. Biochem. Pharmacol. 2006, 71, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Faber, A.C.; Chiles, T.C. Resveratrol induces apoptosis in transformed follicular lymphoma OCI-LY8 cells: Evidence for a novel mechanism involving inhibition of BCL6 signaling. Int. J. Oncol. 2006, 29, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Dey, A.; Sen, R.; Chatterjee, M.; Chattopadhyay, S.; Bandyopadhyay, S.K. Intracellular GSH Depletion Triggered Mitochondrial Bax Translocation to Accomplish Resveratrol-Induced Apoptosis in the U937 Cell Line. J. Pharmacol. Exp. Ther. 2011, 336, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.S.; Bhat, A.A.; Krishnankutty, R.; Mohammad, R.M.; Uddin, S. Therapeutic Potential of Resveratrol in Lymphoid Malignancies. Nutr. Cancer 2016, 68, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Solary, E.; Latruffe, N. Resveratrol, a phytochemical inducer of multiple cell death pathways: Apoptosis, autophagy and mitotic catastrophe. Curr. Med. Chem. 2011, 18, 1100–11021. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Singh, G.; Srivastava, R.K. Chemoprevention by resveratrol: Molecular mechanisms and therapeutic potential. Front. Biosci. 2007, 12, 4839–4854. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Choi, Y.J.; Suh, S.I.; Baek, W.K.; Suh, M.H.; Jin, I.N.; Min, D.S.; Woo, J.H.; Chang, J.S.; Passaniti, A.; et al. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis 2001, 22, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.V.; Hirpara, J.L.; Chawdhury, S.H.; Pervaiz, S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998, 92, 996–1002. [Google Scholar] [PubMed]
- Krammer, P.H. CD95’s deadly mission in the immune system. Nature 2000, 407, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.A.; Hermoso, M.A.; Pozo-Guisado, E.; Fernandez-Salguero, P.M.; Castellon, E.A. Regulation of cell survival by resveratrol involves inhibition of NF kappa B-regulated gene expression in prostate cancer cells. Prostate 2009, 69, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Nonn, L.; Duong, D.; Peehl, D.M. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis 2007, 28, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, C.Y.; Huang, J.; Hong, L.; Zhang, L.; Chu, Z.B. Antimyeloma effects of resveratrol through inhibition of angiogenesis. Chin. Med. J. 2007, 120, 1672–1677. [Google Scholar] [PubMed]
- Lee, J.H.; Guo, Z.; Myler, L.R.; Zheng, S.; Paull, T.T. Direct activation of ATM by resveratrol under oxidizing conditions. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, B.; Hermant, M.; Castrogiovanni, C.; Staudt, C.; Dumont, P. Resveratrol induces DNA damage in colon cancer cells by poisoning topoisomerase II and activates the ATM kinase to trigger p53-dependent apoptosis. Toxicol. In Vitro 2015, 29, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, A.; Marko, D. Resveratrol modulates the topoisomerase inhibitory potential of doxorubicin in human colon carcinoma cells. Molecules 2014, 19, 20054–20072. [Google Scholar] [CrossRef] [PubMed]
- Basso, E.; Fiore, M.; Leone, S.; Degrassi, F.; Cozzi, R. Effects of resveratrol on topoisomerase II-alpha activity: Induction of micronuclei and inhibition of chromosome segregation in CHO-K1 cells. Mutagenesis 2013, 28, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Bredemeyer, A.L.; Helmink, B.A.; Innes, C.L.; Calderon, B.; McGinnis, L.M.; Mahowald, G.K.; Gapud, E.J.; Walker, L.M.; Collins, J.B.; Weaver, B.K.; et al. DNA double-strand breaks activate a multi-functional genetic program in developing lymphocytes. Nature 2008, 456, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Kuraishy, A.I.; Deshpande, C.; Hong, J.S.; Cacalano, N.A.; Gatti, R.A.; Manis, J.P.; Damore, M.A.; Pellegrini, M.; Teitell, M.A. AID-induced genotoxic stress promotes B cell differentiation in the germinal center via ATM and LKB1 signaling. Mol. Cell 2010, 39, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.C.; Teitell, M. B-cell differentiation stimulated by physiologic DNA double strand breaks. Cell Cycle 2011, 10, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Jazirehi, A.R.; Bonavida, B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol. Cancer Ther. 2004, 3, 71–84. [Google Scholar] [PubMed]
- Ghorbani, A.; Zand, H.; Jeddi-Tehrani, M.; Koohdani, F.; Shidfar, F.; Keshavarz, S.A. PTEN over-expression by resveratrol in acute lymphoblastic leukemia cells along with suppression of AKT/PKB and ERK1/2 in genotoxic stress. J. Nat. Med. 2015, 69, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Jeggo, P.A. DNA double-strand break repair in a cellular context. Clin. Oncol. (R. Coll. Radiol.) 2014, 26, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Baer, R.; Gautier, J. DNA double-strand break repair pathway choice and cancer. DNA Repair 2014, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Faber, A.C.; Dufort, F.J.; Blair, D.; Wagner, D.; Roberts, M.F.; Chiles, T.C. Inhibition of phosphatidylinositol 3-kinase-mediated glucose metabolism coincides with resveratrol-induced cell cycle arrest in human diffuse large B-cell lymphomas. Biochem. Pharmacol. 2006, 72, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Gao, Y.Y.; Liu, B.Q.; Niu, X.F.; Zhuang, Y.; Wang, H.Q. Resveratrol-induced cytotoxicity in human Burkitt’s lymphoma cells is coupled to the unfolded protein response. BMC Cancer 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, D.; Zamo, A.; Parisi, A.; Bianchi, E.; Parolini, C.; Timperio, A.M.; Zolla, L.; Chilosi, M. Induction of apoptosis in Jeko-1 mantle cell lymphoma cell line by resveratrol: A proteomic analysis. J. Proteome Res. 2008, 7, 2670–2680. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Obando, P.; Ojeda, L.; Ojeda, P.; Perez, A.; Vargas-Uribe, M.; Rivas, C.I.; Vera, J.C.; Reyes, A.M. Resolution of the direct interaction with and inhibition of the human GLUT1 hexose transporter by resveratrol from its effect on glucose accumulation. Am. J. Physiol. Cell. Physiol. 2013, 305, C90–C99. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, S.; Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.A.; Meek, K. Choosing the right path: Does DNA-PK help make the decision? Mutat. Res. 2011, 711, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.R.; Ferguson, D.O.; Renard, M.; Hoyer, K.K.; Kim, U.; Hao, X.; Alt, F.W.; Roeder, R.G.; Morse, H.C., 3rd; Teitell, M.A. Dysregulated TCL1 requires the germinal center and genome instability for mature B-cell transformation. Blood 2006, 108, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Huang, C.; Geng, H.; Chen, Z.; Harvey, R.; Kang, H.; Ng, C.; Titz, B.; Hurtz, C.; Sadiyah, M.F.; et al. BACH2 mediates negative selection and p53-dependent tumor suppression at the pre-B cell receptor checkpoint. Nat. Med. 2013, 19, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.A.; Kirchhof, N.; Carlson, C.S.; Van Ness, B.G. Targeted overexpression of an activated N-ras gene results in B-cell and plasma cell lymphoproliferation and cooperates with c-myc to induce fatal B-cell neoplasia. Exp. Hematol. 2012, 40, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kuraishy, A.I.; French, S.W.; Sherman, M.; Herling, M.; Jones, D.; Wall, R.; Teitell, M.A. TORC2 regulates germinal center repression of the TCL1 oncoprotein to promote B cell development and inhibit transformation. Proc. Natl. Acad. Sci. USA 2007, 104, 10175–10180. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound resveratrol (RSV), and the antibodies are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara, P.; Spies, J.; Cárcamo, C.; Arancibia, Y.; Vargas, G.; Martin, C.; Salas, M.; Otth, C.; Zambrano, A. The Effect of Resveratrol on Cell Viability in the Burkitt’s Lymphoma Cell Line Ramos. Molecules 2018, 23, 14. https://doi.org/10.3390/molecules23010014
Jara P, Spies J, Cárcamo C, Arancibia Y, Vargas G, Martin C, Salas M, Otth C, Zambrano A. The Effect of Resveratrol on Cell Viability in the Burkitt’s Lymphoma Cell Line Ramos. Molecules. 2018; 23(1):14. https://doi.org/10.3390/molecules23010014
Chicago/Turabian StyleJara, Paola, Johana Spies, Constanza Cárcamo, Yennyfer Arancibia, Gabriela Vargas, Carolina Martin, Mónica Salas, Carola Otth, and Angara Zambrano. 2018. "The Effect of Resveratrol on Cell Viability in the Burkitt’s Lymphoma Cell Line Ramos" Molecules 23, no. 1: 14. https://doi.org/10.3390/molecules23010014
APA StyleJara, P., Spies, J., Cárcamo, C., Arancibia, Y., Vargas, G., Martin, C., Salas, M., Otth, C., & Zambrano, A. (2018). The Effect of Resveratrol on Cell Viability in the Burkitt’s Lymphoma Cell Line Ramos. Molecules, 23(1), 14. https://doi.org/10.3390/molecules23010014





