New Thiazoline-Tetralin Derivatives and Biological Activity Evaluation
Abstract
:1. Introduction
2. Results and Discussion
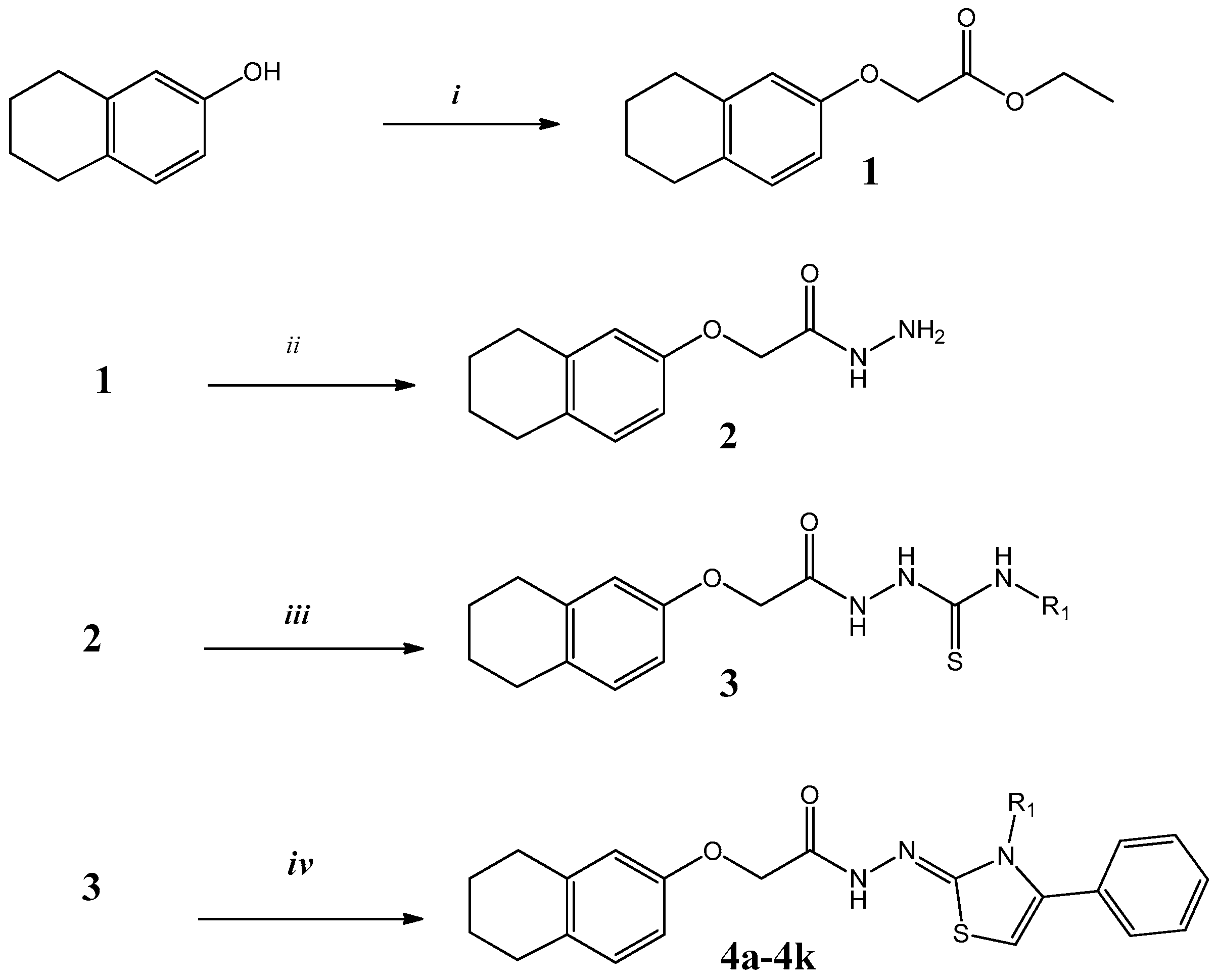
2.1. Chemistry
2.2. Biological Results
2.2.1. Cytotocity
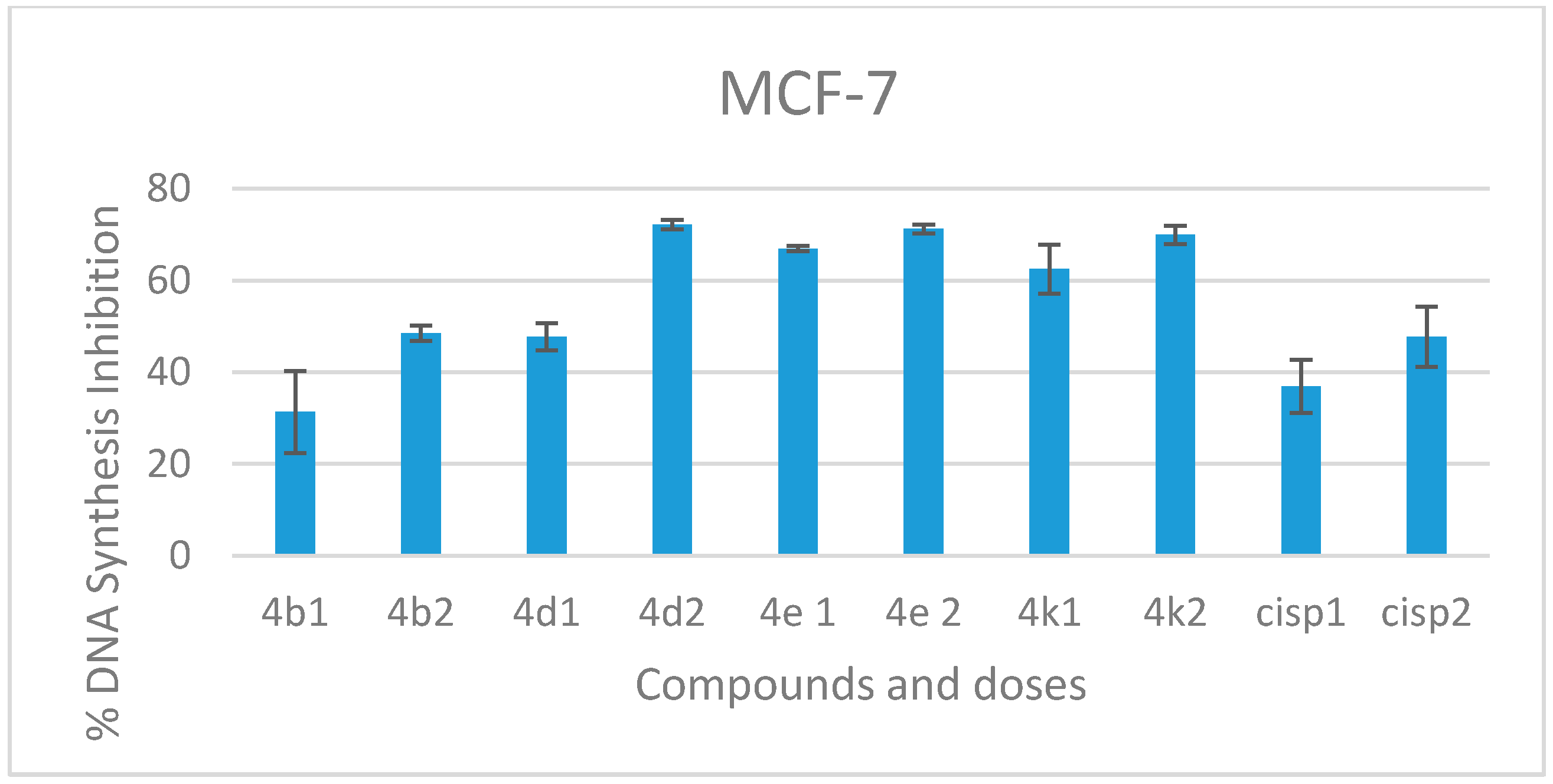
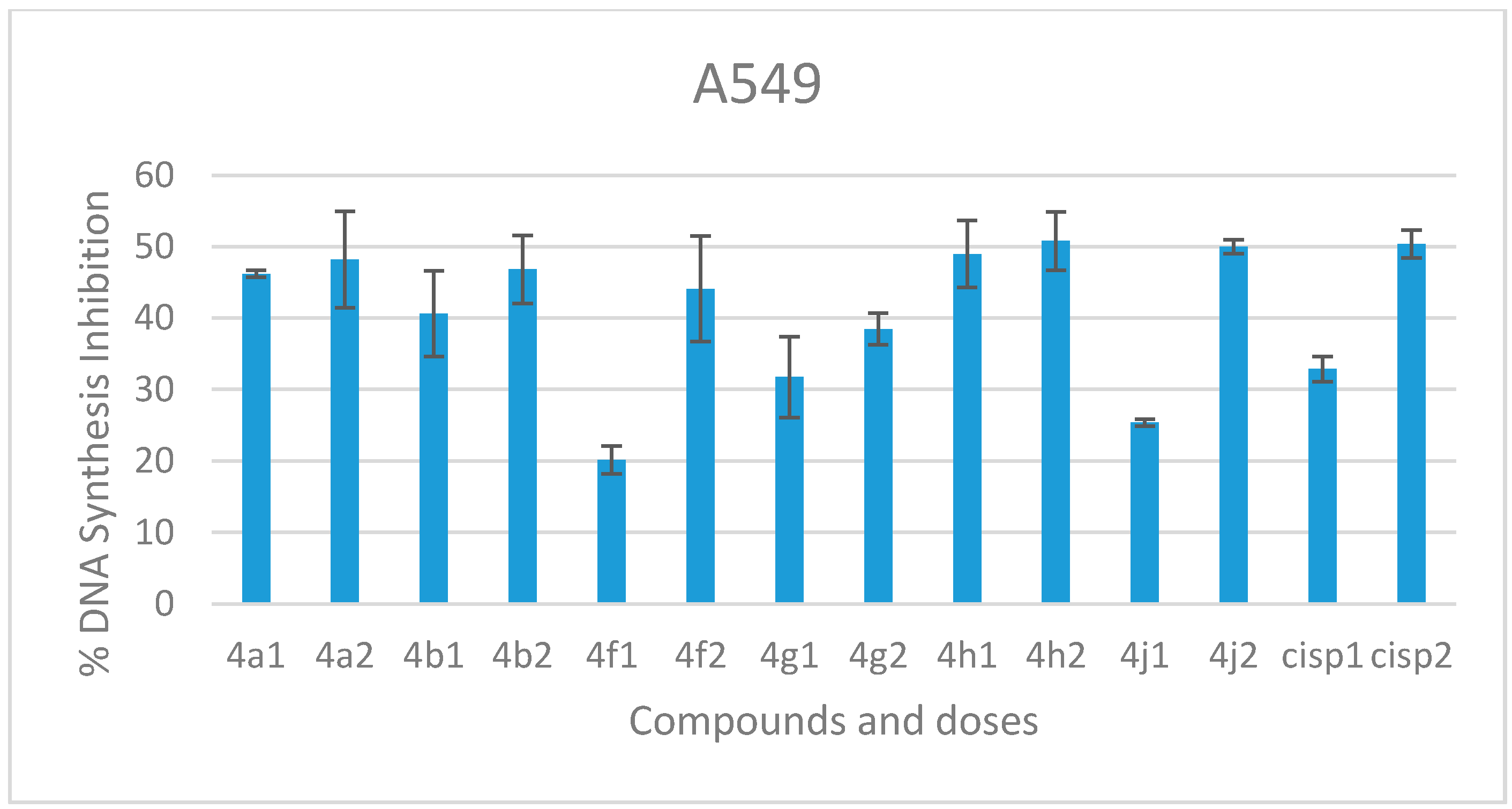
2.2.2. DNA Synthesis Inhibition
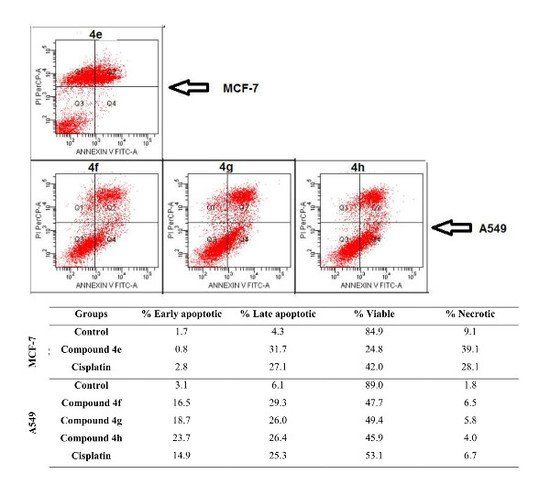
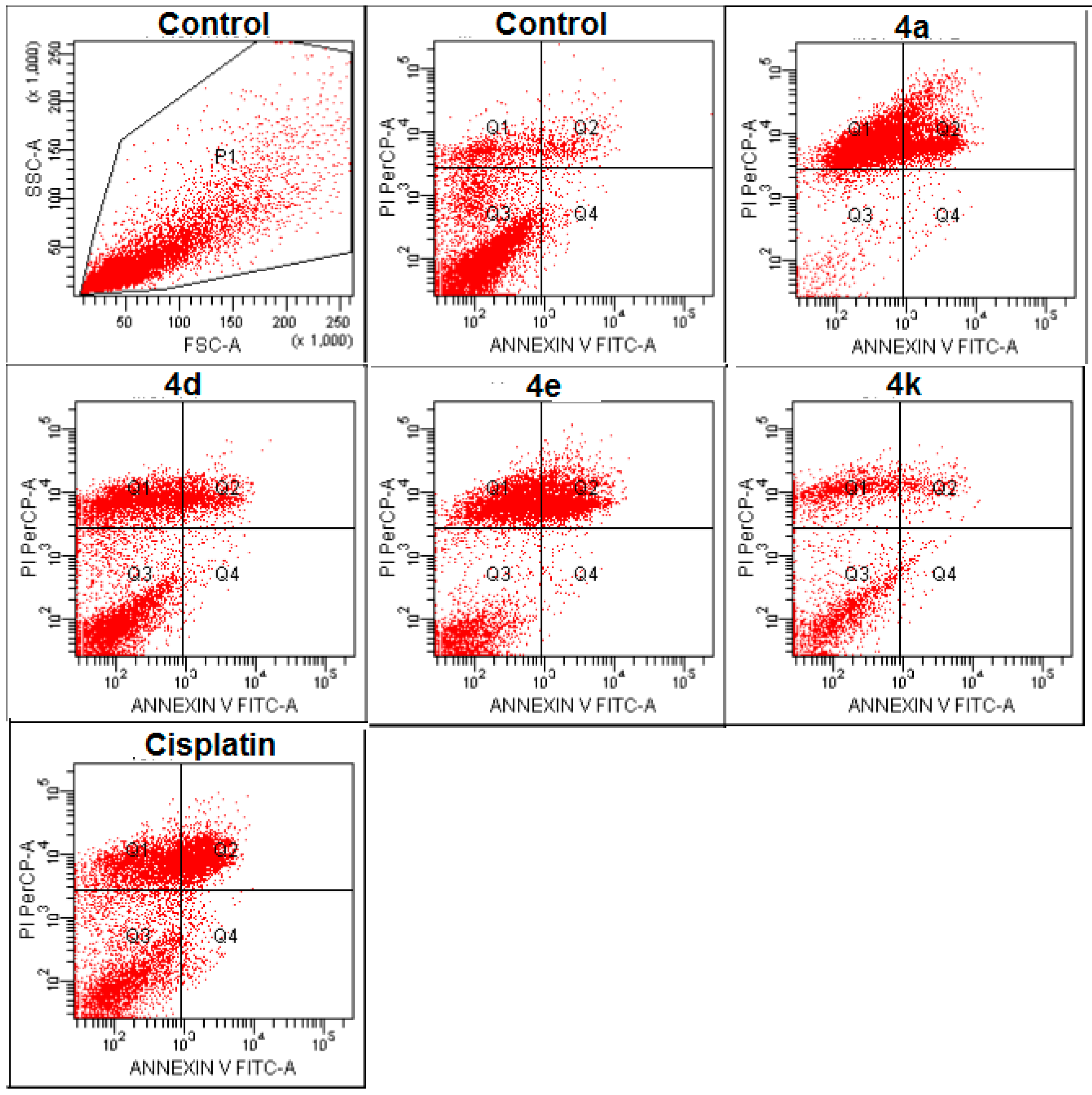
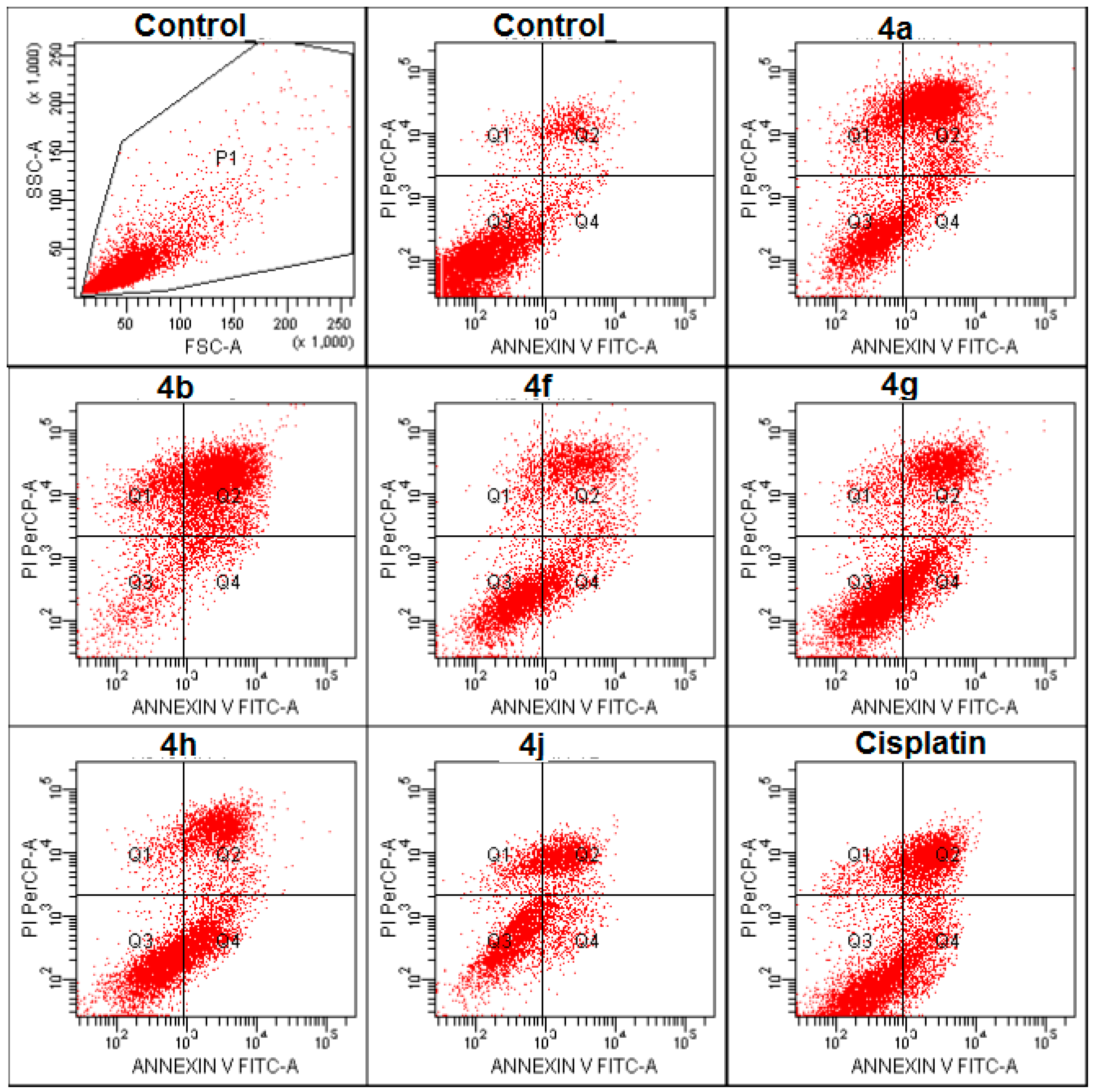
2.2.3. Apoptosis/Necrosis Detection
2.2.4. Anticholinesterase Activity
3. Materials and Methods
3.1. Chemistry
3.2. Biochemistry
3.2.1. MTT Assay (Mitochondrial Activity)
3.2.2. DNA Synthesis Inhibition
3.2.3. Flow Cytometric Analysis
3.2.4. Inhibition of AChE/BuChE Enzymes
3.2.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zaharia, V.; Ignat, A.; Palibroda, N.; Ngameni, B.; Kuete, V.; Fokunang, C.N.; Moungang, M.L.; Ngadjui, B.T. Synthesis of some p-toluenesulfonyl-hydrazinothiazoles and hydrazino-bis-thiazoles and their anticancer activity. Eur. J. Med. Chem. 2010, 45, 5080–5085. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, Z.; Zhang, C.; Xin, T.; Wang, Y.; Song, H.; Jiang, Y.; Chen, Y.; Xu, Y.; Tan, C. Synthesis and cytotoxic activity of some novel N-pyridinyl-2-(6-phenylimidazo[2,1-b]thiazol-3-yl)acetamide derivatives. Molecules 2012, 17, 4703–4716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Gong, J.; Fang, H.; Liu, A.; Du, G.; Xu, W. Design, synthesis, and biological activity of thiazole derivatives as novel influenza neuraminidase inhibitors. J. Enzym. Inhib. Med. Chem. 2011, 26, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.X.; Liu, A.L.; Li, Z.M.; Dong, W.L.; Liu, X.H.; Sun, N.B. Synthesis and anticancer activity of novel thiazole-5-carboxamide derivatives. Appl. Sci. 2016, 6, 8. [Google Scholar] [CrossRef]
- Kashyap, S.J.; Kumar Garg, V.; Kumar Sharma, P.; Kumar, N.; Dudhe, R.; Kumar Gupta, J. Thiazoles: Having diverse biological activities. Med. Chem. Res. 2012, 21, 2123–2132. [Google Scholar] [CrossRef]
- Nastasa, C.; Tiperciuc, B.; Duma, M.; Benedec, D.; Oniga, O. New hydrazones bearing thiazole scaffold: Synthesis, characterization, antimicrobial, and antioxidant investigation. Molecules 2015, 20, 17325–17338. [Google Scholar] [CrossRef] [PubMed]
- Bhole, R.P.; Bhusari, K.P. Synthesis and antitumor activity of (4-hydroxyphenyl)[5-substituted alkyl/aryl)-2-thioxo-1,3,4-thiadiazol-3-yl]methanone and [(3,4-disubstituted)-1,3-thiazol-2ylidene]-4-hydroxybenzohydrazide. Med. Chem. Res. 2011, 20, 695–704. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Rani, R.; Gupta, P.P.; Agrawal, S.K.; Saxena, A.K. Synthesis, anticancer, and anti-inflammatory activity evaluation of methanesulfonamide and amidine derivatives of 3,4-diaryl-2-imino-4-thiazolines. Mol. Divers. 2009, 13, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Boltneva, N.P.; Lushchekina, S.V.; Serebryakova, O.G.; Stupina, T.S.; Terentiev, A.A.; Serkov, I.V.; Proshin, A.N.; Bachurin, S.O.; Richardson, R.J. Synthesis, molecular docking and biological evaluation of N,N-disubstituted 2-aminothiazolines as a new class of butyrylcholinesterase and carboxylesterase inhibitors. Bioorg. Med. Chem. 2016, 24, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Han, F.S.; Osajima, H.; Cheung, M.; Tokuyama, H.; Fukuyama, T. Novel structural motifs consisting of chiral thiazolines: Synthesis, molecular recognition, and anticancer activity. Chemistry 2007, 13, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, S.; Moore, R.E.; Patterson, G.L. Mirabazoles, minor tantazole-related cytotoxins from the terrestrial blue-green alga scytonema mirabil. Tetrahedron Lett. 1991, 32, 2593–2596. [Google Scholar] [CrossRef]
- Igarashi, Y.; Asano, D.; Sawamura, M.; In, Y.; Ishida, T.; Imoto, M. Ulbactins F and G, polycyclic thiazoline derivatives with tumor cell migration inhibitory activity from Brevibacillus sp. Org. Lett. 2016, 18, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Marson, C.M.; Matthews, C.J.; Yiannaki, E.; Atkinson, S.J.; Soden, P.E.; Shukla, L.; Lamadema, N.; Thomas, N.S. Discovery of potent, isoform-selective inhibitors of histone deacetylase containing chiral heterocyclic capping groups and a N-(2-aminophenyl)benzamide binding unit. J. Med. Chem. 2013, 56, 6156–6174. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.A.; Amnerkar, N.D.; Pathare, S.S.; Bhusari, K.P. Docking study of p-hydroxybenzohydrazide derivatives as tyrosine kinase inhibitors and anticancer agents. J. Comput. Methods Mol. Des. 2015, 5, 109–114. [Google Scholar]
- Wang, W.; Zhao, B.; Xu, C.; Wu, W. Synthesis and antitumor activity of the thiazoline and thiazine multithioether. Int. J. Org. Chem. 2012, 2, 117–120. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Johar, M.; Singh, N.; Dastidar, S.G. Synthesis of biscoupled hemin-thiazoline derivatives and their anticancer activity evaluation. Indian J. Chem. 2004, 43B, 162–167. [Google Scholar] [CrossRef]
- Mahani, N.M.; Sabermahani, F.; Jahani, P.M.; Jalali, N. A density function theory based quantitative structure activity relationships study of thiazoline derivatives as anticancer agents. Iran. J. Anal. Chem. 2015, 2, 70–76. [Google Scholar]
- Taher, A.T.; Khalil, N.A.; Ahmed, E.M. Synthesis of novel isatin-thiazoline and isatin-benzimidazole conjugates as anti-breast cancer agents. Arch. Pharm. Res. 2011, 34, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Dalton, J.T.; Gududuru, V.; Hurh, E. Thiazoline analogs as cell proliferation inhibitors. Chem. Abstr. 2005, 143, 326352. [Google Scholar]
- Ng, R.A.; Sui, Z. Preparation of thiazoline derivatives as selective androgen receptor modulators (SARMs). Chem. Abstr. 2005, 142, 74557. [Google Scholar]
- Pine, M.J.; Mirand, E.A.; Ambrus, J.L.; Bock, F.G. Antitumor studies of 2- amino-2-thiazoline and other tumor-modifying agents. J. Med. 1983, 14, 433–449. [Google Scholar] [CrossRef]
- Randazzo, A.; Bifulco, G.; Giannini, C.; Bucci, M.; Debitus, C.; Cirino, G.; Gomez-Paloma, L. Halipeptins A and B: Two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J. Am. Chem. Soc. 2001, 123, 10870–10876. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Perarnau, A.; Preciado, S.; Palmeri, C.M.; Moncunill-Massaguer, C.; Iglesias-Serret, D.; González-Gironès, D.M.; Miguel, M.; Karasawa, S.; Sakamoto, S.; Cosialls, A.M.; et al. A trifluorinated thiazoline scaffold leading to pro-apoptotic agents targeting prohibitins. Angew. Chem. Int. Ed. 2014, 53, 10150–10154. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.H.; Ke, F.Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004, 12, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Monneret, C. Recent developments in the field of antitumour anthracyclines. Eur. J. Med. Chem. 2001, 36, 483–493. [Google Scholar] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Lamshöft, M.; Spiteller, M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009, 107, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Chaitramallu, M.; Devaraju, P. Synthesis of organic nanoparticle of aryl tetralin compounds and study of their biological activities for targeted delivery system. Indian J. Adv. Chem. Sci. 2014, 2, 61–63. [Google Scholar]
- Chen, H.; Zuo, S.; Wang, X.; Tang, X.; Zhao, M.; Lu, Y.; Chen, L.; Liu, J.; Liu, Y.; Liu, D.; et al. Synthesis of 4b-triazole-podophyllotoxin derivatives by azideealkyne cycloaddition and biological evaluation as potential antitumor agents. Eur. J. Med. Chem. 2011, 46, 4709–4714. [Google Scholar] [PubMed]
- Hamdy, N.A.; Gamel-Eldeen, A.M.; Abdel-Aziz, H.A.; El-Hussieny, A.A.; Fakhr, I.A. Modulation of carcinogen metabolizing enzymes by new fused heterocycles pendant to 5,6,7,8-tetrahydronaphthalene derivatives. Eur. J. Med. Chem. 2010, 45, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Shi, Q.; Nakagawa-Goto, K.; Wua, P.; Bastow, K.F.; Morris-Natschke, S.L.; Lee, K. Antitumor agents 269. Non-aromatic ring-A neotanshinlactone analog, TNO, asa new class of potent antitumor agents. Bioorg. Med. Chem. Lett. 2009, 19, 6289–6292. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Hamdy, N.A.; Abdel-Aziz, H.A.; El-Hussieny, A.A.; Fakhr, I.A. Induction of intrinsic apoptosis pathway in colon cancer HCT-116 cells by novel 2-substituted-5,6,7,8-tetrahydronaphthalene derivatives. Eur. J. Med. Chem. 2014, 77, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.A.; Anwar, M.M.; Abu-Zied, K.M.; Awad, H.M. Synthesis, tumor inhibitory and antioxidant activity of new polyfuncctionnaly 2-substituted-5,6,7,8-tetrahydronaphthalene derivatives containing pyridine, thioxopyridine and pyrazolopyridine moieties. Acta Pol. Pharm. 2013, 70, 987–1001. [Google Scholar] [PubMed]
- Gurkan-Alp, A.S.; Mumcuoglu, M.; Andac, C.A.; Dayanc, E.; Cetin-Atalay, R.; Buyukbingol, E. Synthesis, anticancer activities and molecular modeling studies of novel indole retinoid derivatives. Eur. J. Med. Chem. 2012, 58, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullah, E.S. Synthesis and anticancer activity of some novel tetralin-6-yl-pyrazoline, 2-thioxopyrimidine, 2-oxopyridine, 2-thioxo-pyridine and 2-iminopyridine derivatives. Molecules 2011, 16, 3410–3419. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, M.Y.; El Zahar, M.I.; Kamel, M.M.; Omar, M.T.; Anwar, M.M. Novel 5,6,7,8-tetrahydronaphth-2-yl heterocycles of possible biological activity. Egypt. Pharmaceut. J. 2004, 3, 49–65. [Google Scholar]
- Gouhar, R.S.; Raafat, E. Synthesis of (3-(naphthalen-1-yl)oxiran-2-yl)(5,6,7,8 tetrahydronaphthalen-2-yl)methanone and reaction with some nucleophiles for anticancer evaluation. Res. Chem. Intermed. 2015, 41, 5529–5543. [Google Scholar] [CrossRef]
- Amin, K.M.; El-Zahar, M.I.; Anwar, M.M.; Kamel, M.M.; Mohamed, M.H. Synthesis and anticancer actıvıty of novel tetralin-6-ylpyridine and tetralin-6-ylpyrimidine derivatives. Acta Pol. Pharm. 2009, 66, 279–291. [Google Scholar] [PubMed]
- Ali, M.M.; Hassan, S.A. Role of some newly synthesized tetrahydronaphthalenthiazol derivatives as anticancer compounds. Int. J. Cancer Res. 2007, 3, 103–110. [Google Scholar]
- Solimana, E.A.; El-Zahar, M.I.; El-Masry, A.H.; Kamel, M.; Gohar, R.S. Synthesis and anticancer evaluation of novel tetrahydronaphthalen-6-ylthiazole heterocycles against human HePG2 and MCF7 cell lines. Der Pharm. Chem. 2010, 2, 507–521. [Google Scholar]
- Čolović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Zovko, A.; Sepcic, K.; Turk, T.; Faimali, M.; Garaventa, F.; Chelossi, E.; Paleari, L.; Falugi, C.; Aluigi, M.G.; Angelini, C.; et al. New aspects of the relationship between acethylcholinesterase activity and cancer I: Poly-Aps experiments. WSEAS Trans. Biol. Biomed. 2009, 6, 58–69. [Google Scholar]
- Ciftci, G.A.; Temel, H.E.; Yildirim, S.U.; Kaplancikli, Z.A.; Altintop, M.D.; Genc, L. Apoptotic effects of some carbazole derivatives on lung carcinoma and glioma cell lines. Med. Chem. Res. 2013, 22, 3751–3759. [Google Scholar] [CrossRef]
- Turan-Zitouni, G.; Ozdemir, A.; Kaplancikli, Z.A.; Revial, G.; Demirci, F. Synthesis and antimicrobial activity evaluation of new hydrazide derivatives. Turk. J. Pharm. Sci. 2011, 8, 199–206. [Google Scholar]
- Turan-Zitouni, G.; Kaplancıklı, Z.A.; Erol, K.; Kılıç, F.S. Synthesis and analgesic activity of some triazoles and triazolothiazines. II Farmaco 1999, 54, 218–223. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Mossmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Maghni, K.; Nicolescu, O.M.; Martin, J.G. Suitability of cell metabolic colorimetric assays for assessment of CD4+ T cell proliferation: Comparison to 5-bromo-2-deoxyuridine (BrdU) ELISA. J. Immunol. Methods 1999, 223, 185. [Google Scholar] [CrossRef]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a–4k are available from the authors. |
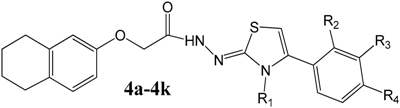 | ||||
|---|---|---|---|---|
| Compounds | R1 | R2 | R3 | R4 |
| 4a | Cyclohexyl | H | H | H |
| 4b | Cyclohexyl | H | H | OCH3 |
| 4c | Phenyl | H | H | H |
| 4d | Phenyl | H | H | CH3 |
| 4e | Phenyl | H | H | OCH3 |
| 4f | Phenyl | H | H | Br |
| 4g | Phenyl | H | H | Cl |
| 4h | Phenyl | H | H | F |
| 4i | Phenyl | H | NO2 | H |
| 4j | Phenyl | H | H | NO2 |
| 4k | Phenyl | Cl | Cl | H |
| Compounds | MCF-7 | A549 | NIH/3T3 | SIMCF-7 | SIA549 |
|---|---|---|---|---|---|
| 4a | >1000 | 157.6 ± 14.8 | 206.1 ± 15.3 | 0.2 | 1.3 |
| 4b | 69.2 ± 5.8 | 137.5 ± 7.2 | 55.7 ± 1.2 | 0.8 | 0.4 |
| 4c | 296.7 ± 46.6 | >1000 | >1000 | 3.4 | … |
| 4d | 71.8 ± 12.5 | >1000 | >1000 | 13.9 | … |
| 4e | 91.4 ± 9.8 | 707.9 ± 83.3 | >1000 | 10.9 | 1.4 |
| 4f | 280.9 ± 56.2 | 54.3 ± 6.8 | 146.7 ± 5.4 | 0.5 | 2.7 |
| 4g | 483.0 ± 31.2 | 28.6 ± 4.1 | 224.5 ± 20.5 | 0.5 | 7.8 |
| 4h | 243.1 ± 14.9 | 48.6 ± 6.0 | 246.7 ± 12.2 | 1.0 | 5.1 |
| 4i | >1000 | >1000 | >1000 | … | … |
| 4j | >1000 | 49.0 ± 1.41 | >1000 | … | 20.4 |
| 4k | 152.7 ± 27.5 | >1000 | 165.4 ± 11.0 | 1.1 | … |
| Cisplatin | 100.0 ± 3.3 | 108.3 ± 11.8 | - | … | … |
| Groups | % Early Apoptotic | % Late Apoptotic | % Viable | % Necrotic | |
|---|---|---|---|---|---|
| MCF-7 cell line | Control | 1.7 | 4.3 | 84.9 | 9.1 |
| Compound 4b | 0.7 | 29.1 | 6.3 | 63.8 | |
| Compound 4d | 1.1 | 12.1 | 50.7 | 36.1 | |
| Compound 4e | 0.8 | 31.7 | 24.8 | 39.1 | |
| Compound 4k | 2.4 | 8.9 | 54.3 | 34.4 | |
| Cisplatin | 2.8 | 27.1 | 42.0 | 28.1 | |
| A549 cell line | Control | 3.1 | 6.1 | 89.0 | 1.8 |
| Compound 4a | 7.4 | 48.3 | 31.5 | 12.8 | |
| Compound 4b | 6.6 | 67.0 | 10.9 | 15.6 | |
| Compound 4f | 16.5 | 29.3 | 47.7 | 6.5 | |
| Compound 4g | 18.7 | 26.0 | 49.4 | 5.8 | |
| Compound 4h | 23.7 | 26.4 | 45.9 | 4.0 | |
| Compound 4j | 11.2 | 30.3 | 47.0 | 11.4 | |
| Cisplatin | 14.9 | 25.3 | 53.1 | 6.7 |
| Compounds | AChE % Inhibition (80 µg/mL) | BuChE % Inhibition (80 µg/mL) |
|---|---|---|
| 4a | 38.26 ± 2.18 | 28 ± 1.88 |
| 4b | 11.08 ± 1.48 | 15.89 ± 1.72 |
| 4c | 19.30 ± 1.87 | 16.48 ± 0.08 |
| 4d | 43.22 ± 1.56 | 33.19 ± 2.38 |
| 4e | 10.81 ± 0.94 | --- |
| 4f | 42.87 ± 2.84 | --- |
| 4g | 24.37 ± 1.20 | --- |
| 4h | 49.92 ± 1.44 | --- |
| 4i | 41.85 ± 0.23 | --- |
| 4j | 44.96 ± 1.60 | --- |
| 4k | 15.19 ± 1.99 | --- |
| Donepezil IC50 (µM) | (11.8 ± 0.7) × 10−3 | 4.85 ± 0.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turan-Zitouni, G.; Yurttaş, L.; Tabbi, A.; Akalın Çiftçi, G.; Temel, H.E.; Kaplancıklı, Z.A. New Thiazoline-Tetralin Derivatives and Biological Activity Evaluation. Molecules 2018, 23, 135. https://doi.org/10.3390/molecules23010135
Turan-Zitouni G, Yurttaş L, Tabbi A, Akalın Çiftçi G, Temel HE, Kaplancıklı ZA. New Thiazoline-Tetralin Derivatives and Biological Activity Evaluation. Molecules. 2018; 23(1):135. https://doi.org/10.3390/molecules23010135
Chicago/Turabian StyleTuran-Zitouni, Gülhan, Leyla Yurttaş, Aouatef Tabbi, Gülşen Akalın Çiftçi, Halide Edip Temel, and Zafer Asım Kaplancıklı. 2018. "New Thiazoline-Tetralin Derivatives and Biological Activity Evaluation" Molecules 23, no. 1: 135. https://doi.org/10.3390/molecules23010135
APA StyleTuran-Zitouni, G., Yurttaş, L., Tabbi, A., Akalın Çiftçi, G., Temel, H. E., & Kaplancıklı, Z. A. (2018). New Thiazoline-Tetralin Derivatives and Biological Activity Evaluation. Molecules, 23(1), 135. https://doi.org/10.3390/molecules23010135