Novel 4-Methylumbelliferone Amide Derivatives: Synthesis, Characterization and Pesticidal Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Acaricidal Activities
2.3. Herbicidal Activities
2.4. Antifungal Activities
3. Experimental Section
3.1. Chemistry
3.1.1. Synthesis of 1
3.1.2. Synthesis of 2a and 2b
3.1.3. Synthesis of 3a and 3b
3.1.4. General Procedure for Synthesis of 4-Methylumbelliferone Amide Derivatives (4aa–4ah, 4ba–4bq)
3.2. Procedures for ActivityEvaluation
3.2.1. Acaricidal Activity
3.2.2. Herbicidal Activity
3.2.3. Antifungal Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, A. Coumarin Synthesis, Reactions and Pharmacological Activities; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2013; ISBN 3659503603. [Google Scholar]
- Zhang, G.H.; Zheng, H.; Guo, M.Y.; Du, L.; Liu, G.J.; Wang, P. Synthesis of polymeric fluorescent brightener based on coumarin and its performances on paper as light stabilizer, fluorescent brightener and surface sizing agent. Appl. Surf. Sci. 2016, 367, 167–173. [Google Scholar] [CrossRef]
- Mishra, V.R.; Sekar, N. Photostability of coumarin laser dyes—A mechanistic study using global and local reactivity descriptors. J. Fluoresc. 2017, 27, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Xue, Y.B.; Liu, Z.; Peng, S.S.; He, Y.; Zhang, Y.; Fang, R.; Wang, J.P.; Luo, Z.W.; Yao, G.M.; et al. Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 2015, 5, 13544. [Google Scholar] [CrossRef] [PubMed]
- Bertin, R.; Chen, Z.; Martínez-Vázquez, M.; García-Argaéz, A.; Froldi, G. Vasodilation and radical-scavenging activity of imperatorin and selected coumarinic and flavonoid compounds from genus Casimiroa. Phytomedicine 2014, 21, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Kuk, H.S.; Won, S.W. Effects of psoralen and angelicin on hepatic drug-metabolizing enzyme activities. Arch. Pharm. Res. 1988, 11, 122–126. [Google Scholar]
- Srivastava, P.; Vyas, V.K.; Variya, B.; Patel, P.; Qureshi, G.; Ghate, M. Synthesis, anti-inflammatory, analgesic, 5-lipoxygenase (5-LOX) inhibition activities, and molecular docking study of 7-substituted coumarin derivatives. Bioorg. Chem. 2016, 67, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, Y.; Laïos, I.; Cleeren, A.; Nonclercq, D.; Bermont, L.; Refouvelet, B.; Boubekeur, K.; Xicluna, A.; Leclercq, G.; Laurent, G. Synthesis, structure, and estrogenic activity of 4-amino-3-(2-methylbenzyl) coumarins on human breast carcinoma cells. Bioorg. Med. Chem. 2007, 15, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Pavithra, G.; Ajay, K.K. Synthesis of coumarin appended 1,3-oxazines as potent antimicrobial and antioxidant agents. Pharm. Chem. J. 2017, 51, 582–589. [Google Scholar]
- Kumar, R.; Banerjee, S.K.; Handa, K.L. Coumarins of Heracleum canescens and Heracleum pinnatum. Sources for dermal photosensitizing agents. PlantaMedica 1976, 30, 291–295. [Google Scholar]
- Shakeel-u-Rehman; Khan, R.; Bhat, K.; Raja, A.; Shawl, A.; Alam, M. Isolation, characterisation and antibacterial activity studies of coumarins from Rhododendron lepidotum Wall. ex G. Don, Ericaceae. Braz. J. Pharmacogn. 2010, 20, 886–890. [Google Scholar] [CrossRef]
- Song, P.P.; Zhao, J.; Liu, Z.L.; Duan, Y.B.; Hou, Y.P.; Zhao, C.Q.; Wu, M.; Wei, M.; Wang, N.H.; Lv, Y.; et al. Evaluation of antifungal activities and structure-activity relationships of coumarin derivatives. Pest Manag. Sci. 2017, 73, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Hu, Y.; Chen, X.H.; Wang, G.X.; Ling, F. Synthesis and anthelmintic activity of coumarin-imidazole hybrid derivatives against Dactylogyrus intermedius in goldfish. Bioorg. Med. Chem. Lett. 2016, 26, 5039–5043. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Wang, J.B.; Shen, J.J.; Bi, C.; Zhou, H.W. Coumarin-based Hg2+ fluorescent probe: Synthesis and turn-on fluorescence detection in neat aqueous solution. Sens. Actuators B Chem. 2017, 243, 678–683. [Google Scholar] [CrossRef]
- Pang, L.; Zhou, Y.; Gao, W.; Song, H.; Wang, X.; Wang, Y. A highly selective and sensitive fluorescence probe for rapid detection of hypochlorite in tap water and cancer cells. RSC Adv. 2016, 6, 105795–105800. [Google Scholar] [CrossRef]
- Anna, Z.; Dominik, K.; Filip, B.; Ryszard, O. Mixed carbonates as useful substrates for a fluorogenic assay for lipases and esterases. ChemBioChem 2015, 16, 677–682. [Google Scholar]
- Arora, P.; Ranawat, M.S.; Arora, N. Synthesis and screening of some novel coumarin derivatives for antipsychotic activity. Res. J. Pharm. Technol. 2012, 5, 968–972. [Google Scholar]
- Ostrowska, K.; Młodzikowska, K.; Głuch-Lutwin, M.; Gryboś, A.; Siwek, A. Synthesis of a new series of aryl/heteroarylpiperazinyl derivatives of 8-acetyl-7-hydroxy-4-methylcoumarin with low nanomolar 5-HT 1A affinities. Eur. J. Med. Chem. 2017, 137, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.T.; Park, Y.H.; Kim, H.W.; Kim, H.S.; Lee, D.; Lee, M.B.; Kim, Y.M.; Choi, W.S. Suppression of IgE-mediated mast cell activation and mouse anaphylaxis via inhibition of Syk activation by 8-formyl-7-hydroxy-4-methylcoumarin, 4μ8C. Toxicol. Appl. Pharmacol. 2017, 332, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Wang, X.B.; Kong, L.Y. α-Glucosidase inhibitors via green pathway: Biotransformation for bicoumarins catalyzed by Momordica charantia peroxidase. J. Agric. Food Chem. 2013, 61, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, B.; Chen, Z.; Pan, Y.; Wang, H.; Liang, H.; Yi, X. Synthesis and antioxidant activities of novel 4-Schiff base-7-benzyloxy-coumarin derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 6811–6815. [Google Scholar] [CrossRef] [PubMed]
- Radwan, E.; Reem, I.A. Anti-inflammatory screening and molecular modeling of some novel coumarin derivatives. Molecules 2015, 20, 5374–5391. [Google Scholar]
- Li, Y.J.; Wang, C.Y.; Ye, M.Y.; Yao, G.Y.; Wang, H.S. Novel coumarin-containing aminophosphonatesas antitumor agent: Synthesis, cytotoxicity, DNA-binding and apoptosis evaluation. Molecules 2015, 20, 14791–14809. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Ye, M.Y.; Huang, R.Z.; Yao, G.Y.; Pan, Y.M.; Liao, Z.X.; Wang, H.S. Coumarin-containing aminophosphonates bridged with chiral side chain: Synthesis and influence of chirality on cytotoxicity and DNA binding. Med. Chem. Res. 2014, 23, 3144–3156. [Google Scholar] [CrossRef]
- Cong, N.T.; Nhan, H.T.; Van, H.L.; Thang, T.D.; Kuo, P.C. Synthesis and antibacterial activity of analogs of 5-arylidene-3-(4-methylcoumarin-7-yloxyacetylamino)-2-thioxo-1,3-thiazoli-din-4-one. Molecules 2014, 19, 13577–13586. [Google Scholar] [CrossRef] [PubMed]
- Šarkanj, B.; Molnar, M.; Cacic, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food Chem. 2013, 139, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.; Yates, T.; Shamaldevi, N.; Bowen, T.; Lokeshwa, V. Dietary supplement hymecromone and sorafenib: A novel combination for the control of renal cell carcinoma. J. Urol. 2013, 190, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Kuipers, H.; Frymoyer, A.; Ishak, H.; Bollyky, J.; Wight, T.; Bollyky, P. 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front. Immunol. 2015, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, X.Z.; Yan, Z.Q.; Guo, H.R.; Qin, B. Phytotoxicity of umbelliferone and its analogs: Structure-activity relationships and action mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, X.; Jin, H.; Yang, X.; Qin, B. Antifungal activity of umbelliferone derivatives: Synthesis and structure-activity relationships. Microb. Pathog. 2017, 104, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Pawar, P.; Joseph, M.; Phalgune, M.; Kashalkar, U.; Deshpande, R.; Nirmala, R. Efficacy of 4-methy-7-hydroxy coumarin derivatives against vectors Aedesa egypti and Culexquinque fasciatus. Indian J. Exp. Biol. 2008, 46, 788–792. [Google Scholar] [PubMed]
- Huang, H.Z.; Yao, H.W.; Liu, J.Y.; Aman, I.S.; Shizuo, G.K.; Anthony, J.C.; Bruce, D.H. Development of pyrethroid-like fluorescent substrates for glutathione S-transferase. Anal. Biochem. 2012, 431, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chetan, T.P.; Sangamesh, A.P.; Ajaykumar, D.K.; Vinod, H.N.; Manjunathad, M.; Shivshankar, M.K.; Prema, S.B. Synthesis, spectral, thermal, fluorescence, antimicrobial, anthelmintic and DNA cleavage studies of mononuclear metal chelates of bi-dentate 2H-chromene-2-one Schiff base. J. Photochem. Photobiol. B 2015, 148, 322–332. [Google Scholar]
- Tang, C.C.; Li, Y.C.; Chen, B.; Yang, H.Z.; Jin, G.Y. Pesticide Chemistry; Nankai University: Tianjin, China, 1998; ISBN 7-310-01010-8. (In Chinese) [Google Scholar]
- Qiao, L.L.; Wei, Y.; Hao, S.H. Synthesis and biological activity of novel fluorinated amide hydroxy methyl coumarin derivatives. Chin. J. Org. Chem. 2017. (In Chinese) [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Hao, S.H. Synthesis and Herbicidal Activity of N-Acyl-N-(m-fluoro-benzyl)-6-amino-coumarin. Chin. J. Organ. Chem. 2015, 35, 1691–1699. (In Chinese) [Google Scholar] [CrossRef]
- Shah, N.M.; Mehta, D.H. Nitration of 7-hydroxy-4-methylcoumarin and its methyl ether. J. Indian Chem. Soc. 1954, 31, 784–786. [Google Scholar]
- Banal, V.; Khanna, R.N. Regioselective mononitration of coumarins using chromium nitrate as nitrating agent. Synth. Commun. 2002, 32, 1345–1350. [Google Scholar] [CrossRef]
- Ramana, M.M.V.; Malik, S.S.; Parihar, J.A. Guanidinium nitrate: A novel reagent for aryl nitrations. Tetrahedron Lett. 2004, 45, 8681–8683. [Google Scholar] [CrossRef]
- Ganguly, N.; Sukai, A.K.; De, S. Cerium (IV) ammonium nitrate mediated nitration of coumarins. Synth. Commun. 2001, 31, 301–309. [Google Scholar] [CrossRef]
- Kozlova, I.K. Synthesis of o-acylamino-4-methyl-7-hydroxycoumarins (4-methylumbelliferones). Chem. Heterocycl. Compd. 1985, 21, 750–753. [Google Scholar] [CrossRef]
- Tyagi, Y.K.; Kumar, A.; Raj, H.G.; Vohra, P.; Gupta, G.; Kumari, R.; Kumar, P.; Gupta, R.K. Synthesis of novel amino and acetyl amino-4-methylcoumarins and evaluation of their antioxidant activity. Eur. J. Med. Chem. 2005, 40, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, H.K.; Pahelkar, A.; Takale, B.S. Preparative-scale synthesis of amino coumarins through new sequential nitration and reduction protocol. Tetrahedron Lett. 2017, 58, 4107–4110. [Google Scholar] [CrossRef]
- Hao, S.H.; Miao, K.L.; Wei, Y.; Wang, T. Synthesis and Acaricidal Activity of a Kind of Novel Amidating Hydroxymethyl Coumarin Derivatives. CN 107011306A; 4 August 2017. Available online: http://epub.sipo.gov.cn/pam.action (accessed on 20 December 2017). (In Chinese)
- Dennehy, T.J.; Farnham, A.W.; Denholm, I. The microimmersion bioassay: A novel method for the topical application of pesticides to spider mites. Pest Manag. Sci. 2010, 39, 47–54. [Google Scholar] [CrossRef]
- Wei, Y.; Li, S.Q.; Hao, S.H. New angular oxazole-fused coumarin derivatives: Synthesis and biological activities. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Compd. | R | Compd. | R | Compd. | R |
|---|---|---|---|---|---|
| 4aa | -CH2CH2CH3 | 4ba | -CH2CH2CH3 | 4bi | 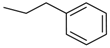 |
| 4ab | 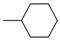 | 4bb | -CH(CH3)CH3 | 4bj | 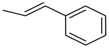 |
| 4ac | 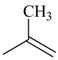 | 4bc | -CH2(CH2)9CH3 | 4bk | 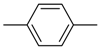 |
| 4ad | 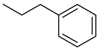 | 4bd | -CH2CH2CH2Cl | 4bl |  |
| 4ae |  | 4be |  | 4bm | 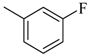 |
| 4af | 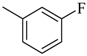 | 4bf | 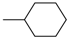 | 4bn | 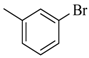 |
| 4ag | 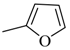 | 4bg |  | 4bo | 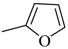 |
| 4ah |  | 4bh | 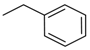 | 4bp | 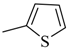 |
| 4bq | 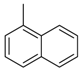 |
| Compd. | 200 mg/L | 1000 mg/L | Compd. | 200 mg/L | 1000 mg/L |
|---|---|---|---|---|---|
| 4aa | 4.2 ± 5.2 | 12.9 ± 5.0 | 4bf | 18.4 ± 3.0 | 29.2 ± 6.3 |
| 4ab | 4.5 ± 3.2 | 27.1 ± 6.5 | 4bg | 40.3 ± 3.7 | 71.7 ± 5.4 |
| 4ac | 51.9 ± 9.9 | 88.3 ± 4.9 | 4bh | 54.0 ± 13.1 | 65.1 ± 6.6 |
| 4ad | 9.1 ± 5.0 | 19.4 ± 2.7 | 4bi | 87.8 ± 8.1 | 98.6 ± 6.1 |
| 4ae | 23.0 ± 4.9 | 42.1 ± 10.9 | 4bj | 26.6 ± 5.8 | 38.0 ± 8.0 |
| 4af | 33.9 ± 2.9 | 56.9 ± 4.7 | 4bk | 57.4 ± 10.9 | 79.8 ± 8.3 |
| 4ag | 32.8 ± 5.6 | 52.6 ± 7.3 | 4bl | 23.0 ± 6.3 | 31.7 ± 9.7 |
| 4ah | 30.0 ± 3.5 | 53.1 ± 7.5 | 4bm | 7.1 ± 8.6 | 55.0 ± 10.7 |
| 4ba | 20.4 ± 9.6 | 30.0 ± 3.4 | 4bn | 47.4 ± 10.3 | 58.8 ± 7.4 |
| 4bb | 11.8 ± 7.1 | 29.7 ± 10.0 | 4bo | 53.8 ± 8.0 | 63.4 ± 4.6 |
| 4bc | 61.5 ± 2.4 | 69.2 ± 2.4 | 4bp | 48.7 ± 9.5 | 62.6 ± 3.0 |
| 4bd | 75.1 ± 7.6 | 83.5 ± 5.6 | 4bq | 41.9 ± 3.0 | 72.6 ± 4.2 |
| 4be | 65.1 ± 3.2 | 73.8 ± 2.3 | Bifenazate | 93.5 ± 5.7 | 100.0 ± 0.0 |
| Compd. | C. glaucum | Compd. | D. sanguinalis | Compd. | D. sanguinalis | |||
|---|---|---|---|---|---|---|---|---|
| Taproot | Caulis | Taproot | Caulis | Taproot | Caulis | |||
| 4af | 31.2 ± 9.7 | - | 4aa | 41.6 ± 2.6 | 67.4 ± 4.4 | 4bj | 10.6 ± 5.7 | 61.5 ± 12.4 |
| 4ah | - | 40.0 ± 5.3 | 4ab | 17.4 ± 7.4 | 33.6 ± 9.1 | 4bk | 41.9 ± 8.5 | 54.8 ± 8.7 |
| 4bb | 37.1 ± 9.6 | 16.8 ± 6.5 | 4ad | 25.5 ± 4.4 | 53.1 ± 8.9 | 4bl | 27.8 ± 5.2 | 48.5 ± 7.9 |
| 4bc | 40.1 ± 8.6 | 15.3 ± 6.5 | 4ag | 30.6 ± 3.6 | - | 4bm | 44.8 ± 7.7 | 12.2 ± 13.0 |
| 4be | 35.2 ± 3.4 | - | 4ba | 57.1 ± 4.3 | 70.1 ± 5.9 | 4bo | 64.6 ± 4.4 | 17.7 ± 5.1 |
| 4bf | 61.1 ± 9.7 | 50.6 ± 4.8 | 4bb | 66.7 ± 5.1 | 49.0 ± 12.8 | 4bp | 15.5 ± 3.3 | 42.5 ± 12.3 |
| 4bh | 76.3 ± 3.3 | 39.3 ± 1.9 | 4bc | - | 45.4 ± 11.7 | 4bq | 54.8 ± 4.3 | 60.9 ± 10.9 |
| 4bk | 41.8 ± 6.0 | - | 4bd | 3.4 ± 12.1 | 62.6 ± 10.4 | Acetochlor | 84.2 ± 7.0 | 78.1 ± 8.4 |
| 4bm | 51.1 ± 7.9 | - | 4be | 18.9 ± 6.8 | 40.5 ± 5.6 | |||
| 4bn | 31.1 ± 2.2 | 10.9 ± 8.1 | 4bf | 86.4 ± 10.6 | 71.8 ± 8.1 | |||
| 4bo | 30.1 ± 2.6 | 63.0 ± 5.8 | 4bg | 44.4 ± 11.7 | - | |||
| 4bq | 41.8 ± 6.0 | 9.4 ± 10.5 | 4bh | 96.2 ± 7.0 | 52.0 ± 6.4 | |||
| Acetochlor | 88.2 ± 2.1 | 78.5 ± 7.0 | 4bi | 48.0 ± 6.7 | 47.7 ± 10.2 | |||
| Compd. | F1 | Compd. | F2 | Compd. | F3 | Compd. | F4 | Compd. | F4 |
|---|---|---|---|---|---|---|---|---|---|
| 4bf | 45.8± 8.6 | 4ab | 48.2± 7.4 | 4ac | 31.8± 7.9 | 4ab | 32.6± 1.1 | 4bj | 39.8± 2.1 |
| 4bh | 32.1±5.5 | 4ag | 45.9± 9.0 | 4bh | 38.4± 3.3 | 4ae | 32.6± 8.9 | 4bk | 88.7± 1.9 |
| 4bk | 56.6± 5.0 | 4bd | 54.6± 7.8 | 4bj | 36.1± 4.2 | 4ba | 47.3± 1.6 | 4bl | 48.1± 2.2 |
| 4bl | 39.3± 6.1 | 4be | 69.4± 6.2 | 4bk | 46.6± 1.9 | 4bd | 36.5± 1.6 | 4bm | 51.0± 2.5 |
| 4bq | 60.8± 3.6 | 4bf | 32.9± 9.8 | 4bn | 48.9± 4.1 | 4bf | 46.5± 4.0 | 4bn | 72.7± 4.1 |
| Carb. | 88.9± 4.7 | 4bi | 57.8± 3.8 | 4bp | 47.9± 4.2 | 4bg | 66.1± 5.2 | 4bo | 34.8± 8.6 |
| 4bl | 34.5± 9.9 | 4bq | 71.2± 4.8 | 4bh | 84.9± 1.6 | 4bp | 81.8± 4.2 | ||
| 4bn | 37.7± 6.8 | Carb. | 70.9± 8.4 | 4bi | 58.4±2.7 | 4bq | 61.1± 4.8 | ||
| Carb. | 54.1± 7.1 | Carb. | 76.1± 3.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Miao, K.-L.; Hao, S.-H. Novel 4-Methylumbelliferone Amide Derivatives: Synthesis, Characterization and Pesticidal Activities. Molecules 2018, 23, 122. https://doi.org/10.3390/molecules23010122
Wei Y, Miao K-L, Hao S-H. Novel 4-Methylumbelliferone Amide Derivatives: Synthesis, Characterization and Pesticidal Activities. Molecules. 2018; 23(1):122. https://doi.org/10.3390/molecules23010122
Chicago/Turabian StyleWei, Yan, Kai-Long Miao, and Shuang-Hong Hao. 2018. "Novel 4-Methylumbelliferone Amide Derivatives: Synthesis, Characterization and Pesticidal Activities" Molecules 23, no. 1: 122. https://doi.org/10.3390/molecules23010122
APA StyleWei, Y., Miao, K.-L., & Hao, S.-H. (2018). Novel 4-Methylumbelliferone Amide Derivatives: Synthesis, Characterization and Pesticidal Activities. Molecules, 23(1), 122. https://doi.org/10.3390/molecules23010122




