Improving the Efficiency of New Automatic Dishwashing Detergent Formulation by Addition of Thermostable Lipase, Protease and Amylase
Abstract
1. Introduction
2. Results and Discussion
2.1. Stability of T1 Lipase, Rand Protease and Maltogenic Amylase In Detergent Components
2.2. Efficiency of Detergent Formulation
2.3. Efficiency of Individual Enzyme Concentration
2.4. Enzymes Encapsulation Performance
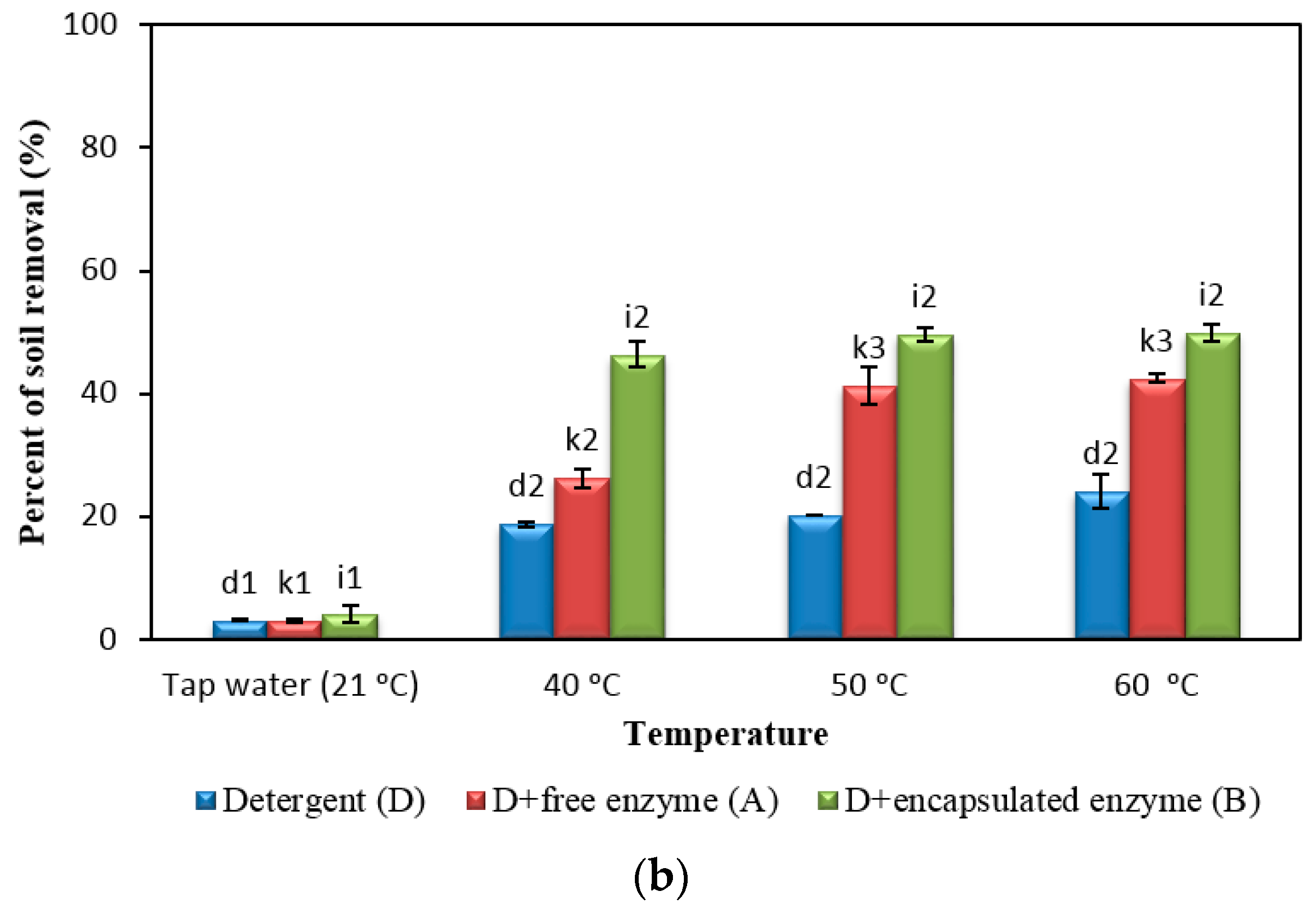
2.5. Comparison of Detergent with Free and Encapsulated Enzymes
2.6. Effect of Detergent Concentration on Removal of Soil
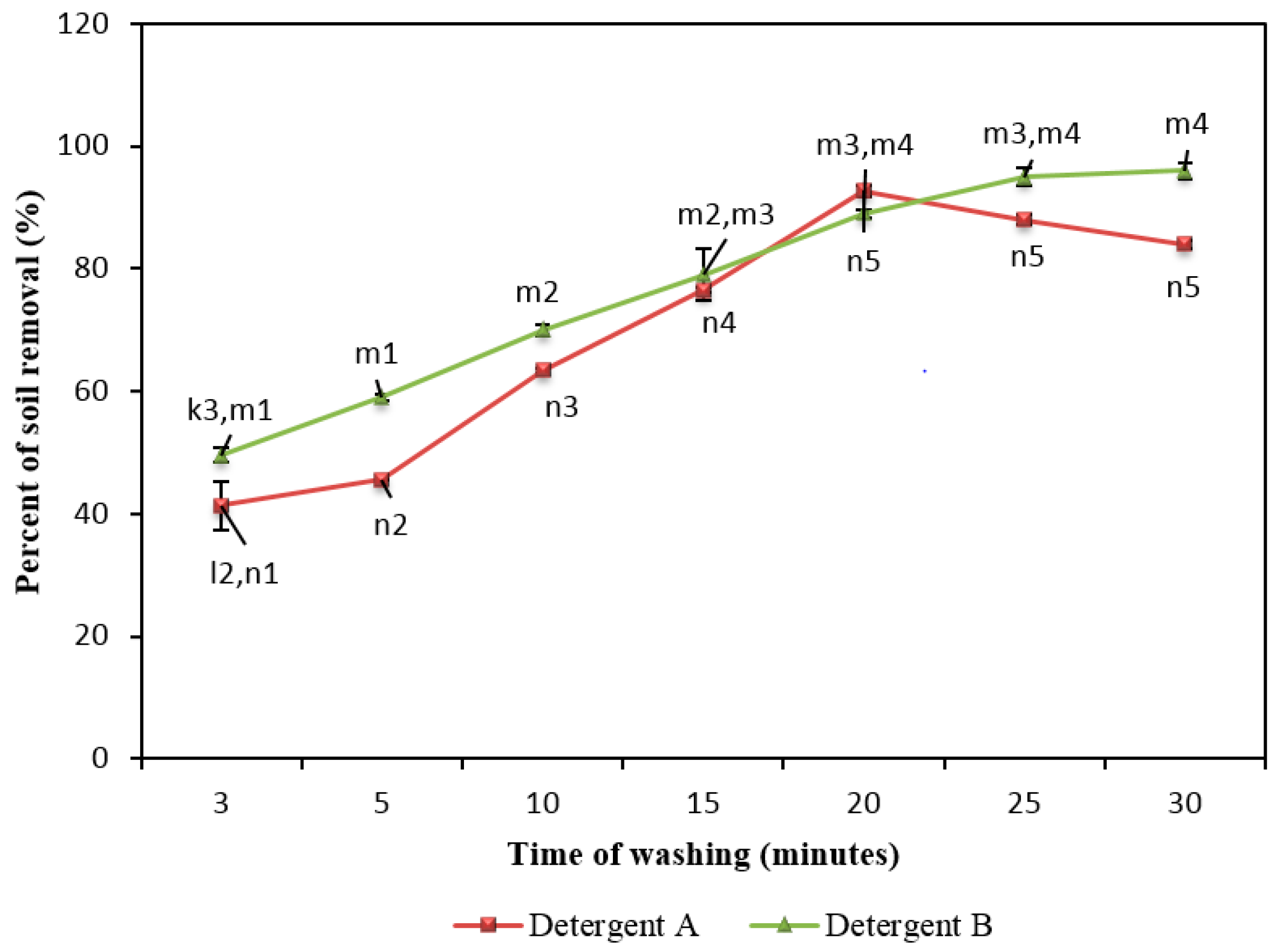
2.7. Effect of Washing Time on Removal of Soil
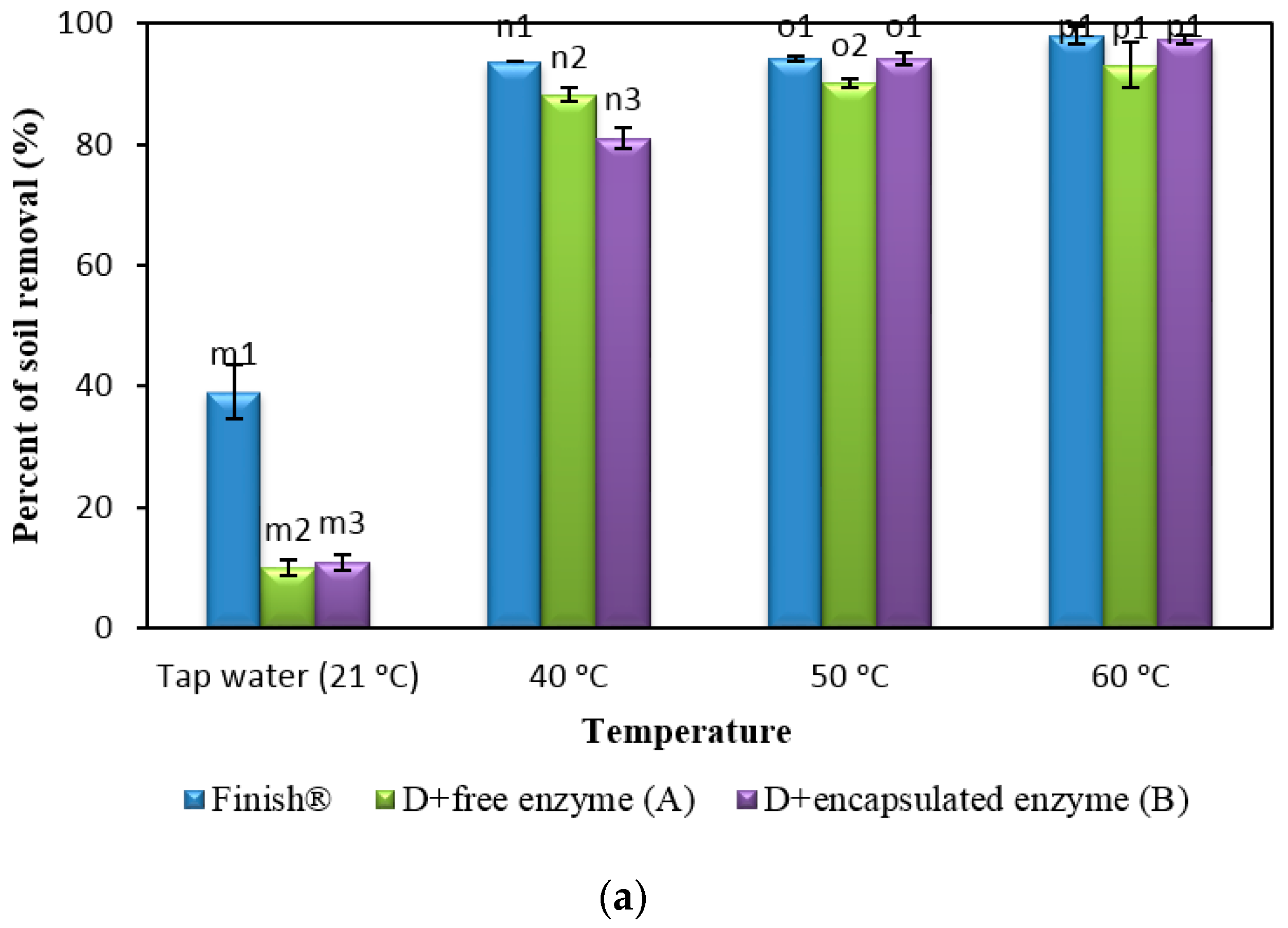
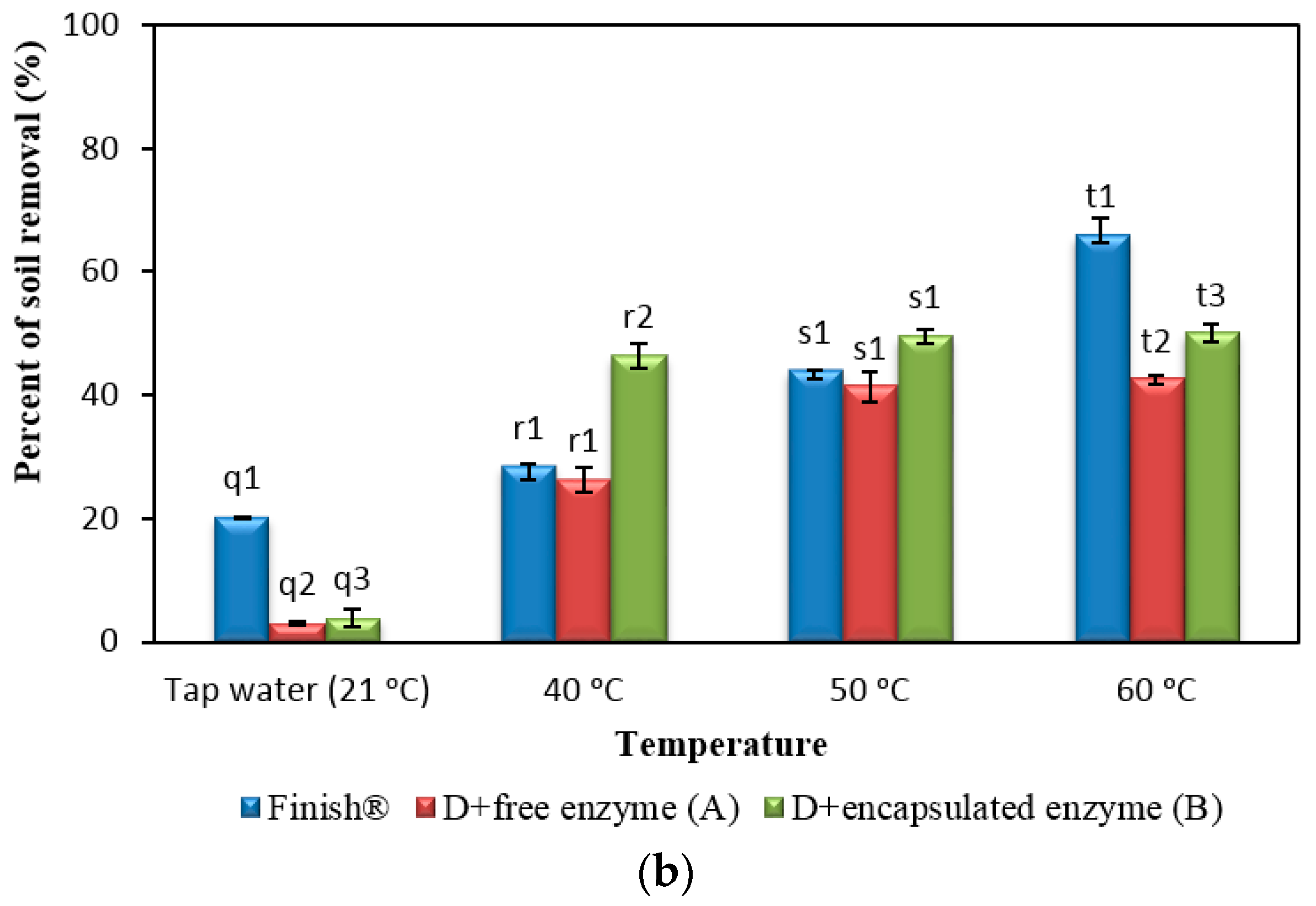
2.8. Comparison of Formulated Detergents Containing Enzymes with Commercial
3. Materials and Methods
3.1. Materials
3.2. Enzyme Production
3.2.1. T1 Lipase
3.2.2. Rand Protease
3.2.3. Maltogenic Amylase
3.3. Enzyme Analysis
3.3.1. Lipase
3.3.2. Rand Protease
3.3.3. Maltogenic Amylase
3.4. Protein Assay
3.5. Encapsulation of Enzymes
3.5.1. T1 Lipase
3.5.2. Rand Protease
3.5.3. Maltogenic Amylase
3.5.4. Scanning Electron Microscope
3.6. Enzymes Compatibility Tests
3.7. Detergent Formulation
3.8. Hard Water Preparation
3.9. Dishwashing Test
3.9.1. Efficiency of Detergent Formulation
3.9.2. Determination of Individual Enzyme Concentration
3.9.3. Comparison of Detergent with Free and Encapsulated Enzymes
3.9.4. Effect of Detergent Concentration on Removal of Soil
3.9.5. Effect of Washing Time on Removal of Soil
3.9.6. Comparison of Formulated Detergent and Commercial
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCoy, M. Goodbye, Phosphates. Chem. Eng. News 2011, 89, 12–17. [Google Scholar] [CrossRef]
- Bjorkling, F.; Godtfredsen, S.E.; Kirk, O. The future impact of industrial lipases. Trends Biotechnol. 1991, 9, 360–363. [Google Scholar] [CrossRef]
- Rahman, I.A.; Rahman, R.N.Z.R.A.; Salleh, A.B.; Basri, M. Formulation and evaluation of an automatic dishwashing detergent containing T1 lipase. J. Surfactants Deterg. 2013, 16, 427–434. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzym. Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Olsen, H.S.; Falholt, P. The role of enzymes in modern detergency. J. Surfactants Deterg. 1998, 1, 555–567. [Google Scholar] [CrossRef]
- Zyzyck, L.; Gorlin, P.A.; Dixit, N.; Lai, K.Y. Liquid automatic dishwasher detergents. In Liquid Detergents, 2nd ed.; Lai, K.Y., Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 129, pp. 319–376. [Google Scholar]
- Hauthal, H. Types and Typical Ingredients of Detergents. In Handbook Of Detergents, Part C; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–99. [Google Scholar]
- Eggert, T.; van Pouderoyen, G.; Pencreac'h, G.; Douchet, I.; Verger, R.; Dijkstra, B.W.; Jaeger, K.-E. Biochemical properties and three-dimensional structures of two extracellular lipolytic enzymes from Bacillus subtilis. Colloids Surf. B Biointerfaces 2002, 26, 37–46. [Google Scholar] [CrossRef]
- Von Rybinski, W.; Hill, K. Alkyl Polyglycosides—Properties and Applications of a new Class of Surfactants. Angew. Chem. Int. Ed. 1998, 37, 1328–1345. [Google Scholar] [CrossRef]
- Grbavčić, S.; Bezbradica, D.; Izrael-Živković, L.; Avramović, N.; Milosavić, N.; Karadžić, I.; Knežević-Jugović, Z. Production of lipase and protease from an indigenous Pseudomonas aeruginosa strain and their evaluation as detergent additives: Compatibility study with detergent ingredients and washing performance. Bioresour. Technol. 2011, 102, 11226–11233. [Google Scholar]
- Gupta, R.; Beg, Q.; Lorenz, P. Bacterial alkaline proteases: Molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002, 59, 15–32. [Google Scholar] [PubMed]
- Misset, O. Stability of Industrial Enzymes. In Stability and Stabilization of Enzymes; van den Tweel, W.J.J., Harder, A., Buitelaar, R.M., Eds.; Elsevier Wordmark: Maastricht, The Netherlands, 1992. [Google Scholar]
- Rathi, P.; Saxena, R.K.; Gupta, R. A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem. 2001, 37, 187–192. [Google Scholar] [CrossRef]
- Abusham, R.A.; Rahman, R.N.Z.R.; Salleh, A.B.; Basri, M. Optimization of physical factors affecting the production of thermo-stable organic solvent-tolerant protease from a newly isolated halo tolerant Bacillus subtilis strain Rand. Microb. Cell Fact. 2009, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leow, T.C.; Rahman, R.N.Z.R.A.; Basri, M.; Salleh, A.B. A thermoalkaliphilic lipase of Geobacillus sp. T1. Extremophiles 2007, 11, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Sulong, M.R. Recombinant Thermostable Maltogenic Amylase from Geobacillus sp. SK70 and Its Variants; Universiti Putra Malaysia: Selangor, Malaysia, 2015. [Google Scholar]
- Lund, H.; Kaasgaard, S.G.; Skagerlind, P.; Jorgensen, L.; Jørgensen, C.I.; van de Weert, M. Protease and Amylase Stability in the Presence of Chelators Used in Laundry Detergent Applications: Correlation Between Chelator Properties and Enzyme Stability in Liquid Detergents. J. Surfactants Deterg. 2012, 15, 265–276. [Google Scholar] [CrossRef]
- Hemachander, C.; Puvanakrishnan, R. Lipase from Ralstonia pickettii as an additive in laundry detergent formulations. Process Biochem. 2000, 35, 809–814. [Google Scholar] [CrossRef]
- Mehrnoush, A.; Tan, C.P.; Hamed, M.; Aziz, N.A.; Ling, T.C. Optimisation of freeze drying conditions for purified serine protease from mango (Mangifera indica Cv. Chokanan) peel. Food Chem. 2011, 128, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Chen, Y.; Cui, Z.; R Foster, P. Effect of freezing and thawing rates on denaturation of protein in aqueous solutions. Biotechnol. Bioeng. 2003, 82, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Eijsink, V.G.; Vriend, G.; van den Burg, B.; van der Zee, J.R.; Venema, G. Increasing the thermostability of a neutral protease by replacing positively charged amino acids in the N-terminal turn of alpha-helices. Protein Eng. 1992, 5, 165–170. [Google Scholar]
- Eisenberg, D.; Luthy, R.; Bowie, J.U.; Charles, W.; Carter, R.M.S., Jr. VERIFY3D: Assessment of protein models with three-dimensional profiles. In Methods in Enzymology; Academic Press: New York, NY, USA, 1997; Volume 277, pp. 396–404. [Google Scholar]
- Haki, G.D.; Rakshit, S.K. Developments in industrially important thermostable enzymes: A review. Bioresour. Technol. 2003, 89, 17–34. [Google Scholar] [CrossRef]
- Lim, W.H.; Salmiah, A. Dishwashing performance of mixed palm stearin sulfonated methyl esters—Nonylphenol ethoxylate alcohol. J. Surfactants Deterg. 2004, 7, 53–58. [Google Scholar] [CrossRef]
- Abeliotis, K.; Candan, C.; Amberg, C.; Ferri, A.; Osset, M.; Owens, J.; Stamminger, R. Impact of water hardness on consumers’ perception of laundry washing result in five European countries. Int. J. Consum. Stud. 2015, 39, 60–66. [Google Scholar] [CrossRef]
- Sengupta, P. Potential health impacts of hard water. Int. J. Prev. Med. 2013, 4, 866–875. [Google Scholar] [PubMed]
- Chauhan, M.; Garlapati, V.K. Production and Characterization of a Halo-, Solvent-, Thermo-tolerant Alkaline Lipase by Staphylococcus arlettae JPBW-1, Isolated from Rock Salt Mine. Appl. Biochem. Biotechnol. 2013, 171, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Leow, T.C.; Rahman, R.N.Z.R.A.; Basri, M.; Salleh, A.B. High level expression of thermostable lipase from Geobacillus sp. strain T1. Biosci. Biotechnol. Biochem. 2004, 68, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sulong, M.R.; Leow, T.C.; Rahman, R.N.Z.R.A.; Basri, M.; Salleh, A.B. Characteristics of recombinant maltogenic amylase from Geobacillus sp. SK70. Indian J. Biotechnol. 2017, 19, 91–99. [Google Scholar]
- Eggert, T.; Pouderoyen, G.v.; Dijkstra, B.W.; Jaeger, K.-E. Lipolytic enzymes LipA and LipB from Bacillus subtilis differ in regulation of gene expression, biochemical properties, and three-dimensional structure. FEBS Lett. 2001, 502, 89–92. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Rhee, J.S. A simple and rapid colorimetric method for determination of free fatty acid for lipase assay. J. Am. Oil Chem. Soc. 1986, 63, 89–92. [Google Scholar] [CrossRef]
- Winn-Deen, E.S.; David, H.; Sigler, G.; Chavez, R. Development of a direct assay for alpha-amylase. Clin. Chem. 1988, 34, 2005. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microorganism quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Edman, P.; Begg, G. A protein sequenator. Eur. J. Biochem. 1967, 1, 80–91. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Parameter | Components | Types of Enzymes (Relative Activity (%)) | ||
|---|---|---|---|---|
| T1 Lipase | Rand Protease | Maltogenic Amylase | ||
| Control | - | 100 | 100 | 100 |
| surfactants | PEG (non-ionic) | 84.58 ± 0.04 | 94.96 ± 0.07 | 113.5 ± 0.01 |
| G600 (non-ionic) | 108.57± 0.07 | 99.33 ± 0.05 | 101 ± 0.07 | |
| Tween 80 (non-ionic) | 98.8 ± 0.04 | 115.51 ± 0.06 | 93.39 ± 0.07 | |
| SDS (anionic) | 14 ± 0.03 | 10 ± 0.001 | 1.08 ± 0.08 | |
| Bleach | Sodium percarbonate | 5.44 ± 0.06 | 5.2 ± 0.05 | 21.81 ± 0.60 |
| Sodium perborate | 6.40 ± 0.07 | 24.32 ± 0.19 | 1.2 ± 0.50 | |
| Dispersing agent | Sodium polyacrylate | 54 ± 0.18 | 48 ± 0.13 | 71.9 ± 0.03 |
| Builders | Sodium citrate | 48 ± 0.03 | 44.74 ± 0.04 | 96 ± 0.05 |
| Sodium metasillicate | 7.55 ± 0.06 | 16.65 ± 0.02 | 0.3 ± 0.05 | |
| Sodium silicate | 20.8 ± 0.30 | 16.43 ± 0.04 | 0.68 ± 0.04 | |
| Control | Glycine-NaOH, pH 9.0 | 100 | 100 | 100 |
| Alkalinity agents | Phosphate, pH 7.0 | 88.4 ± 0.09 | 100.3 ± 0.01 | 125 ± 0.02 |
| Tris-HCl, pH 7.0 | 42 ± 0.04 | 106 ± 0.10 | 64.4 ± 0.21 | |
| Sodium citrate, pH 8.3 | 48 ± 0.03 | 54.74 ± 0.04 | 96 ± 0.05 | |
| Sodium bicarbonate (SB), pH 8.6 | 80.7 ± 0.04 | 83.3 ± 0.27 | 129 ± 0.06 | |
| Sodium carbonate (SC): glycine (30:70), pH 9.25 | 120 ± 0.17 | 92 ± 0.01 | 119.1 ± 0.2 | |
| SC:SB (30:70), pH 9.5 | 5 ± 0.05 | 67.9 ± 0.02 | 70 ± 0.08 | |
| Enzymes | Encapsulated Enzyme | Powdered Free Enzyme | Control (Liquid Free Enzyme) | |
|---|---|---|---|---|
| T1 lipase | Total activity (U) | 1048.3 | 420 | 1098 |
| Activity retained (%) | 95.5 | 38.25 | 100 | |
| Rand protease | Total activity (U) | 10289 | 5032.5 | 11250 |
| Activity retained (%) | 91.4 | 44.73 | 100 | |
| Maltogenic amylase | Total activity (U) | 744.4 | 31.26 | 990 |
| Activity retained (%) | 75.2 | 3.2 | 100 |
| Detergent Concentration (%) | Percentage of Soil Removal | |
|---|---|---|
| Detergent A | Detergent B | |
| 0 | 8.3 ± 1.2 p1 | 8.3 ± 1.2 q1 |
| 1.5 | 41.4 ± 1.8 p2 | 49.6 ± 0.3 q2 |
| 2 | 44.0 ± 1.7 p2 | 51.0 ± 1.4 q2 |
| 2.5 | 44.6 ± 1.8 p2 | 51.3 ± 1.4 q2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naganthran, A.; Masomian, M.; Rahman, R.N.Z.R.A.; Ali, M.S.M.; Nooh, H.M. Improving the Efficiency of New Automatic Dishwashing Detergent Formulation by Addition of Thermostable Lipase, Protease and Amylase. Molecules 2017, 22, 1577. https://doi.org/10.3390/molecules22091577
Naganthran A, Masomian M, Rahman RNZRA, Ali MSM, Nooh HM. Improving the Efficiency of New Automatic Dishwashing Detergent Formulation by Addition of Thermostable Lipase, Protease and Amylase. Molecules. 2017; 22(9):1577. https://doi.org/10.3390/molecules22091577
Chicago/Turabian StyleNaganthran, Ashwini, Malihe Masomian, Raja Noor Zaliha Raja Abd. Rahman, Mohd Shukuri Mohamad Ali, and Hisham Mohd Nooh. 2017. "Improving the Efficiency of New Automatic Dishwashing Detergent Formulation by Addition of Thermostable Lipase, Protease and Amylase" Molecules 22, no. 9: 1577. https://doi.org/10.3390/molecules22091577
APA StyleNaganthran, A., Masomian, M., Rahman, R. N. Z. R. A., Ali, M. S. M., & Nooh, H. M. (2017). Improving the Efficiency of New Automatic Dishwashing Detergent Formulation by Addition of Thermostable Lipase, Protease and Amylase. Molecules, 22(9), 1577. https://doi.org/10.3390/molecules22091577





