Cytotoxic Evaluation of (2S)-5,7-Dihydroxy-6-prenylflavanone Derivatives Loaded PLGA Nanoparticles against MiaPaCa-2 Cells
Abstract
:1. Introduction
2. Results and Discussion
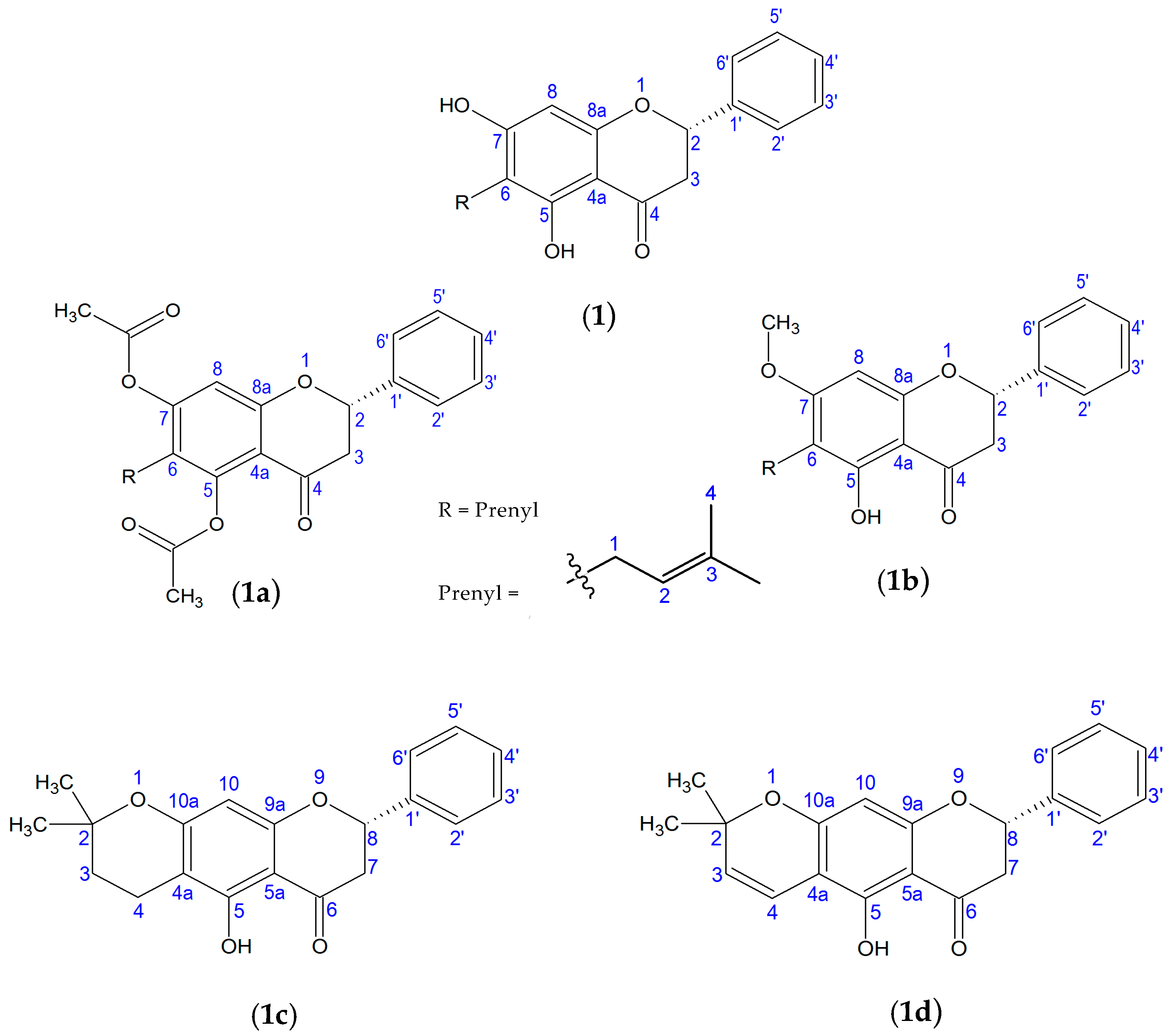
2.1. Chemical Characterization
2.2. Pharmacomodulation
2.2.1. Esterification
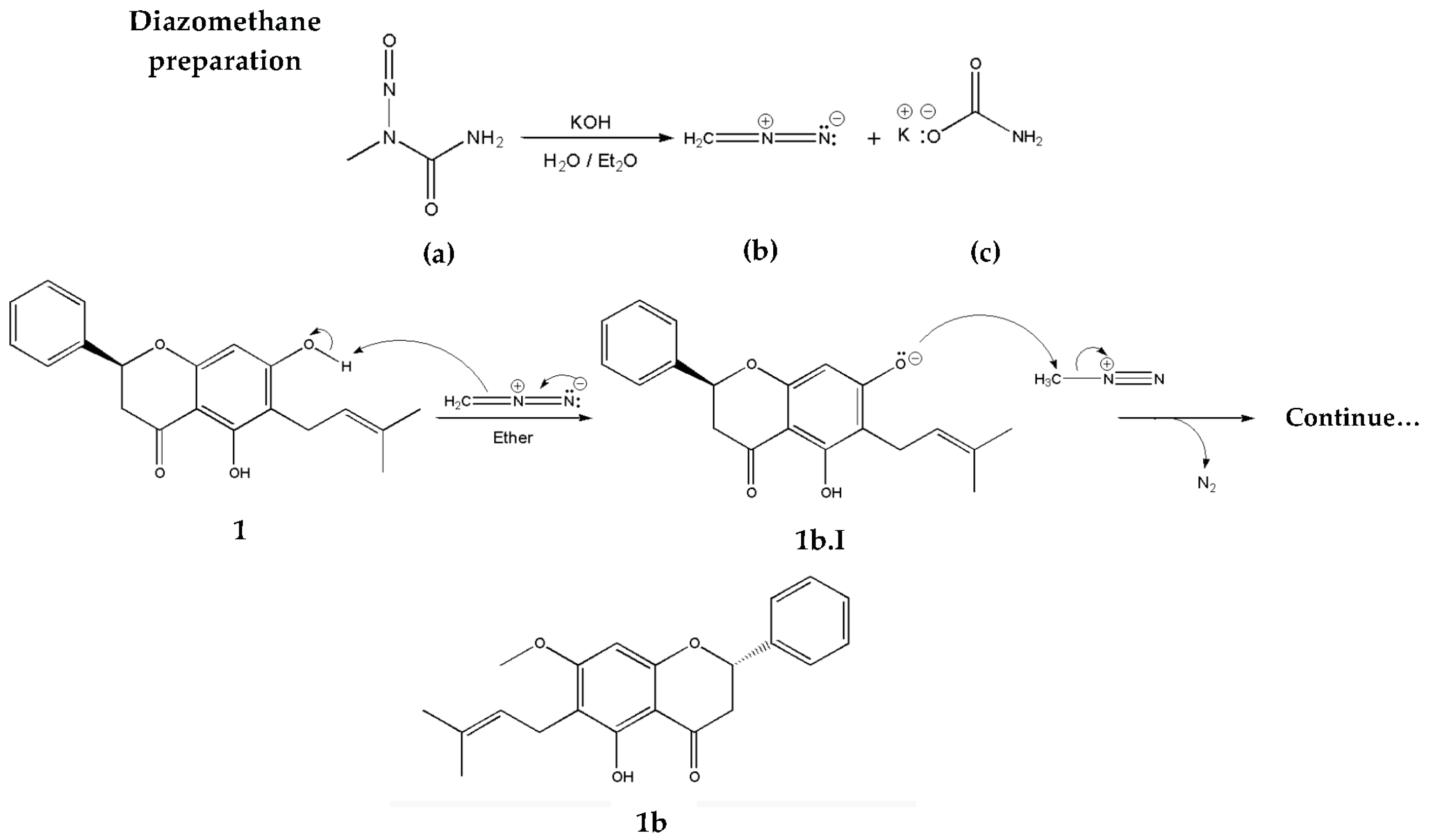
2.2.2. Methylation
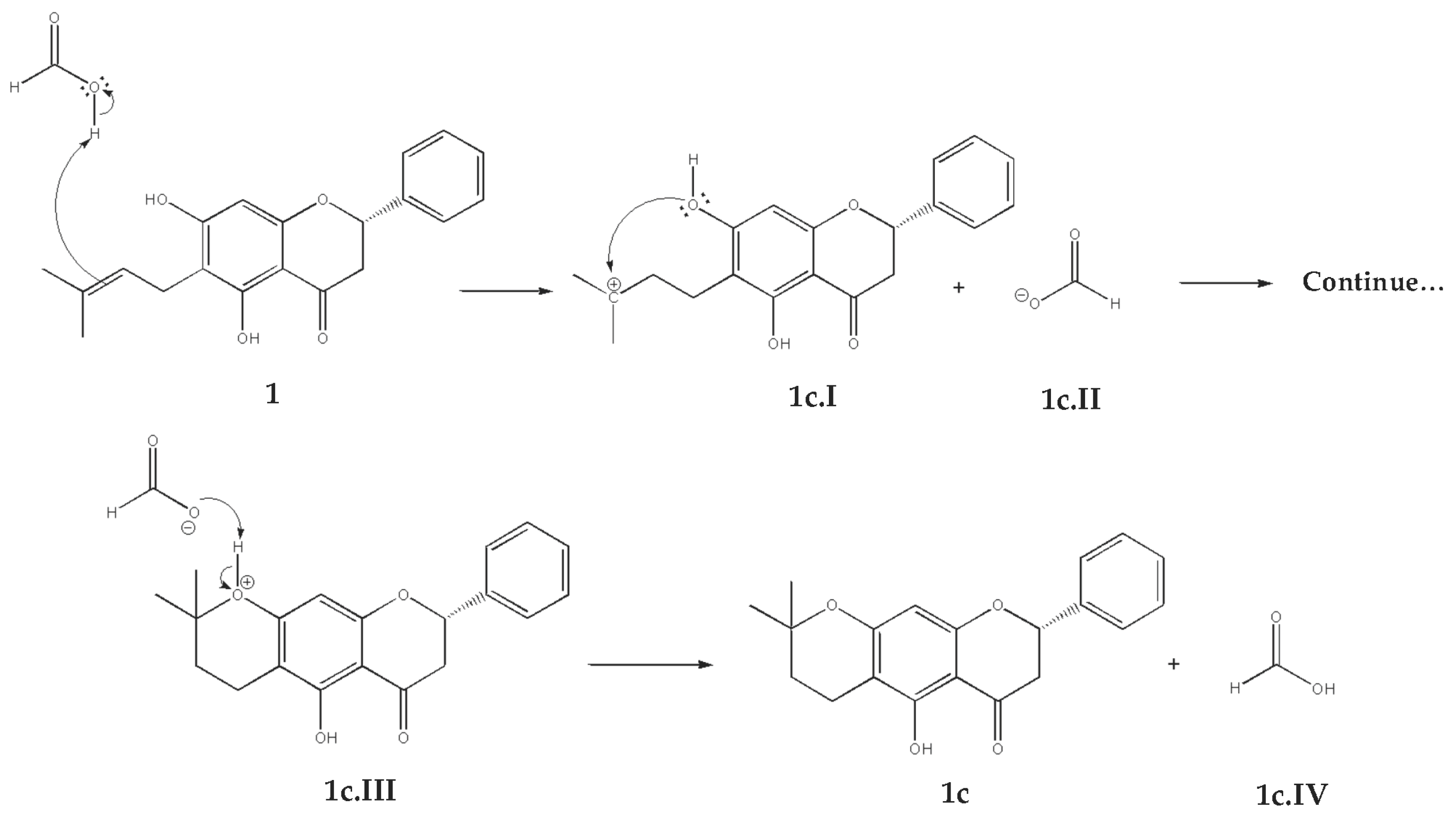
2.2.3. Cyclization
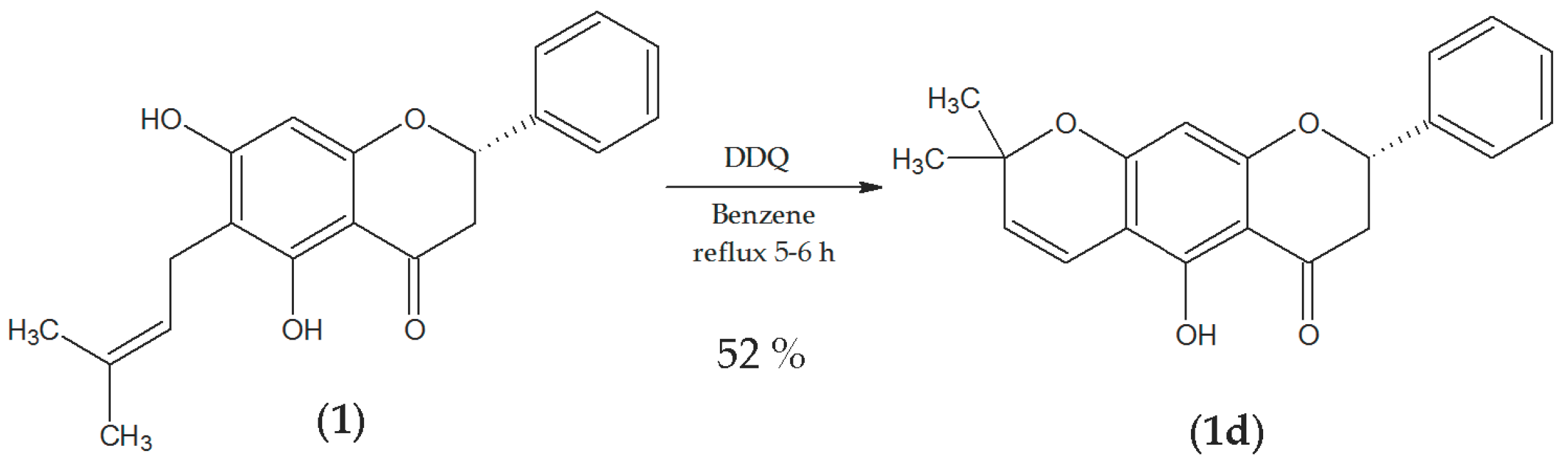
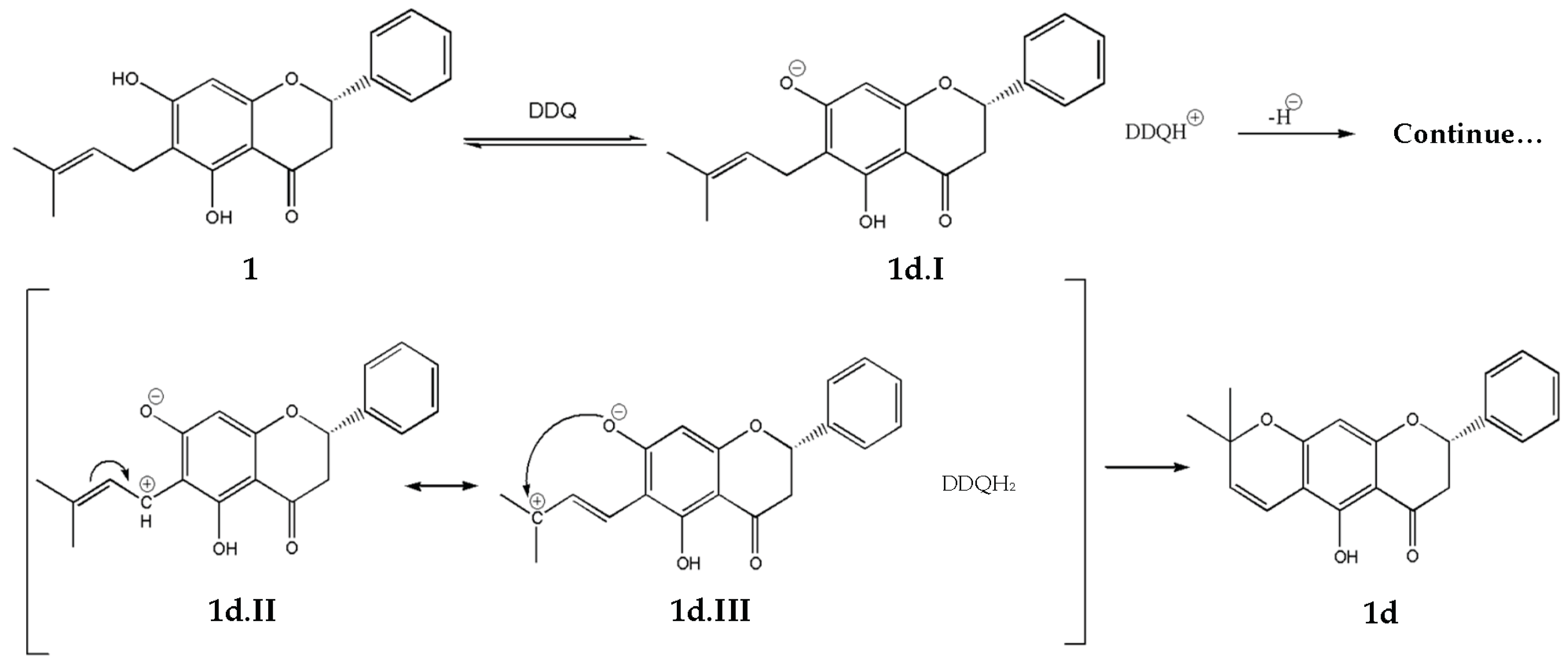
2.2.4. Vinylogous-Cyclization
2.3. Cytotoxic Assay of Free Compounds
2.3.1. Brine Shrimp (Artemia salina)
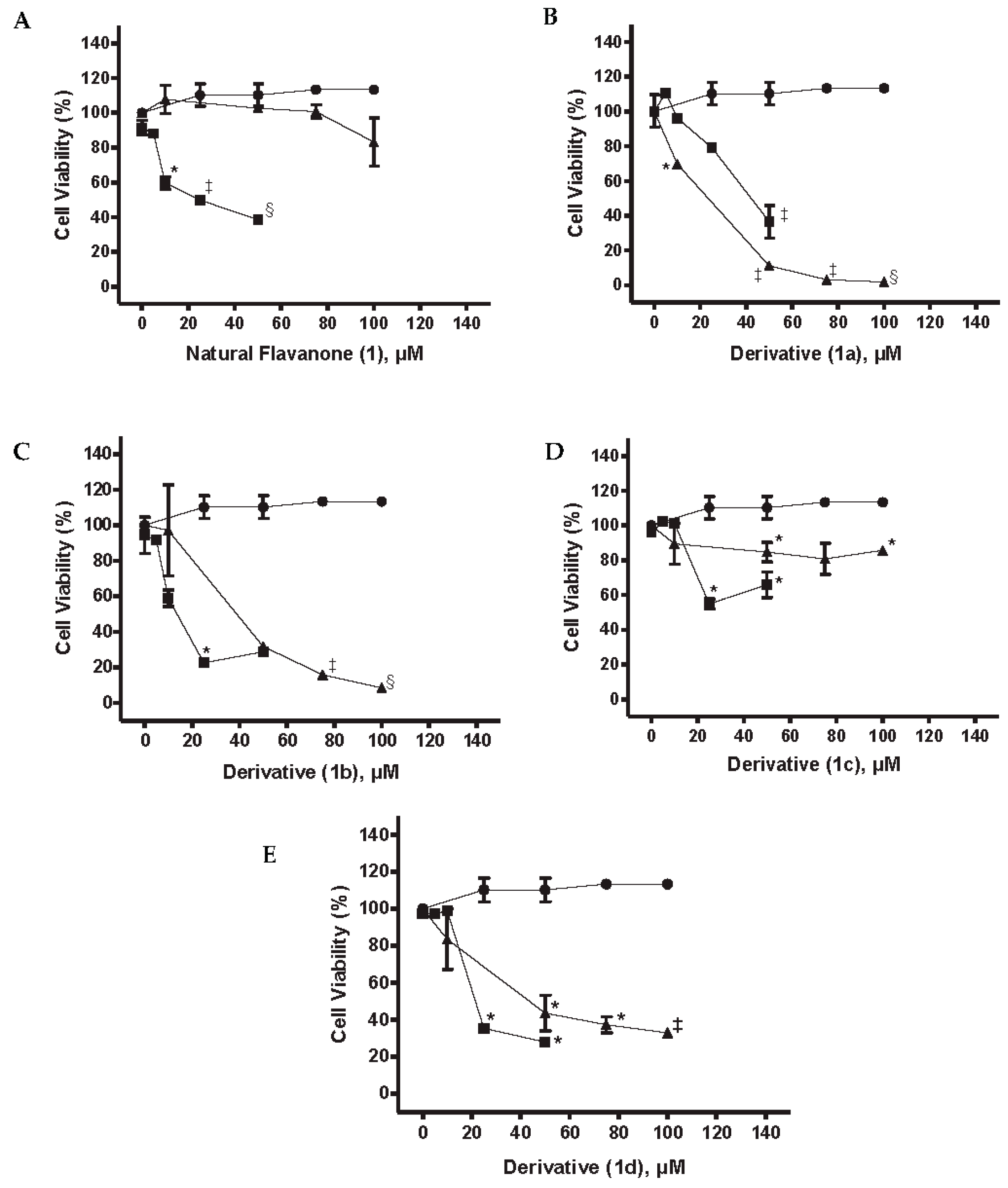
2.3.2. Cell Viability MiaPaCa-2 Cells
2.4. PLGA NPs
2.5. Cytotoxic Activity of NPs
3. Materials and Methods
3.1. Plant Material
3.2. Materials and Instrumentation
3.3. Preparation of Methanolic Extract
3.4. Isolation of Compound (2S)-5,7-Dihydroxy-6-(3-methyl-2-buten-1-yl)-2-phenyl-2,3-dihydro-4H-1-benzopyran-4-one (1)
3.5. Preparation of Derivatives
3.6. Preparations PLGA NPs
3.6.1. Particle Size Analysis
3.6.2. Zeta Potential Measurements
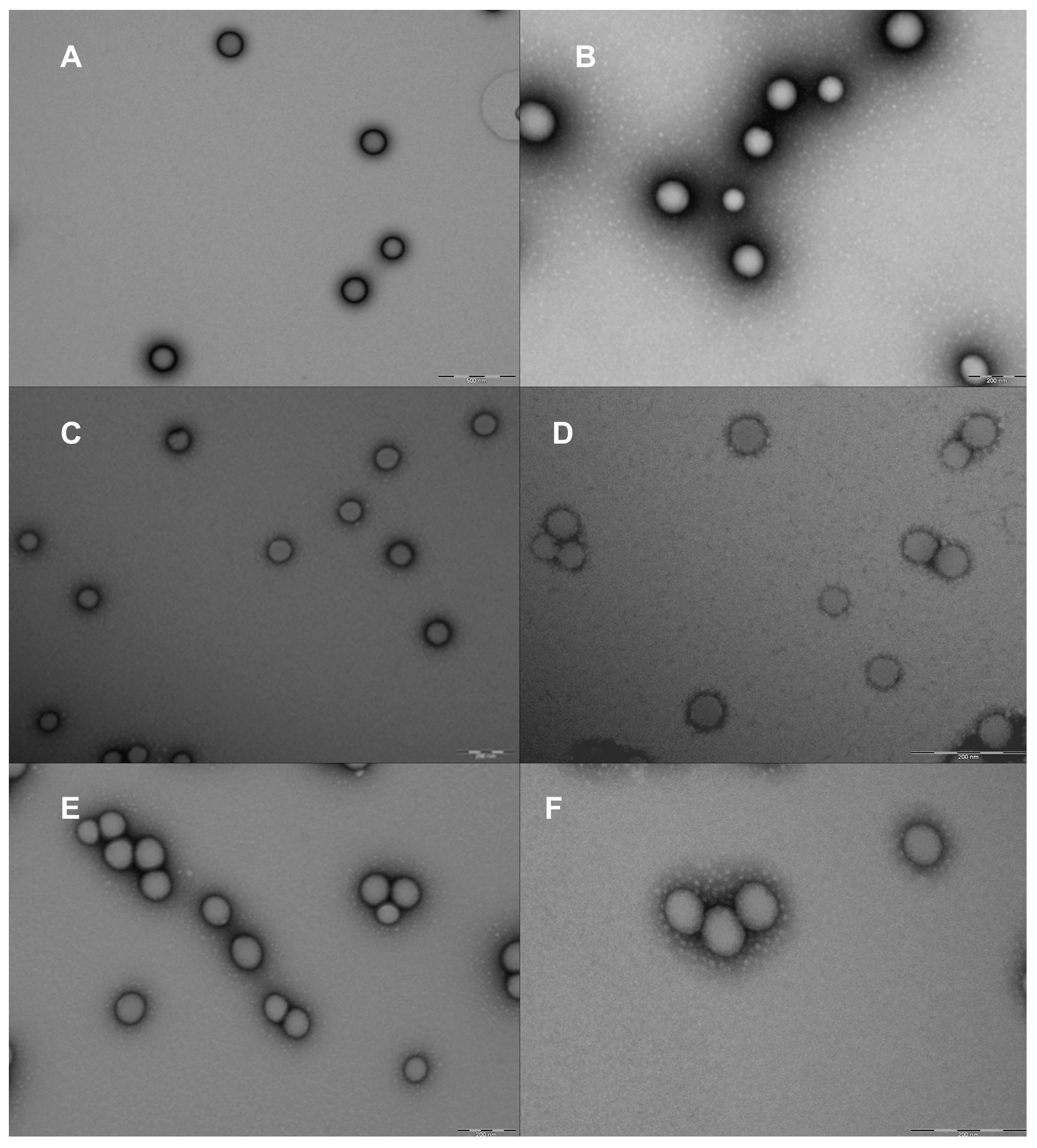
3.6.3. Morphological Studies
3.6.4. Entrapment Efficiency (EE %)
3.7. Cytotoxic Assays
3.7.1. Brine Shrimp, Artemia salina Assay
3.7.2. Cell Culture
3.7.3. Cell Viability Studies
3.8. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Delle Monache, G. Prenylated flavonoids: Pharmacology and biotechnology. Curr. Med. Chem. 2005, 12, 717–739. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Villegas, V.; García, M.L.; Calpena, A.; Clares-Naveros, B.; Garduño-Reamírez, M.L. Anti-Inflammatory, antioxidant and cytotoxicity activities of methanolic extract prenylated flavanones isolated from leaves of Eysehardtia platycarpa. Nat. Prod. Commun. 2013, 8, 177–180. [Google Scholar] [PubMed]
- Śmejkal, K. Cytotoxic potential of C-prenylated flavonoids. Phytochem. Rev. 2014, 13, 245–275. [Google Scholar] [CrossRef]
- Cano, A.; Espinoza, M.; Ramos, C.H.; Delgado, G. New prenylated flavanones from Esenbeckia berlandieri ssp. acapulcensis. J. Mex. Chem. Soc. 2006, 50, 71–75. [Google Scholar]
- Bohm, B.A. Flavanones and dihydroflavonols. In The Flavonoids, 1st ed.; Harborne, J.B., Mabry, T.J., Mabry, H., Eds.; Springer-Science Business Media: Boston, MA, USA, 1975; pp. 560–631. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Swami, A.; Shi, J.; Gadde, S.; Votruba, A.R.; Kolishetti, N.; Farokhzar, O.C. Nanoparticles for targeted and temporally controlled drug delivery. In Multifunctional Nanoparticles for Drug Delivery, Applications: Imaging, Targeting and Delivery, Nanostructure Science Technology; Svenson, S., Prud’homme, R.K., Eds.; Springer: Boston, MA, USA, 2012; Volume 2, pp. 9–25. ISBN 978-1-4614-2304-1. [Google Scholar]
- Domínguez-Villegas, V.; Clares-Naveros, B.; García, M.L.; Calpena-Campmany, C.; Bustos-Zagal, P.; Garduño-Ramirez, M.L. Development and characterization of two nano-structured systems for topical application of flavanones isolated from Eysenhardtia platycarpa. Colloids Surf. B Biointerface 2014, 116, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-Assambled lipip-polymer hybrid nanoparticles: A robust drug delivery plataform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Cabeza, L.; Arias, J.L.; Melguizo, C.; Álvarez, P.J.; Vélez, C.; Clares, B.; Áranega, A.; Prados, J. Poly(butylcyanoacrylate) and poly(ε-caprolactone) nanoparticles loaded with 5-fluorouracil increase the cytotoxic effect of the drug in experimental colon cancer. AAPS J. 2015, 17, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, V.K.; Sharma, N.D.; Mittal, B.; Gupta, S.R. Novel prenylated flavanoids from M. philippensis Muell. Arg. Indian J. Chem. Sect. B 1988, 27, 238–241. [Google Scholar] [CrossRef]
- Narváez-Mastache, J.M.; Garduño-Ramírez, M.L.; Alvarez, L.; Delgado, G. Antihyperglycemic activity and chemical constituents of Eysenhardtia platycarpa (Fabaceae). J. Nat. Prod. 2006, 27, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.I.; Juliani, H.R; Gros, E.G. Quercetin 3,7,3′-trisulphate from Flaveria bidentis. Phytochemistry 1985, 24, 1394–1395. [Google Scholar] [CrossRef]
- Delgado, A.; Minguillon, C.; Joglar, J. Introducción a la Química Terapéutica, 2nd ed.; Ediciones Díaz de Santos, S. A.: Madrid, España, 2004; pp. 119–142. [Google Scholar]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Ta, N.; Kawamori, T.; Wen, X.; Tsuji, P.A.; Walle, U.K. Cancer chemopreventive properties of orally bioavailable flavonoids—Methylated versus unmethylated flavones. Biochem. Pharmacol. 2007, 73, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.G.; Fuzzati, N.; Li, Q.S.; Yang, C.R.; Stoeckli-Evans, H.; Hostettmann, K. Polyphenols from Eriosema tuberosum. Phytochemistry 1995, 39, 1049–1061. [Google Scholar] [CrossRef]
- Filho, R.B.; Gottlieb, O.R.; Mourao, A.P. A stilbene and two flavanones from Derris rariflora. Phytochemistry 1975, 14, 261–263. [Google Scholar] [CrossRef]
- Ying, H.; Hu, Y.; He, Q.; Li, R.; Yang, B. Synthesis and anticancer activity of a novel class of flavonoids: 2,4-Diarylchromane[4,3-d]-Δ1,9b-1,2,3-thiadiazolines. Eur. J. Med. Chem. 2007, 42, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.C.; Gupta, R.C.; Sarpal, P.D. Synthesis of (±) lupinifolin, di-O-methyl xanthohumol and isoxanthohumol and related compounds. Tetrahedron 1978, 34, 3563–3567. [Google Scholar] [CrossRef]
- Ahluwalia, V.K.; Jain, A.; Gupta, R.A. A convenient synthesis of linear 2-methylpyrianochromones. Bull. Chem. Soc. Jpn. 1982, 55, 2649–2652. [Google Scholar] [CrossRef]
- Ahluwalia, V.K.; Jolly, R.S. Reaction of 2,2-dimethylchromans with 2,3-dichloro-5,6-dicyanobenzoguinone (DDQ). Synthesis 1982, 1, 74–75. [Google Scholar] [CrossRef]
- Manchanda, V.P.; Batta, A.K.; Khanna, P.L.; Khanna, R.N. Synthesis of glabranine, 5,7-dihydroxy-6-prenyl-flavanone and 5-hydroxy-7-methoxy-6-prenyl flavanone. Curr. Sci. 1976, 45, 322–323. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: New York, NY, USA, 1971. [Google Scholar]
- Sohna, H.Y.; Sona, K.H.; Kwona, C.S.; Kwonb, G.S.; Kange, S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Gradiz, R.; Silva, H.C.; Carvalho, L.; Botelho, M.F.; Mota-Pinto, A. MIA PaCa-2 and PANC-1-pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci. Rep. 2016, 6, 21648. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health. Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, O.; Wíniewski, J.; Środa-Pomianek, K.; Bielawska-Pohl, A.; Paprocka, M.; Duś, D.; Duarte, N.; Ferreira, M.J.U.; Michalak, K. Multidrug resistance reversal and apoptosis induction in human colon cancer cells by some flavonoids present in Citrus plants. J. Nat. Prod. 2012, 75, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Chapter 27. Bioisosterism in Drug Desing. Annu. Rep. Med. Chem. 1986, 21, 283–291. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Gourdeau, H.; Meerovitch, K.; Drewe, J.; Reddy, S.; Qiu, L.; Zhang, H.; Bergeron, F.; Bouffard, D.; Yang, Q.; et al. Discovery and mechanism of action of a novel series of apoptosis inducers with potential vascular targeting activity. Mol. Cancer Ther. 2004, 3, 1365–1373. [Google Scholar] [PubMed]
- Roginsky, A.B.; Ujiki, M.B.; Ding, X.Z.; Adrian, T.E. On the potential use of flavonoids in the treatment and prevention of pancreatic cancer. In Vivo 2005, 19, 61–68. Available online: http://iv.iiarjournals.org/content/19/1/61.long (accessed on 5 September 2017). [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Nat. Inst. Health 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Singh-Joy, S.D.; McLain, V.C. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int. J. Toxcol. 2008, 27, 93–128. [Google Scholar] [CrossRef]
- Vandervoot, J.; Ludwig, A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: A factorial design study. Int. J. Pharm. 2002, 238, 77–92. [Google Scholar] [CrossRef]
- Sierra, A.F.; Ramírez, M.L.; Campmany, A.C.; Martínez, A.R.; Naveros, B.C. In vivo and in vitro evaluation of the use of a newly developed melatonin loaded emulsion combined with UV filters as a protective agent against skin irradiation. J. Dermatol. Sci. 2013, 69, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Govender, T.; Stolnik, S.; Garnett, M.C.; Illum, L.; David, S.S. PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water soluble drug. J. Control. Release 1999, 57, 171–185. [Google Scholar] [CrossRef]
- Acharya, S.; Dilnawas, F.; Sahoo, S.K. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials 2009, 30, 5737–5750. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, M.G.; Gukasyan, H.J.; Davda, J.; Labhasetwar, V.; Kim, K.J.; Lee, V.H. Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: Role in PLGA nanoparticle endocytosis. Mol. Vis. 2003, 9, 559–568. [Google Scholar] [PubMed]
- Sahoo, S.K.; Labhasetwar, V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol. Pharmacol. 2005, 2, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.V.; Iyer, C.S.R.; Iyer, P.R. Cinnamoylation of chromans: Formation of flavonoids and neoflavonoids. Heterocycles 1986, 24, 1925–1930. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, 1–4. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Influence of concentration, ionic strength and pH on zeta potential and mean hydrodynamic diameter of edible polysaccharide solutions envisaged for multinanolayered films production. Carbohydr. Polym. 2011, 85, 522–528. [Google Scholar] [CrossRef]
- Deshiikan, S.; Papadopoulos, K. Modified Booth equation for the calculation of zeta potential. Colloid Polym. Sci. 1998, 276, 117–124. [Google Scholar] [CrossRef]
- Abramoff, M.D. ImageJ as an image processing tool and library. Microsc. Microanal. 2007, 13, 1672–1673. [Google Scholar] [CrossRef]
- Mayer, B.N.; Ferrigni, N.R.; Potnam, J.E.; Jacobson, L.B.; Nicholas, D.E.; Mclaughin, J.L. Brine shrimp: A general convenient bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, D. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Villalobos, X.; Dakhel, S.; Padilla, L.; Hervas, R.; Hernández, J.L.; Ciudad, C.J.; Noé, V. Polypurine reverse Hoogsteen hairpins prostate cancer PC3 cells in vitro and in vivo. Biochem. Pharm. 2013, 86, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| Compounds | Concentration (ppm/µM) | Percentage Mortality Values (%) |
|---|---|---|
| 1 | 0/0 | 0.0 |
| 10/0.03083 | 0.0 | |
| 100/0.3083 | 11.5 | |
| 1000/3.0828 | 43.2 | |
| 1a | 0/0 | 0.0 |
| 10/0.0245 | 36.7 | |
| 100/0.245 | 40.0 | |
| 1000/2.45 | 30.0 | |
| 1b | 0/0 | 0.0 |
| 10/0.02955 | 10.0 | |
| 100/0.29551 | 0.0 | |
| 1000/2.9551 | 0.0 | |
| 1c | 0/0 | 0.0 |
| 10/0.03083 | 50.0 | |
| 100/0.3083 | 45.0 | |
| 1000/3.0828 | 40.0 | |
| 1d | 0/0 | 0.0 |
| 10/0.03102 | 13.3 | |
| 100/0.3102 | 0.0 | |
| 1000/3.102 | 0.0 |
| Concentration | Percentage Cell Viability (%) of MiaPaCa-2 | ||||
|---|---|---|---|---|---|
| 1 | 1a | 1b | 1c | 1d | |
| DMSO and Control | 91.35 ± 4.18 | 100.28 ± 9.39 | 94.27 ± 10.30 | 95.99 ± 1.14 | 97.47 ± 2.22 |
| 5 μM | 88.08 ± 1.99 | 110.67 ± 2.03 | 91.65 ± 2.04 | 102.18 ± 1.05 | 97.24 ± 1.20 |
| 10 μM | 59.63 ± 3.54 | 96.19 ± 1.40 | 58.94 ± 4.58 | 101.23 ± 1.45 | 98.81 ± 1.70 |
| 25 μM | 49.80 ± 1.19 | 79.22 ± 1.16 | 22.56 ± 1.69 | 54.82 ± 2.84 | 35.35 ± 2.50 |
| 50 μM | 38.48 ± 1.52 | 36.54 ± 9.34 | 28.74 ± 2.53 | 65.85 ± 7.46 | 27.86 ± 1.25 |
| PLGA Nanoparticles | Concentration (mM) | Entrapment Efficiency (EE %) |
|---|---|---|
| NPs1 | 4.62 | 80.00 ± 4.75 |
| NPs1a | 3.67 | 88.47 ± 4.18 |
| NPs1b | 4.43 | 85.00 ± 5.80 |
| NPs1c | 4.62 | 78.28 ± 5.85 |
| NPs1d | 4.65 | 78.75 ± 4.34 |
| Nanoparticles | Z-Average (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| Blank | 195.667 ± 1.069 | 0.054 ± 0.016 | −4.237 ± 0.242 |
| NPs1 | 205.200 ± 0.265 | 0.058 ± 0.053 | −8.250 ± 0.346 |
| NPs1a | 178.033 ± 1.332 | 0.080 ± 0.004 | −9.053 ± 0.323 |
| NPs1b | 141.633 ± 0.777 | 0.086 ± 0.017 | −10.633 ± 0.231 |
| NPs1c | 175.167 ± 0.603 | 0.101 ± 0.031 | −6.480 ± 0.377 |
| NPs1d | 173.400 ± 1.587 | 0.064 ± 0.007 | −6.647 ± 0.405 |
| Nanoparticles | Z-Average (nm) |
|---|---|
| Blank | 157.295 ± 10.405 |
| NPs1 | 112.779 ± 19.232 |
| NPs1a | 76.430 ± 7.197 |
| NPs1b | 54.351 ± 7.156 |
| NPs1c | 102.228 ± 11.656 |
| NPs1d | 81.933 ± 8.500 |
| Concentration | Percentage Cell Viability (%) of MiaPaCa-2 against Developed NPs | |||||
|---|---|---|---|---|---|---|
| Blank NPs | NPs1 | NPs1a | NPs1b | NPs1c | NPs1d | |
| 0 μM | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 10 μM | 111.24 ± 6.44 | 107.75 ± 8.18 | 69.62 ± 2.26 | 97.12 ± 25.64 | 89.44 ± 11.74 | 83.51 ± 16.33 |
| 50 μM | 111.24 ± 6.44 | 102.68 ± 1.64 | 11.22 ± 2.36 | 31.88 ± 2.43 | 84.66 ± 5.60 | 43.59 ± 9.60 |
| 75 μM | 113.40 ± 15.50 | 100.75 ± 4.00 | 3.20 ± 1.88 | 15.88 ± 2.40 | 80.68 ± 9.03 | 37.30 ± 4.33 |
| 100 μM | 113.40 ± 15.50 | 83.24 ± 13.81 | 1.76 ± 1.01 | 8.47 ± 1.05 | 85.69 ± 2.41 | 32.85 ± 2.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade-Carrera, B.; Clares, B.; Noé, V.; Mallandrich, M.; Calpena, A.C.; García, M.L.; Garduño-Ramírez, M.L. Cytotoxic Evaluation of (2S)-5,7-Dihydroxy-6-prenylflavanone Derivatives Loaded PLGA Nanoparticles against MiaPaCa-2 Cells. Molecules 2017, 22, 1553. https://doi.org/10.3390/molecules22091553
Andrade-Carrera B, Clares B, Noé V, Mallandrich M, Calpena AC, García ML, Garduño-Ramírez ML. Cytotoxic Evaluation of (2S)-5,7-Dihydroxy-6-prenylflavanone Derivatives Loaded PLGA Nanoparticles against MiaPaCa-2 Cells. Molecules. 2017; 22(9):1553. https://doi.org/10.3390/molecules22091553
Chicago/Turabian StyleAndrade-Carrera, Berenice, Beatriz Clares, Véronique Noé, Mireia Mallandrich, Ana C. Calpena, María Luisa García, and María Luisa Garduño-Ramírez. 2017. "Cytotoxic Evaluation of (2S)-5,7-Dihydroxy-6-prenylflavanone Derivatives Loaded PLGA Nanoparticles against MiaPaCa-2 Cells" Molecules 22, no. 9: 1553. https://doi.org/10.3390/molecules22091553
APA StyleAndrade-Carrera, B., Clares, B., Noé, V., Mallandrich, M., Calpena, A. C., García, M. L., & Garduño-Ramírez, M. L. (2017). Cytotoxic Evaluation of (2S)-5,7-Dihydroxy-6-prenylflavanone Derivatives Loaded PLGA Nanoparticles against MiaPaCa-2 Cells. Molecules, 22(9), 1553. https://doi.org/10.3390/molecules22091553







