Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion
Abstract
1. Introduction
2. Results and Discussion
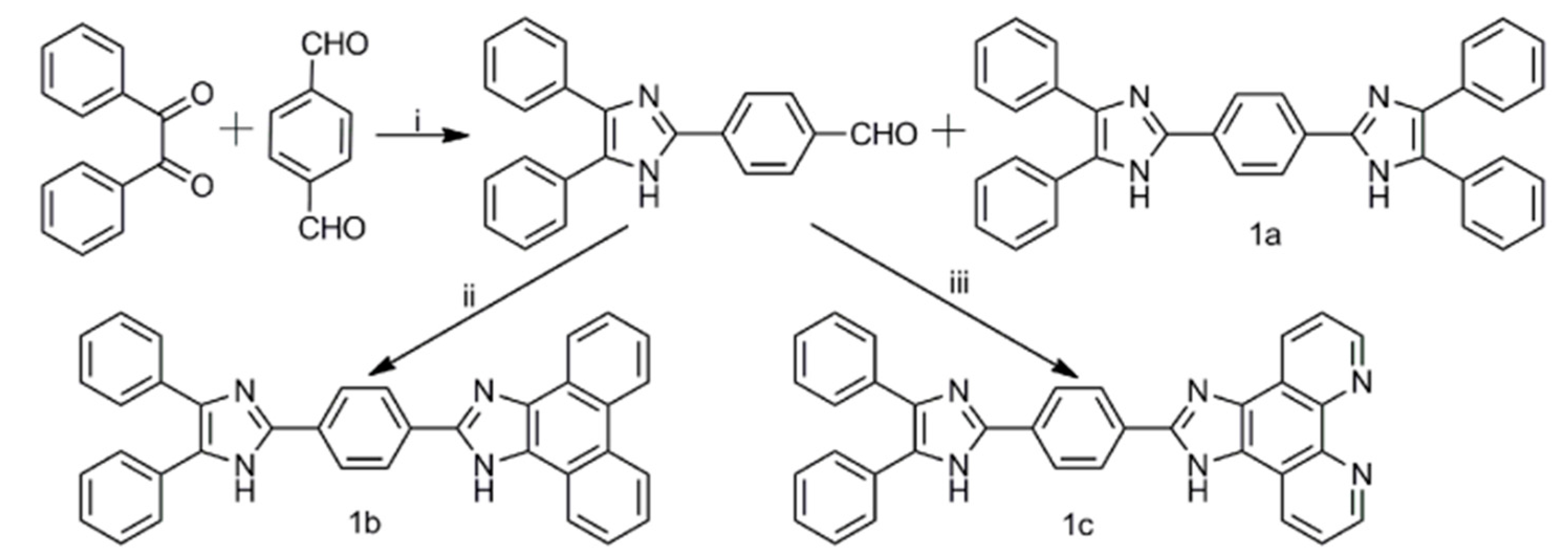
2.1. Synthesis of Compounds 1a–1c
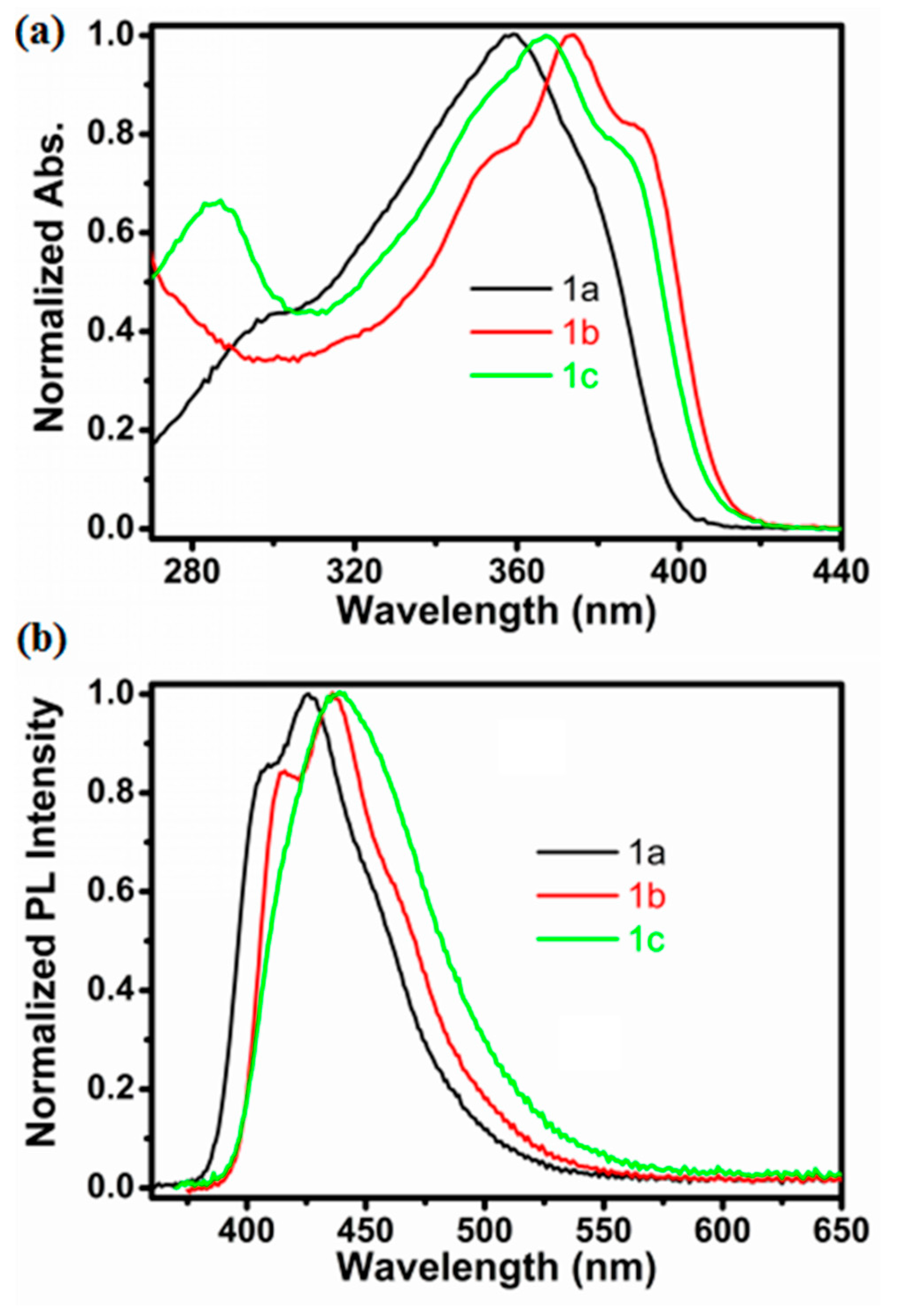
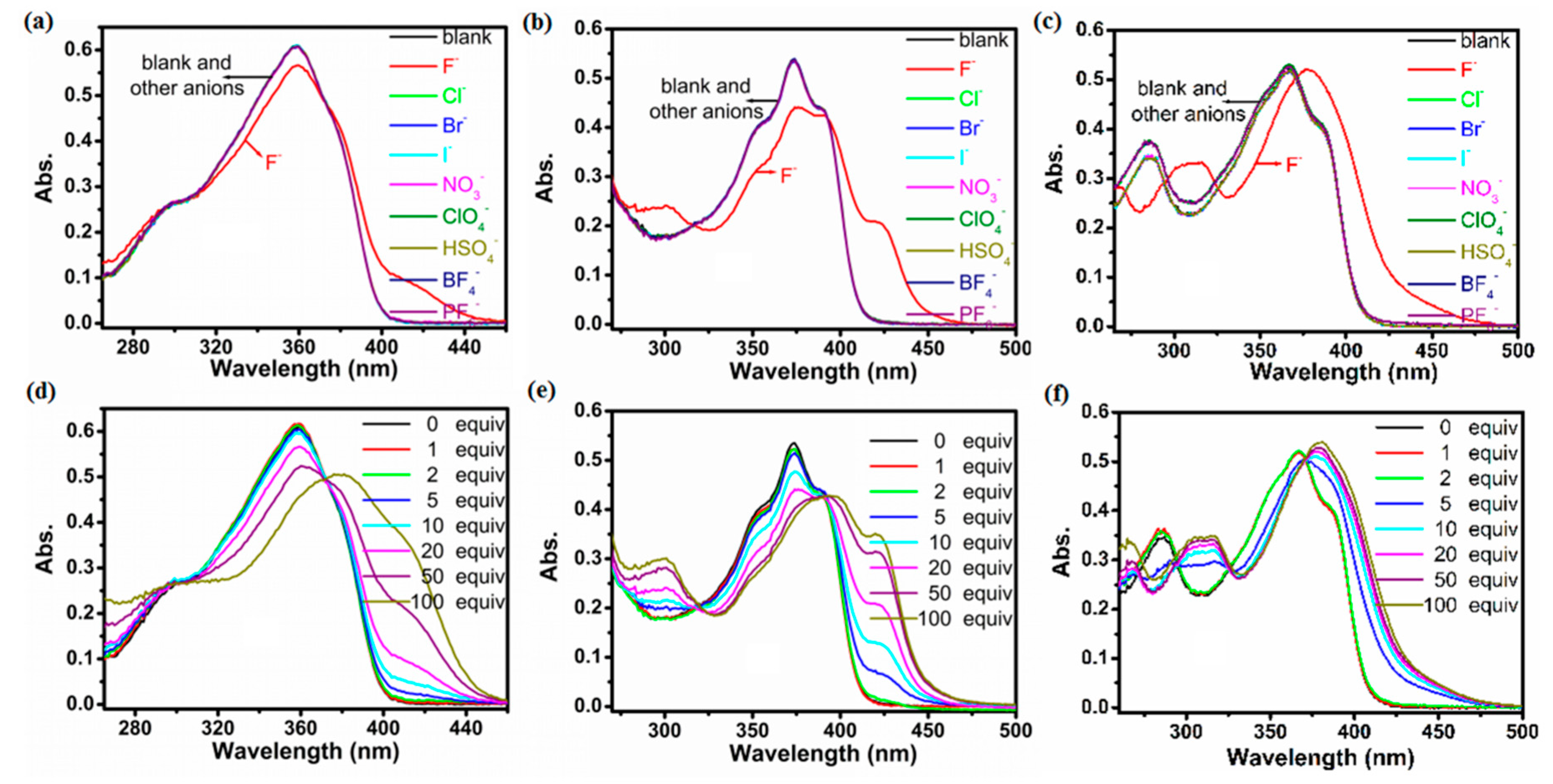
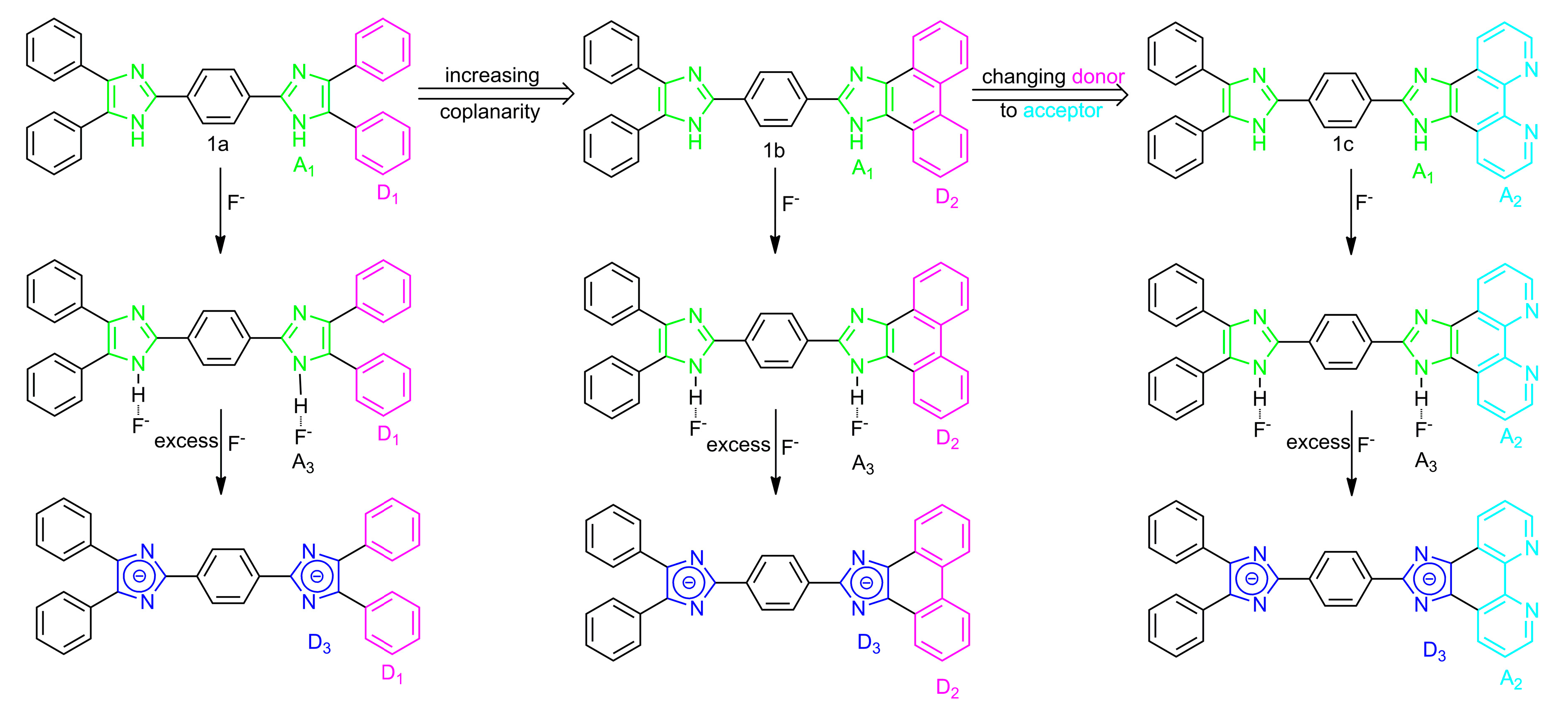
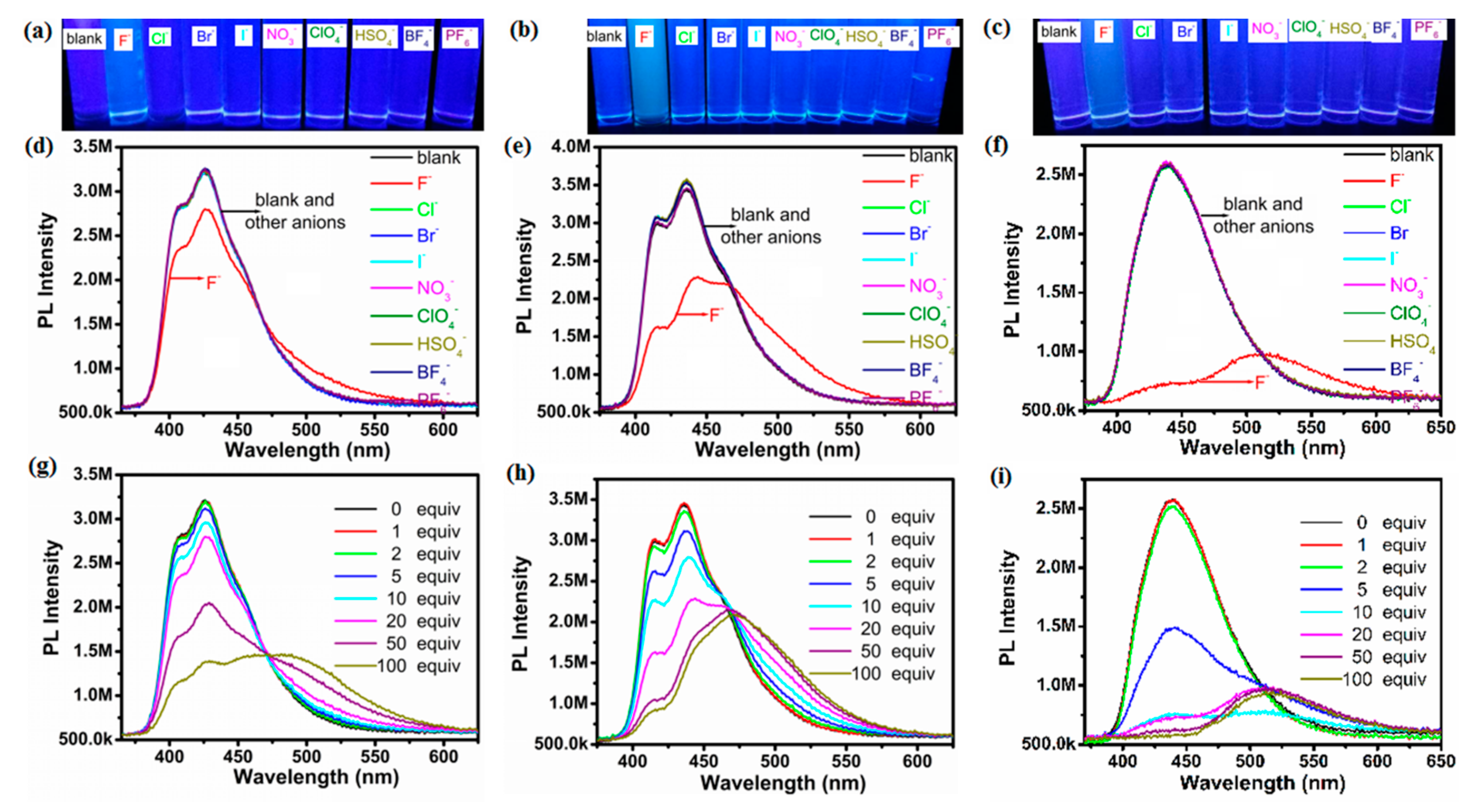
2.2. Optical Properties of Compounds 1a–1c
3. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Descalzo, A.B.; Rurack, K.; Weisshoff, H.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P.; Hoffmann, K.; Soto, J. Rational design of a chromo- and fluorogenic hybrid chemosensor material for the detection of long-chain carboxylates. J. Am. Chem. Soc. 2005, 127, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Vetrichelvan, M.; Nagarajan, R.; Valiyaveettil, S. Carbazole-containing conjugated copolymers as colorimetric/fluorimetric sensor for iodide anion. Macromolecules 2006, 39, 8303–8310. [Google Scholar] [CrossRef]
- Lin, T.-P.; Chen, C.-Y.; Wen, Y.-S.; Sun, S.-S. Synthesis, photophysical, and anion-sensing properties of quinoxalinebis (sulfonamide) functionalized receptors and their metal complexes. Inorg. Chem. 2007, 46, 9201–9212. [Google Scholar] [CrossRef] [PubMed]
- Ajayakumar, M.; Mukhopadhyay, P.; Yadav, S.; Ghosh, S. Single-electron transfer driven cyanide sensing: A new multimodal approach. Org. Lett. 2010, 12, 2646–2649. [Google Scholar] [CrossRef] [PubMed]
- Eun Jun, M.; Roy, B.; Han Ahn, K. “Turn-on” fluorescent sensing with “reactive” probes. Chem. Commun. 2011, 47, 7583–7601. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef] [PubMed]
- Ros-Lis, J.V.; Martinez-Manez, R.; Soto, J. Subphthalocyanines as fluoro-chromogenic probes for anions and their application to the highly selective and sensitive cyanide detection. Chem. Commun. 2005, 5260–5262. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Müller, A.M.; Bardeen, C.J.; Cheng, Q. Self-assembly combined with photopolymerization for the fabrication of fluorescence “turn-on” vesicle sensors with reversible “on-off” switching properties. Adv. Mater. 2006, 18, 55–60. [Google Scholar] [CrossRef]
- Garcia, F.; Garcia, J.M.; Garcia-Acosta, B.; Martinez-Manez, R.; Sancenon, F.; Soto, J. Pyrylium-containing polymers as sensory materials for the colorimetric sensing of cyanide in water. Chem. Commun. 2005, 2790–2792. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, J.; Ma, J.; Zhu, D.; Zhang, Y.; Liang, L.; Lu, M. An efficient long fluorescence lifetime polymer-based sensor based on europium complex as chromophore for the specific detection of F−, CH3COO−, and H2PO4−. Polym. Chem. 2012, 3, 2640–2648. [Google Scholar] [CrossRef]
- Isaad, J.; Perwuelz, A. New color chemosensors for cyanide based on water soluble azo dyes. Tetrahedron Lett. 2010, 51, 5810–5814. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Feng, J.; Hu, D.; Wang, S.; Li, S.; Li, Y.; Yang, G. A rapid aqueous fluoride ion sensor with dual output modes. Angew. Chem. Int. Ed. 2010, 49, 4915–4918. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Swager, T.M. A fluorescent self-amplifying wavelength-responsive sensory polymer for fluoride ions. Angew. Chem. Int. Ed. 2003, 42, 4803–4806. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Chen, W.; Ni, N.; Cheng, Y.; Dai, C.; Dinh, H.; Wang, B. A fluorescent probe for rapid aqueous fluoride detection and cell imaging. Chem. Commun. 2013, 49, 2494–2496. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Piersanti, G.; Retini, M.; Varrese, M.A.; Zappia, G. New coumarin-urea based receptor for anions: A selective off–on fluorescence response to fluoride. Tetrahedron 2012, 68, 3768–3775. [Google Scholar] [CrossRef]
- Martinez-Manez, R.; Sancenon, F. New advances in fluorogenic anion chemosensors. J. Fluoresc. 2005, 15, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Qi, H.; Wang, L.; Su, Z.; Wang, G. A ratiometric fluorescent probe for fluoride ion employing the excited-state intramolecular proton transfer. Talanta 2009, 80, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Liu, B.; Wang, H.; Tian, J.; Bai, R. A “naked eye” and ratiometric fluorescent chemosensor for rapid detection of F− based on combination of desilylation reaction and excited-state proton transfer. Chem. Commun. 2011, 47, 3957–3959. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, G.; Chellappa, D. Rhodamine based sensor for naked-eye detection and live cell imaging of fluoride ions. J. Mater. Chem. B 2013, 1, 5768–5772. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.; Koh, M.; Park, S.B.; Hong, J.I. Fluorescent probe for detection of fluoride in water and bioimaging in A549 human lung carcinoma cells. Chem. Commun. 2009, 21, 4735–4737. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Mosca, L. The interaction of fluoride with fluorogenic ureas: An ON1-OFF-ON2 response. J. Am. Chem. Soc. 2013, 135, 6345–6355. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Singha, S.; Wang, T.; Seo, E.; Lee, J.H.; Lee, S.J.; Kim, K.H.; Ahn, K.H. In vivo two-photon fluorescent imaging of fluoride with a desilylation-based reactive probe. Chem. Commun. 2012, 48, 10243–10245. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Zhao, Y.G.; Duan, C.Y.; Zhang, B.G.; Bai, Z.P. A highly selective chromo- and fluorogenic dual responding fluoride sensor: Naked-eye detection of F- ion in natural water via a test paper. Dalton Trans. 2006, 30, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, Z.; Zhou, Y.; Li, Y.; Xu, Z. Colorimetric fluoride sensor based on 1,8-naphthalimide derivatives. Dyes Pigments 2011, 91, 442–445. [Google Scholar] [CrossRef]
- Rao, M.R.; Mobin, S.M.; Ravikanth, M. Boron–dipyrromethene based specific chemodosimeter for fluoride ion. Tetrahedron 2010, 66, 1728–1734. [Google Scholar] [CrossRef]
- Hou, P.; Chen, S.; Wang, H.; Wang, J.; Voitchovsky, K.; Song, X. An aqueous red emitting fluorescent fluoride sensing probe exhibiting a large Stokes shift and its application in cell imaging. Chem. Commun. 2014, 50, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, Q.; Li, Z.; Lai, G.; Jiang, J.; Shen, Z. A specific chemodosimeter for fluoride ion based on a pyrene derivative with trimethylsilylethynyl groups. Org. Biomol. Chem. 2011, 9, 4558–4562. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Das, A.K.; Manna, A.; Maity, A.K.; Fun, H.-K.; Quah, C.K.; Saha, P. A colorimetric and ratiometric fluorescent turn-on fluoride chemodosimeter and application in live cell imaging: High selectivity via specific SiO cleavage in semi aqueous media and prompt recovery of ESIPT along with the X-ray structures. Tetrahedron Lett. 2014, 55, 2633–2638. [Google Scholar] [CrossRef]
- Gu, P.-Y.; Wang, Z.; Zhang, Q. Azaacenes as active elements for sensing and bio applications. J. Mater. Chem. B 2016, 4, 7060–7074. [Google Scholar] [CrossRef]
- Gu, P.-Y.; Wang, Z.; Liu, G.; Nie, L.; Ganguly, R.; Li, Y.; Zhang, Q. Synthesis, physical properties, and sensing behaviour of a novel naphthalenediimide derivative. Dyes Pigments 2016, 131, 224–230. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Karmakar, P.; Roy, J.; Manna, S.; Maiti, K.; Sahoo, P.; Mandal, D. Colorimetric and ratiometric fluorescent chemosensor for fluoride ions based on phenanthroimidazole (PI): Spectroscopic, NMR and density functional studies. RSC Adv. 2015, 5, 37935–37942. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Oliveira, E.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Cyanide and fluoride colorimetric sensing by novel imidazo-anthraquinones functionalised with indole and carbazole. Supramol. Chem. 2013, 26, 71–80. [Google Scholar] [CrossRef]
- Kumar, D.; Thomas, K.R.J. 2-Hydroxyarylimidazole-based colorimetric and ratiometric fluoride ion sensors. RSC Adv. 2014, 4, 56466–56474. [Google Scholar] [CrossRef]
- Gwon, S.Y.; Kim, S.H. Anion sensing and F(-)-induced reversible photoreaction of D-pi-A type dye containing imidazole moiety as donor. Spectrochim. Acta A 2014, 117, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, S.; Li, Q.; Lan, H.; Xiao, S.; Li, Y.; Tan, R.; Yi, T. A fluorescent non-conventional organogelator with gelation-assisted piezochromic and fluoride-sensing properties. Dyes Pigments 2017, 137, 111–116. [Google Scholar] [CrossRef]
- Manivannan, R.; Satheshkumar, A.; Elango, K.P. Tuning of the H-bonding ability of imidazole N–H towards the colorimetric sensing of fluoride and cyanide ions as their sodium salts in water. New J. Chem. 2013, 37, 3152–3160. [Google Scholar] [CrossRef]
- Jayasudha, P.; Manivannan, R.; Elango, K.P. Highly selective colorimetric receptors for detection of fluoride ion in aqueous solution based on quinone-imidazole ensemble—Influence of hydroxyl group. Sens. Actuator B Chem. 2016, 237, 230–238. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, R.; Agarwal, M. Rationally designed tri-armed imidazole–indole hybrids as naked eye receptors for fluoride ion sensing. Synth. Commun. 2017, 47, 1307–1318. [Google Scholar] [CrossRef]
- Gu, P.-Y.; Gao, J.; Zhang, Q.; Liu, G.; Zhou, F.; Xu, Q.-F.; Lu, J.-M. Tuning optical properties of phenanthroline derivatives through varying excitation wavelength and pH values. J. Mater. Chem. C 2014, 2, 1539–1544. [Google Scholar] [CrossRef]
- Tian, M.; Wang, C.; Wang, L.; Luo, K.; Zhao, A.; Guo, C. Study on the synthesis and structure-effect relationship of multi-aryl imidazoles with their fluorescence properties. Luminescence 2014, 29, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, X.; Zhou, F.; Weng, L.-P.; Guo, L.-T.; Chen, M.; Chao, H.; Ji, L.-N. pH responsive luminescent switches of ruthenium(II) complexes containing two imidazole groups: Synthesis, spectroscopy, electrochemistry and theoretical calculations. Inorg. Chim. Acta 2009, 362, 4960–4966. [Google Scholar] [CrossRef]
- Xie, N.; Chen, Y. Synthesis and photophysical properties of 1,4-bis(4,5-diarylimidazol) benzene dyes. J. Photochem. Photobiol. A 2007, 189, 253–257. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Liu, R.; Zhu, X.-L.; Cai, X.; Zhu, H.-J. A highly efficient and selective probe for F− detection based on 1H-imidazo[4,5-b]phenazine derivative. Chin. Chem. Lett. 2015, 26, 339–342. [Google Scholar] [CrossRef]
- Niu, H.T.; Su, D.; Jiang, X.; Yang, W.; Yin, Z.; He, J.; Cheng, J.P. A simple yet highly selective colorimetric sensor for cyanide anion in an aqueous environment. Org. Biomol. Chem. 2008, 6, 3038–3040. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–1c are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, F. Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion. Molecules 2017, 22, 1519. https://doi.org/10.3390/molecules22091519
Zhang L, Liu F. Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion. Molecules. 2017; 22(9):1519. https://doi.org/10.3390/molecules22091519
Chicago/Turabian StyleZhang, Liang, and Fang Liu. 2017. "Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion" Molecules 22, no. 9: 1519. https://doi.org/10.3390/molecules22091519
APA StyleZhang, L., & Liu, F. (2017). Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion. Molecules, 22(9), 1519. https://doi.org/10.3390/molecules22091519





