Human Erythrocyte Acetylcholinesterase in Health and Disease
Abstract
:1. Introduction
2. Biochemical Properties of Human Erythrocyte Membrane Acetylcholinesterase
3. Implications of Erythrocyte Membrane Acetylcholinesterase Enzyme Activity in Disease
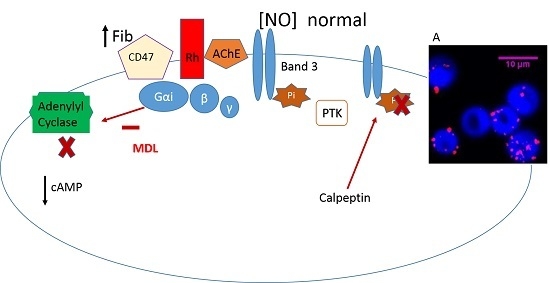
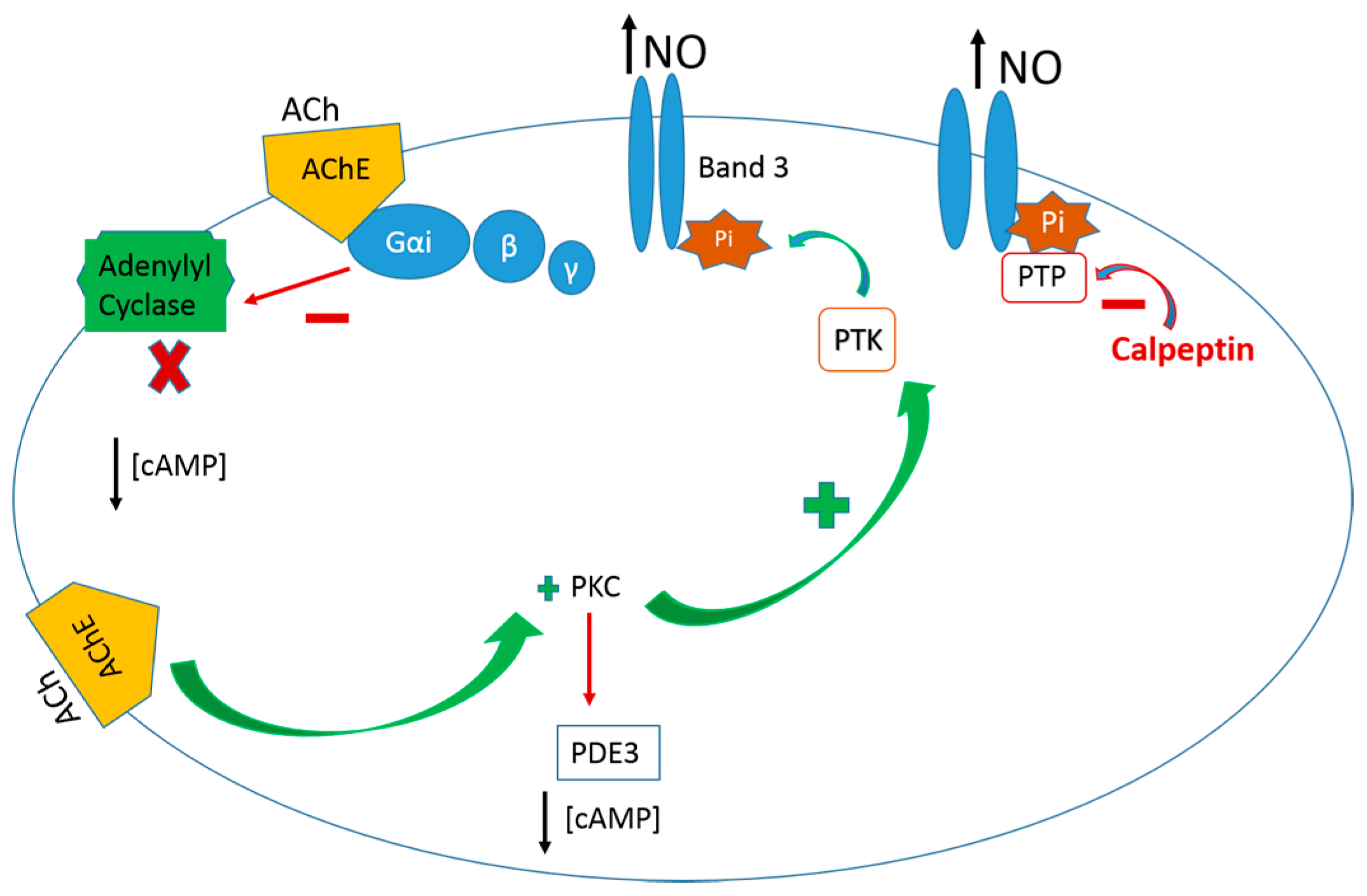
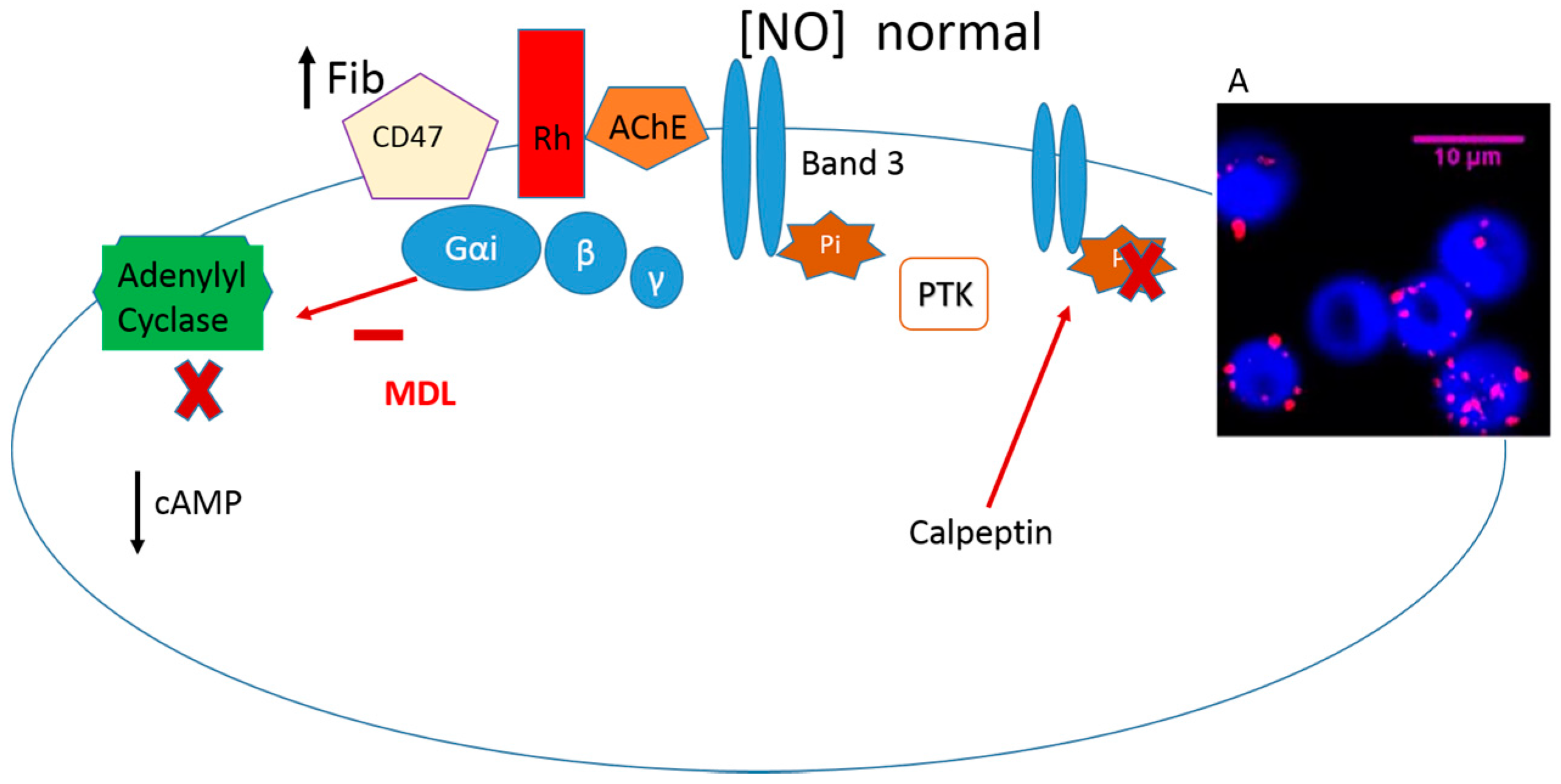
4. Enrolment of Erythrocyte AChE in the Signal Transduction Pathway of Nitric oxide
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mohandas, N.; Gallagher, P.G. Red cell membrane: Past, present, and future. Blood 2008, 112, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Ollon, M.; Nilsson, A.; Oldenborg, P.A. Target cell CD47 regulates macrophages activation and erythrophagocytosis. Transf. Clin. Biol. 2006, 13, 39–43. [Google Scholar]
- Kempe, D.S.; Akel, A.; Lang, P.A.; Hermle, T.; Biswas, R.; Muresanu, J.; Friedrich, B.; Dreischer, P.; Wolz, C.; Schumacher, U.; et al. Suicidal erythrocytes death in sepsis. J. Mol. Med. 2007, 85, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.S.; Puchulu-Campanella, M.E.; Barber, L.A.; Palascak, M.B.; Joiner, C.H.; Low, P.S.; Cohen, R.M. Changes in the properties of normal human red blood cells during in vivo aging. Am. J. Hematol. 2013, 88, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Prall, Y.G.; Gambhir, K.K.; Ampy, F.R. Acetylcholinesterase: An enzymatic marker of human red blood cell aging. Life Sci. 1998, 63, 177–184. [Google Scholar] [CrossRef]
- Aloni, B.; Livne, A. Acetycholinesterase as a probe for erythrocyte-membrane intactness. Biochim. Biophys. Acta 1974, 339, 359–366. [Google Scholar] [CrossRef]
- Lopes de Almeida, J.P.; Oliveira, S.; Saldanha, C. Erythrocyte as a biological sensor. Clin. Hemorheol. Microcirc. 2012, 51, 1–20. [Google Scholar] [PubMed]
- Saldanha, C. Instrumental analysis applied to erythrocyte properties. J. Cell Biotechnol. 2015, 1, 81–93. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [PubMed]
- Zhou, Y.; Varadharaj, S.; Zhao, X.; Parinandi, N.; Flavahan, N.A.; Zweier, J.L. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1027–H1032. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, M.W.; Huang, K.T.; Kuo, L.; Liao, J.C. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J. Biol. Chem. 2000, 275, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Han, T.H.; Hyduke, D.R.; Vaughan, M.W.; Van Herle, H.; Heln, T.W.; Zhang, C.; Kuo, L.; Liao, J.C. Modulation of nitric oxide bioavailability by erythrocytes. Proc. Nat. Acad. Sci. USA 2001, 98, 11771–11776. [Google Scholar] [CrossRef] [PubMed]
- Pawloski, J.R.; Hess, D.T.; Stamler, J.S. Impaired vasodilation by red blood cells in sickle cell disease. Proc. Nat. Acad. Sci. USA 2005, 102, 2531–2536. [Google Scholar] [CrossRef] [PubMed]
- Bordin, L.; Brunati, A.M.; Donella-Deana, V.; Baggio, B.; Toninello, A.; Clari, G. Band 3 is an anchor protein and a target for SHP-2 tyrosine phosphatase in human erythrocytes. Blood 2002, 100, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Campanella, M.E.; Chu, V.; Low, H. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc. Natl. Acad. Sci. USA 2005, 102, 2402–2407. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, C.; Silva, A.S.; Gonçalves, S.; Martins-Silva, J. Modulation of erythrocyte hemorheological properties by band 3 phosphorylation and dephosphorylation. Clin. Hemorheol. Microcirc. 2007, 36, 183–194. [Google Scholar] [PubMed]
- Carvalho, F.A.; Lopes de Almeida, J.P.; Freitas-Santos, T.; Saldanha, C. Modulation of erythrocytes acetylcholinesterase activity and its association with G protein. Band 3 interactions. J. Membr. Biol. 2009, 228, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Rossi, R.; Di Simplicio, P.; Floridi, A.; Canestrari, V. Protein thiols and glutathione influence the nitric oxide-dependent regulation of the red blood cell F metabolism. Nitric Oxide 2002, 6, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Hamaoka, R.; Fujii, J.; Taniguchi, N. Redox capacity of cells affects inactivation of glutathione reductase by nitrosative stress. Arch. Biochem. Biophys. 2000, 378, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Alles, G.A.; Haves, R.C. Cholinesterases in the blood of man. J. Biol. Chem. 1940, 133, 375–390. [Google Scholar]
- Cilliv, G.; Ozand, P.T. Human erythrocyte acetylcholinesterase purification, properties and kinetic behavior. Biochim. Biophys. Acta 1972, 284, 136–156. [Google Scholar] [CrossRef]
- Maulet, Y.; Brodbeck, U.; Fulpius, B. Selective solubilization by melittin of glycophorin A and acetylcholinesterase from human erythrocyte ghosts. Biochim. Biophys. Acta 1984, 778, 594–601. [Google Scholar] [CrossRef]
- Ott, P. Membrane acetylcholinesterases: Purification, molecular properties and interactions with amphiphilic environments. Biochim. Biophys. Acta 1985, 822, 375–392. [Google Scholar] [CrossRef]
- Saldanha, C. Acetilcolinesterase-Contribuição Para o estUdo Cinético da Enzima Eritrocitária de Homem. Ph.D. Thesis, Universidade Nova de Lisboa, Lisboa, Portugal, 28 February 1986. [Google Scholar]
- Di Francesco, C.; Brodbeck, U. Interaction of human red cell membrane acetylcholinesterase with phospholipids. Biochim. Biophys. Acta 1981, 640, 359–364. [Google Scholar] [CrossRef]
- Paulick, M.G.; Bertozzi, C.R. The Glycosylphosphatidylinositol Anchor: A complex membrane-anchoring structure for proteins. Biochemistry 2008, 47, 6991–7000. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Whitsett, C.F.; Oxendine, S.M.; Telen, M.J. Human erythrocyte acetylcholinesterase bears the Yta blood group antigen and is reduced or absent in the Yt(a-b-) phenotype. Blood 1993, 81, 815–819. [Google Scholar] [PubMed]
- Marcel, V.; Palacios, L.G.; Pertuy, C.; Masson, P.; Fournier, D. Two invertebrate acetylcholinesterases show activation followed by inhibition with substrate concentration. Biochem. J. 1998, 15, 329–334. [Google Scholar] [CrossRef]
- Komersa, K.; Ceganb, A.; Linka, M. Kinetics and mechanism of hydrolysis of acetylthiocholine by butyrylcholine esterase. Z. Naturforsch. 2002, 57, 1072–1077. [Google Scholar] [CrossRef]
- Saldanha, C.; Martins e Silva, J. 7-Inhibition of purified human acetylcholinesterase activity by zinc and copper. In Trace Elements in Medicine, Health and Atherosclerosis; Reis, M.F., Miguel, J.M.P., Machado, A.A., Abdulla, M., Eds.; Smith-Gordon Co.: Cambridgeshire, UK, 1995; pp. 117–123. [Google Scholar]
- Ahmed, M.; Latif, N.; Khan, R.A.; Ahmad, A.; Rocha, J.B.T.; Mazzanti, C.M.; Bagatini, M.D.; Morsch, V.M.; Schetinger, M.R.C. Enzymatic and biochemical characterization of bungarus sindanus snake venom acetylcholinesterase. J. Venom. Anim. Toxins Trop. Dis. 2012, 18, 236–243. [Google Scholar] [CrossRef]
- Bütikofer, P.; Brodbeck, U.; Ott, P. Modulation of erythrocyte vesiculation by amphiphilic drugs. Biochim. Biophys. Acta 1987, 901, 291–295. [Google Scholar]
- Saldanha, C.; Santos, N.C.; Martins e Silva, J. A colorimetric process to visualize erythrocyte exovesicles aggregates. Biochem. Mol. Biol. Educ. 2004, 32, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, C.; Santos, N.C.; Martins-Silva, J. Fluorescent probes DPH, TMA-DPH and C17-HC induce erythrocyte exovesiculation. J. Membr. Biol. 2002, 190, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.C.; Martins-Silva, J.; Saldanha, C. Gramicidin D and dithiothreitol effects on erythrocyte exovesiculation. Cell Biochem. Biophys. 2005, 42, 419–430. [Google Scholar] [CrossRef]
- Weiner, L.; Kreimer, D.; Roth, I.; Silman, E. Oxidative stress transforms acetylcholinesterase to a molten-globule-like state. Biochem. Biophys. Res. Commun. 1994, 198, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Parka, Y.-K.; Best, C.A.; Badizadegan, K.; Dasari, R.R.; Feld, M.S.; Kuriabova, T.; Henle, M.L.; Levine, A.J.; Popescu, G. Measurement of red blood cell mechanics during morphological changes. Proc. Nat. Acad. Sci. 2010, 15, 6731–6736. [Google Scholar] [CrossRef] [PubMed]
- Jaromin, A.; Korycińska, M.; Piętka-Ottlik, M.; Musiał, W.; Peczyńska-Czoch, W.; Kaczmarek, L.; Kozubeka, A. Membrane perturbations induced by new analogs of neocryptolepine. Biol. Pharm. 2012, 35, 1432–1439. [Google Scholar] [CrossRef]
- Herz, F.; Kaplan, E. A Review: Human erythrocyte acetylcholinesterase. Pediatr. Res. 1973, 7, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Hilário, S.; Saldanha, C.; Martins e Silva, J. An in vitro study of adrenaline effect on human erythrocyte properties in both gender. Clin. Hemorheol. Microcirc. 2003, 28, 89–98. [Google Scholar] [PubMed]
- Neupane, D.; Jørs, E.; Brandt, L. Pesticide use, erythrocyte acetylcholinesterase level and self-reported acute intoxication symptoms among vegetable farmers in Nepal: A cross-sectional study. Environ. Health 2014, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Paniagua, D.; Gomez-Martín, A.; Gil, F.; Parron, T.; Alarcon, R.; Requena, M.; Lacasaña, M.; Hernandez, A.F. Activity and determinants of cholinesterases and paraoxonase-1 in blood of workers exposed to non-cholinesterase inhibiting pesticides. Chem. Biol. Interact. 2016, 259, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Sargento, L.; Zabala, L.; Saldanha, C.; Ramalho, P.S.; Silva, J.M. The effect of sodium fluorescein angiography on erythrocyte properties. Clin. Hemorheol. Microcirc. 1998, 18, 135–139. [Google Scholar] [PubMed]
- Coelho, H.; Azevedo, M.; Proença, C.; Martins e Silva, J.; Manso, C. Plasma dopamine-beta-hydroxylase and erythrocyte acetylcholinesterase in a group of patients with Parkinson disease. J. Neur. Transm. 1978, 42, 163–166. [Google Scholar] [CrossRef]
- Martins e Silva, J.; Proença, M.C.; Nogueira, J.B.; Clara, J.G.; Costa, J.N.; Manso, C. Erythrocyte acetylcholinesterase in essential hypertension. J. Neural Transm. 1980, 49, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zabala, L.; Saldanha, C.; Martins e Silva, J.; Ramalho, P.S. Red blood cell membrane integrity in primary open angle glaucoma: Ex vivo and in vitro studies. Eye 1999, 13, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, P.S.; Hormigo, A.; Martins, R.; Saldanha, C.; Martins Silva, J. Haematological changes in retinal vasculitis. Eye 1988, 2, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.; Pinto, S.; Napoleão, P.; Pronto-Laborinho, A.C.; Barros, M.A.; Freitas, T.; de Carvalho, M.; Saldanha, C. Identification of erythrocyte biomarkers in amyotrophic lateral sclerosis. Clin. Hemorheol. Microcirc. 2016, 64, 989–994. [Google Scholar]
- Chalkoo, M.A.; Rashid, A.; Kakroo, S.M.; Razvi, S.A.; Wani, A.A. Role of erythrocyte acetylcholinesterase in the diagnosis of hirschsprung’s Disease. J. Pioneer. Med. Stud. 2013, 3, 79–82. [Google Scholar]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, immunity, and hypertension. Hypertension 2011, 57, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Pal, R.; Siddiqi, N.J.; Sharma, B. Acetylcholinesterase from human erythrocytes as a surrogate biomarker of lead induced neurotoxicity. Enzyme Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Vikkey, H.A.; Fidel, D.; Elisabeth, Y.P.; Hilaire, H.; Hervé, L.; Badirou, A.; Alain, K.; Parfait, H.; Fabien, G.; Benjamin, F. Risk factors of pesticide poisoning and pesticide users’ cholinesterase levels in cotton production areas: Glazoué and savè townships, in central republic of benin. Environ. Health Insights 2017, 11, 1–10. [Google Scholar]
- Galassi, F.; Renieri, G.; Sodi, A.; Ucci, F.; Vannozzi, L.; Masini, E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br. J. Ophtalmol. 2004, 88, 757–760. [Google Scholar] [CrossRef]
- Rafael, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of its functional effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar]
- Das, U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med. Sci. Monit. 2007, 12, 214–221. [Google Scholar]
- Silva-Herdade, A.S.; Saldanha, C. Effects of acetylcholine on an animal model of inflammation. Clin. Hemorheol. Microcirc. 2013, 53, 195–202. [Google Scholar]
- Pober, J.S.; Sessa, W.C. Evolving of endothelium cells in inflammation. Nat. Rev. 2007, 7, 803–815. [Google Scholar]
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol. 2012, 34, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000, 86, 29–48. [Google Scholar] [CrossRef]
- Saldanha, C.; Lopes de Almeida, J.P.; Silva-Herdade, A.S. Application of a nitric oxide sensor in biomedicine. Biosensors 2014, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.; Carvalho, F.A.; Martins-Silva, J.; Saldanha, C. The modulation of cyclic nucleotide levels and PKC activity by acetylcholinesterase effectors in human erythrocytes. Acta Bioquímica 2008, 9, 111–114. [Google Scholar]
- Zipser, Y.; Piade, A.; Barbul, A.; Korenstein, R.; Kosower, N.S. Ca2+ promotes erythrocyte band 3 tyrosine phosphorylation via dissociation of phosphotyrosine phosphatase from band 3. Biochem. J. 2002, 368, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Allent, D.P.; Zioncheck, T.F.; Chari, V.; Willardson, B.M.; Geahlen, R.L.; Harrison, M.L. Tyrosine phosphorylation of band 3 inhibits peripheral protein binding. J. Biol. Chem. 1987, 262, 4592–4596. [Google Scholar] [PubMed]
- Saldanha, C.; Teixeira, P.; Santos-Freitas, T.; Napoleão, P. Timolol modulates erythrocyte nitric oxide bioavailability. J. Clin. Exp. Optalmol. 2013, 4, 3. [Google Scholar] [CrossRef]
- Shakur, Y.; Holst, L.S.; Landstrom, T.R.; Movsesian, M.; Degerman, E.; Manganiello, V. Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog. Nucleic Acid Res. Mol. Biol. 2001, 66, 241–277. [Google Scholar] [PubMed]
- Carvalho, F.A.; Almeida, J.P.; Fernandes, I.O.; Freitas-Santos, T.; Saldanha, C. Non-neuronal cholinergic system and signal transduction pathways mediated by band 3 in red blood cells. Clin. Hemorheol. Microcirc. 2008, 40, 207–227. [Google Scholar] [PubMed]
- Carvalho, F.A.; Mesquita, R.; Martins-Silva, J.; Saldanha, C. Acetylcholine and choline effects on erytrocytes nitrite and nitrate levels. J. Appl. Toxicol. 2004, 24, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Almeida, J.P.; Carvalho, F.A.; Silva-Herdade, A.S.; Santos-Freitas, T.; Saldanha, C. Redox thiol status plays a central role in the mobilization and metabolism of nitric oxide in human red blood cells. Cell Biol. Int. 2009, 33, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.; Duro, N.; Napoleão, P.; Saldanha, C. Acetylcholinesterase conformational states influence nitric oxide mobilization in the erythrocyte. J. Membr. Biol. 2015, 248, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Almeida, J.P.; Freitas-Santos, T.; Saldanha, C. Evidence that the degree of band 3 phosphorylation modulates human erythrocytes nitric oxide efflux-in vitro model of hyperfibrinogenemia. Clin. Hemorh. Microc. 2011, 49, 407–416. [Google Scholar]
- Saldanha, C.; Freitas, T.; Almeida, J.P. Fibrinogen effects on erythrocyte nitric oxide mobilization in presence of acetylcholine. Life Sci. 2012, 91, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Silva-Herdade, A.S.; Freitas, T.; Almeida, J.P.; Saldanha, C. Fibrinogen signaling in erythrocyte nitric oxide mobilization in presence of PI3-K and adenylyl cyclase inhibitors. Eur. J. Biomed. Pharm. Sci. 2016, 3, 28–34. [Google Scholar]
- De Oliveira, S.; Vitorino de Almeida, V.; Calado, A.; Rosário, H.S.; Saldanha, C. Integrin-associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochem. Biophys. Acta 2012, 1818, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Almeida, J.P.; Freitas-Santos, T.; Saldanha, C. Fibrinogen-dependent signaling in microvascular erythrocyte function: Implications on nitric oxide efflux. J. Membr. Biol. 2009, 231, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, C.; Silva-Herdade, A.S. Casein kinase effects on nitric oxide metabolism and deformability of human erythocytes, XI. In Proceedings of the Abstract Book of International Conference on Hemorheology and Microcirculation, Yaroslavl, Russia, 3–5 July 2017. [Google Scholar]
- Saldanha, C.; Silva-Herdade, A.S. Physiological properties of erythrocytes in inflammation. J. Cell. Biotechnol. 2017, 3. [Google Scholar] [CrossRef]
- Mutolo, M.G.; Albanese, G.; Rusciano, D.; Pescosolido, N. Oral administration of forskolin, homotaurine, carnosine, and folic acid in patients with primary open angle glaucoma: Changes in intraocular pressure, pattern electroretinogram amplitude and foveal sensitivity. J. Ocul. Pharmacol. Ther. 2016, 32, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, W.; Zalejska-Fiolka, J.; Pojda-Wilczek, D.; Kabiesz, A.; Majewski, W. Oxidative stress in the red blood cells of patients with primary open-angle glaucoma. Clin. Hemorheol. Microcirc. 2016, 62, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Esteves, R.; Freitas, T.; Teixeira, P.; Napoleão, P.; Neves, C.; Saldanha, C. Erythrocyte nitric oxide in glaucoma patients—Ex vivo study. Clin. Hemorh. Microcirc. 2016, 64, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Pawloski, J.R.; Hess, D.T.; Stamler, J.S. Export by red blood cells of nitric oxide bioactivity. Nature 2001, 409, 622–626. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldanha, C. Human Erythrocyte Acetylcholinesterase in Health and Disease. Molecules 2017, 22, 1499. https://doi.org/10.3390/molecules22091499
Saldanha C. Human Erythrocyte Acetylcholinesterase in Health and Disease. Molecules. 2017; 22(9):1499. https://doi.org/10.3390/molecules22091499
Chicago/Turabian StyleSaldanha, Carlota. 2017. "Human Erythrocyte Acetylcholinesterase in Health and Disease" Molecules 22, no. 9: 1499. https://doi.org/10.3390/molecules22091499
APA StyleSaldanha, C. (2017). Human Erythrocyte Acetylcholinesterase in Health and Disease. Molecules, 22(9), 1499. https://doi.org/10.3390/molecules22091499