Quantitative Analysis, Extraction Optimization, and Biological Evaluation of Cudrania tricuspidata Leaf and Fruit Extracts
Abstract
:1. Introduction
2. Results and Discussion
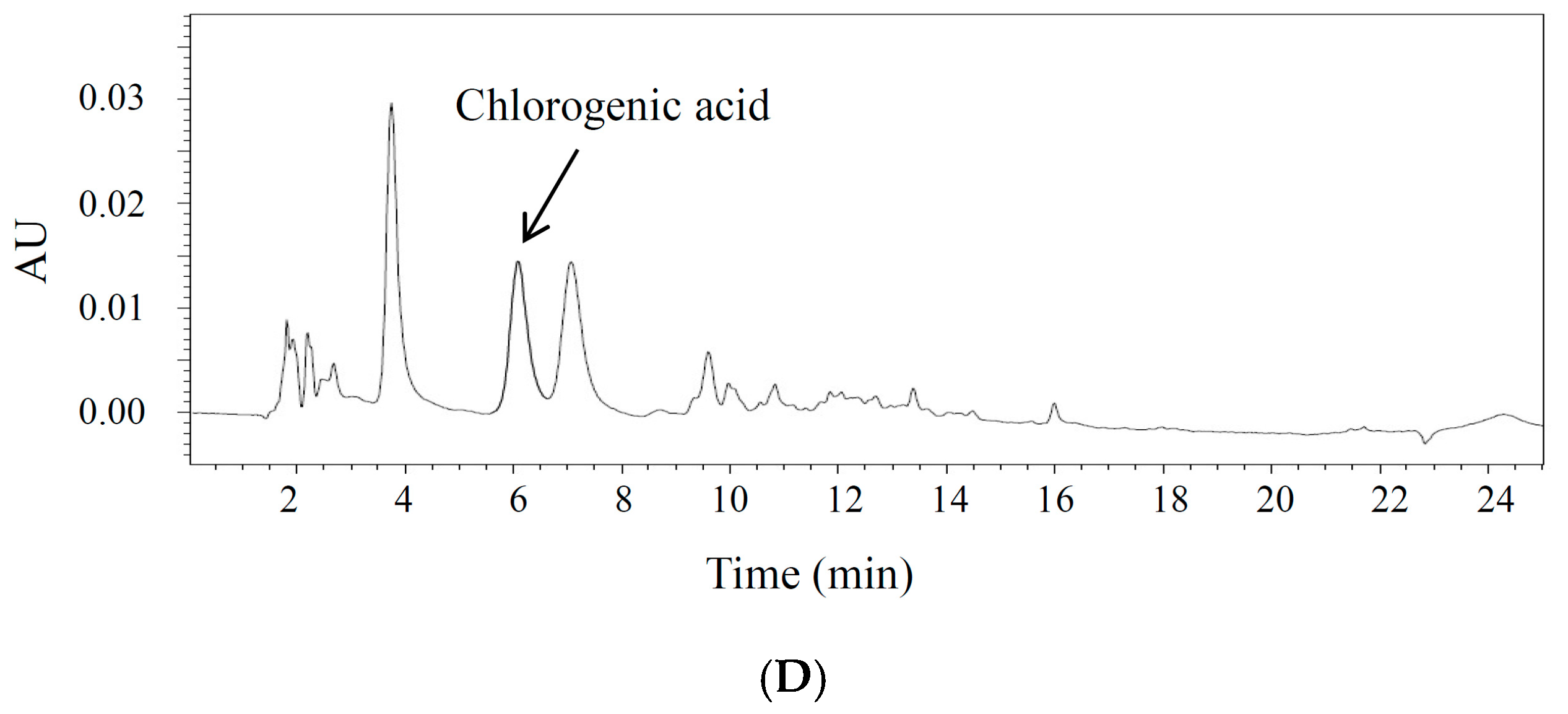
2.1. Optimization of the Chromatographic Conditions
2.2. Method Validation
2.2.1. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.2.2. Linearity
2.2.3. Precision and Accuracy
2.2.4. Repeatability
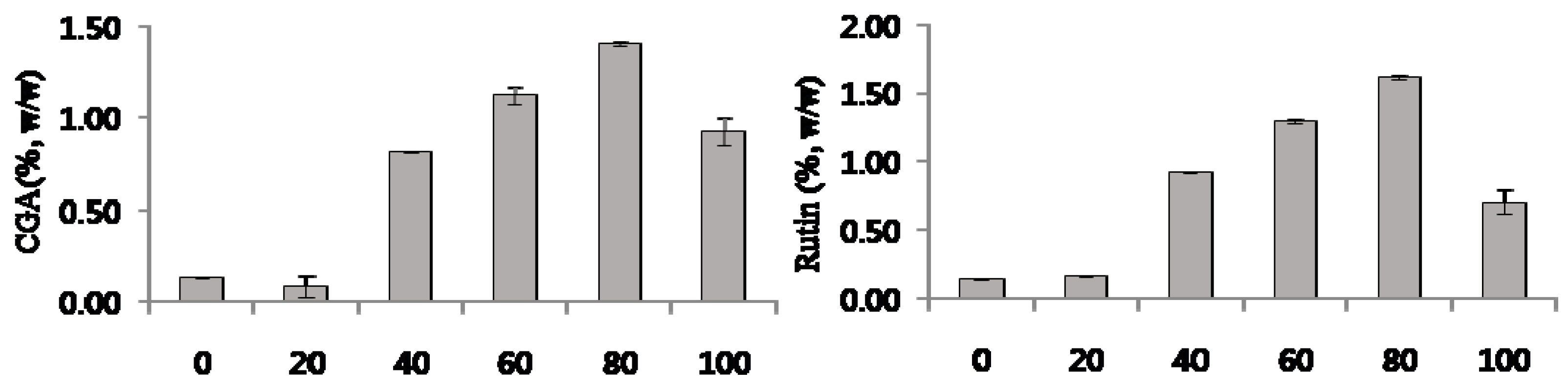
2.3. Contents of Marker Compounds from C. tricuspidata Extracts
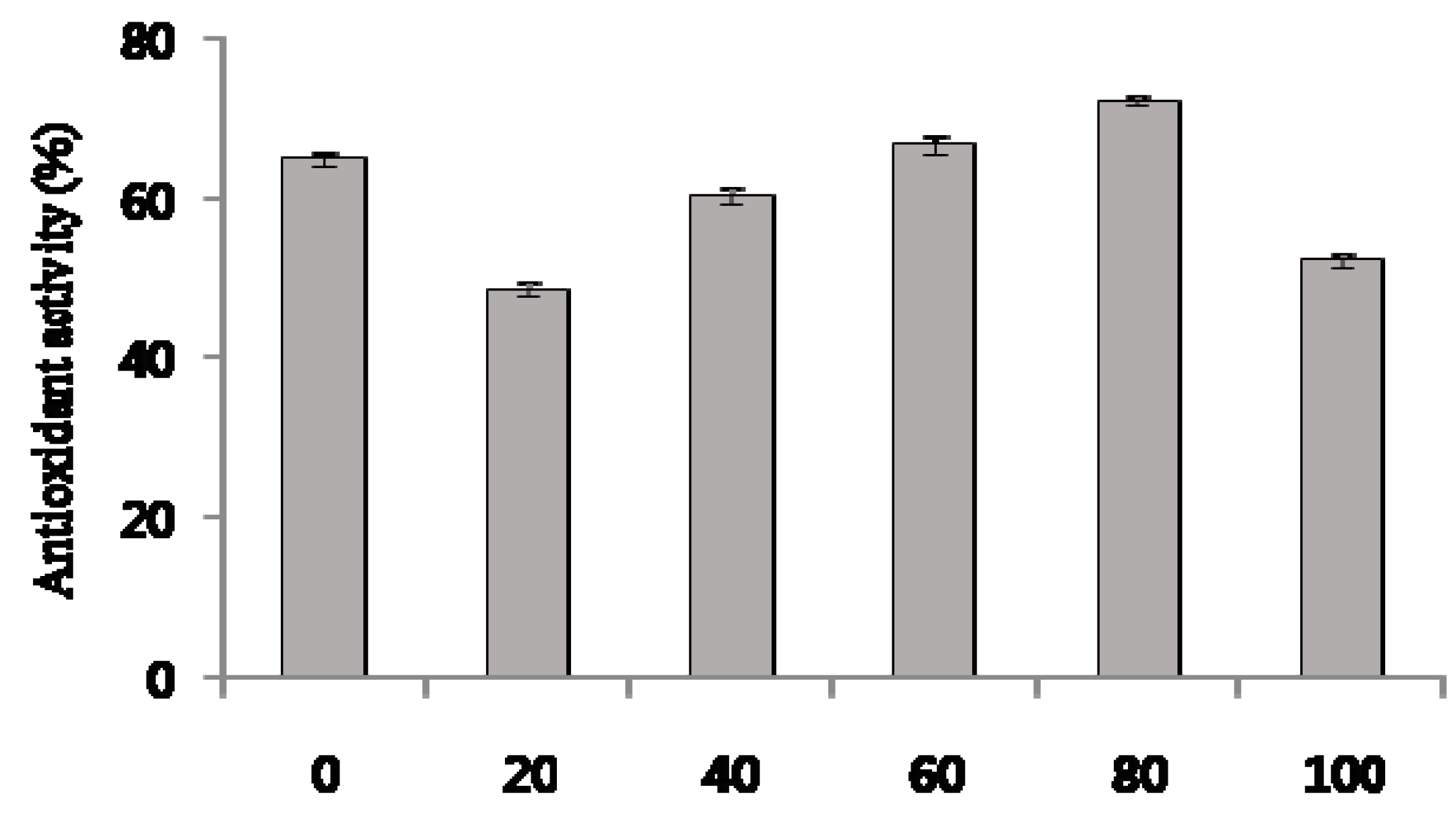
2.4. Antioxidant Activity, Total Phenolic Contents, and Total Flavonoids of C. tricuspidata Extracts
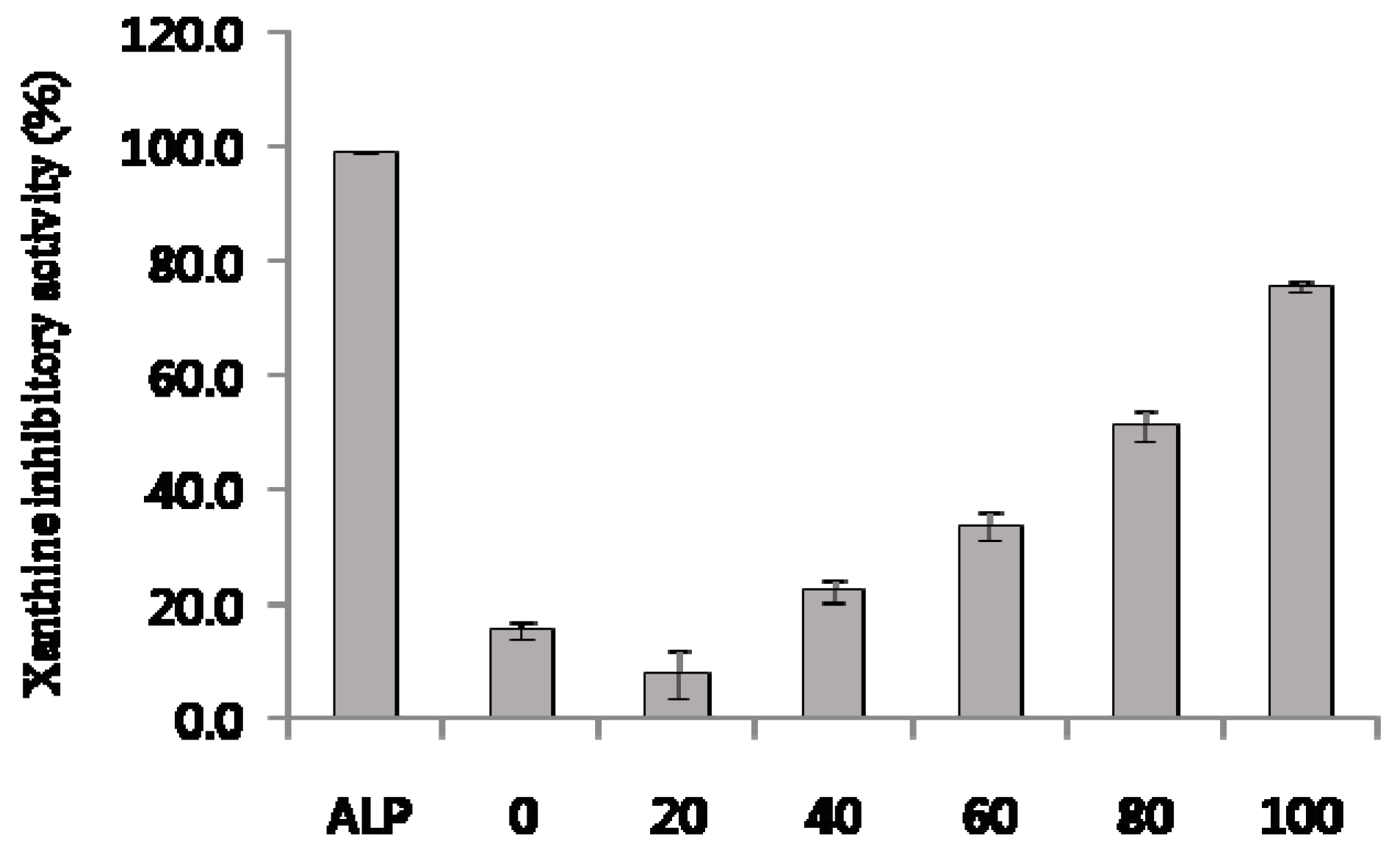
2.5. Xanthine Oxidase Inhibitory Activity of C. tricuspidata Extracts
3. Experimental Section
3.1. Plant Material and Preparation of the Extract
3.2. Instrumentation and Chromatographic Conditions
3.3. Preparation of Standards and Sample Solutions
3.3.1. Standard Solutions
3.3.2. Sample Solutions
3.4. Method Validation
3.4.1. Specificity
3.4.2. Linearity
3.4.3. Sensitivity
3.4.4. Accuracy and Precision
3.4.5. Recovery
3.4.6. Statistical Analysis
3.5. Analysis of the Extract from C. tricuspidata Leaves
3.6. DPPH Free Radical Assay
3.7. Reducing Power
3.8. Determination of Total Phenolic Content
3.9. Determination of Total Flavonoids
3.10. Determination of In Vitro Xanthine Oxidase (XO) Inhibitory Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, S.H.; Jung, E.J.; Lim, D.G.; Oyungerel, B.; Lim, K.I.; Her, E.; Choi, W.S.; Jun, M.H.; Choi, K.D.; Han, D.J. Anti-inflammatory action of Cudrania tricuspidata on spleen cell and T lymphocyte proliferation. J. Pharm. Pharmacol. 2008, 60, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Park, Y.-D.; Han, J.-M.; Im, K.-R.; Lee, B.W.; Jeong, I.Y.; Jeong, T.-S.; Lee, W.S. Anti-atherosclerotic and anti-inflammatory activities of catecholic xanthones and flavonoids isolated from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2006, 16, 5580–5583. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-S.; Lee, D.-S.; Kim, Y.-C. Cudratricusxanthone A from Cudrania tricuspidata suppresses pro-inflammatory mediators through expression of anti-inflammatory heme oxygenase-1 in RAW264.7 macrophages. Int. Immunopharmacol. 2009, 9, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, S.; Lee, S.J.; Ham, I.; Whang, W.K. Antioxidant activities of new flavonoids from Cudrania tricuspidata root bark. Arch. Pharm. Res. 2009, 32, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Hiep, N.T.; Kim, D.-W.; Hong, S.; Guo, Y.; Hwang, B.Y.; Lee, H.J.; Mar, W.; Lee, D. Chemical constituents isolated from the root bark of Cudrania tricuspidata and their potential neuroprotective effects. J. Nat. Prod. 2016, 79, 1938–1951. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-H.; Kim, H.-C.; Cui, J.-M.; Kim, Y.-C. Hepatoprotective constituents of Cudrania tricuspidata. Arch. Pharm. Res. 2005, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Lee, J.H.; Lee, S.-T.; Lee, H.S.; Lee, W.S.; Jeong, T.-S.; Park, K.H. Antioxidant and cytotoxic activities of xanthones from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2005, 15, 5548–5552. [Google Scholar] [CrossRef] [PubMed]
- Hiep, N.T.; Kwon, J.; Kim, D.-W.; Hwang, B.Y.; Lee, H.-J.; Mar, W.; Lee, D. Isoflavones with neuroprotective activities from fruits of Cudrania tricuspidata. Phytochemistry 2015, 111, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ha, H.; Lee, J.K.; Seo, C.S.; Lee, N.H.; Jung, D.Y.; Park, S.J.; Shin, H.K. The fruits of Cudrania tricuspidata suppress development of atopic dermatitis in NC/Nga mice. Phytother. Res. 2012, 26, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Hong, S.S.; Jin, Q.; Li, D.; Kim, H.-K.; Lee, J.; Kwon, S.H.; Lee, D.; Lee, C.-K.; Lee, M.K.; et al. Prenylated and benzylated flavonoids from the fruits of Cudrania tricuspidata. J. Nat. Prod. 2008, 72, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Kim, S.B.; Liu, Q.; Do, S.-G.; Hwang, B.Y.; Lee, M.K. Comparison of pancreatic lipase inhibitory isoflavonoids from unripe and ripe fruits of Cudrania tricuspidata. PLoS ONE 2017, 12, e0172069. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kwon, J.; Lee, D.; Mar, W. Effects of Cudrania tricuspidata fruit extract and its active compound, 5,7,3′,4′-tetrahydroxy-6,8-diprenylisoflavone, on the high-affinity IgE receptor-mediated activation of Syk in mast cells. J. Agric. Food Chem. 2015, 63, 5459–5467. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Hong, S.S.; Hwang, J.S.; Jeong, S.H.; Hwang, J.H.; Lee, M.H.; Lee, M.K.; Lee, D.; Ro, J.S.; Hwang, B.Y. Monoamine oxidase inhibitory constituents from the fruits of Cudrania tricuspidata. Arch. Pharm. Res. 2005, 28, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Choi, K.-M.; Liu, Q.; Kim, S.B.; Ji, H.-J.; Kim, M.; Shin, S.-K.; Do, S.-G.; Shin, E.; Jung, G. Anti-obesity effect of 6,8-diprenylgenistein, an isoflavonoid of Cudrania tricuspidata fruits in high-fat diet-induced obese mice. Nutrients 2015, 7, 10480–10490. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-K.; Nam, D.-E.; Jun, W.; Lee, J. Cudrania tricuspidata water extract improved obesity-induced hepatic insulin resistance in db/db mice by suppressing ER stress and inflammation. Food Nutr. Res. 2015, 59. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Kim, J.-E.; Shim, J.-H.; Yoon, G.; Bang, M.; Bae, C.-S.; Lee, K.-J.; Park, D.-H.; Cho, S.-S. HPLC analysis, optimization of extraction conditions and biological evaluation of Corylopsis coreana Uyeki Flos. Molecules 2016, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.N.; de Dieu Tamokou, J.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.; Dobson, V.; Duthie, S.; Hinselwood, D. Bioavailiability and efficiency of rutin as an antioxidant: A human supplementation study. Eur. J. Clin. Nutr. 2000, 54, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.S.; Park, D.H.; Ki, S.H.; Cho, S.S. Effects of extracts from Corylopsis coreana Uyeki (Hamamelidaceae) flos on xanthine oxidase activity and hyperuricemia. J. Pharm. Pharmacol. 2016, 68, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Citraro, R.; Navarra, M.; Leo, A.; Donato Di Paola, E.; Santangelo, E.; Lippiello, P.; Aiello, R.; Russo, E.; De Sarro, G. The anticonvulsant activity of a flavonoid-rich extract from orange juice involves both NMDA and GABA-benzodiazepine receptor complexes. Molecules 2016, 21, 1261. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Deng, S.; Han, Q.; Zhao, P.; Zhou, Q.; Zheng, S.; Ma, X.; Xu, C.; Yang, J.; Yang, X.; et al. Hypoglycemic activity and the potential mechanism of the flavonoid rich extract from Sophora tonkinensis Gagnep. in KK-Ay mice. Front. Pharmacol. 2016, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-Z.; Yang, X.; Zhou, H.-L.; Pan, S.-X. Safety evaluation, in vitro and in vivo antioxidant activity of the flavonoid-rich extract from Maydis stigma. Molecules 2015, 20, 22102–22112. [Google Scholar] [CrossRef] [PubMed]
- Gajaria, T.K.; Patel, D.K.; Devkar, R.V.; Ramachandran, A. Flavonoid rich extract of Murraya koenigii alleviates in-vitro LDL oxidation and oxidized LDL induced apoptosis in raw 264.7 Murine macrophage cells. J. Food Sci. Technol. 2015, 52, 3367–3375. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, Y.; Han, X.; Liang, C.; Yin, L.; Xu, L.; Qi, Y.; Zhao, Y.; Peng, J.; Sun, C. Potent effects of flavonoid-rich extract from Rosa laevigata Michx fruit against hydrogen peroxide-induced damage in PC12 cells via attenuation of oxidative stress, inflammation and apoptosis. Molecules 2014, 19, 11816–11832. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.-S.; Park, D.-H.; Bae, M.-S.; Oh, D.-S.; Kwon, N.-H.; Kim, J.-E.; Choi, C.-Y.; Cho, S.-S. In vitro and in vivo studies on Quercus acuta Thunb. (Fagaceae) extract: Active constituents, serum uric acid suppression, and xanthine oxidase inhibitory activity. Evid. Based Complement. Altern. Med. 2017, 2017, 4097195. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.S.; Park, D.H.; Kim, J.E.; Yoo, J.C.; Bae, M.S.; Oh, D.S.; Shim, J.H.; Choi, C.Y.; An, K.W.; Kim, E.I.; et al. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Hu, Q.-H.; Zhang, X.; Zhu, Q.; Kong, L.-D. Beneficial effect of rutin on oxonate-induced hyperuricemia and renal dysfunction in mice. Pharmacology 2013, 92, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Wang, Y.; Kong, L.D.; Yang, C.; Zhang, X. Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J. Ethnopharmacol. 2004, 93, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zatorski, H.; Sałaga, M.; Zielińska, M.; Piechota-Polańczyk, A.; Owczarek, K.; Kordek, R.; Lewandowska, U.; Chen, C.; Fichna, J. Experimental colitis in mice is attenuated by topical administration of chlorogenic acid. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.-Q.; Tang, Z.-H.; Yan, Y.-X.; Guo, C.-R.; Cao, L.; Ding, G.; Huang, W.-Z.; Wang, Z.-Z.; Wang, K.D.; Xiao, W.; et al. Study on the anti-gout activity of Chlorogenic acid: Improvement on hyperuricemia and gouty inflammation. Am. J. Chin. Med. 2014, 42, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Lee, D.-M.; Lee, S.-H.; Seong, A.-R.; Gin, D.-W.; Hwang, J.-A.; Park, J.-H. Chlorogenic acid suppresses pulmonary eosinophilia, IgE production, and Th2-type cytokine production in an ovalbumin-induced allergic asthma: Activation of STAT-6 and JNK is inhibited by chlorogenic acid. Int. Immunopharmacol. 2010, 10, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, G.; Pan, J.; Gong, D. Novel insights into the inhibitory mechanism of kaempferol on xanthine oxidase. J. Agric. Food Chem. 2015, 63, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Pandey, V.; Dwivedi, S.; Rao, Y. Antiinflammatory and antiulcer effects of kaempferol, a flavone, isolated from Rhamnus procumbens. Indian J. Exp. Biol. 1988, 26, 121–124. [Google Scholar] [PubMed]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Vats, P.; Verma, S.M.; Monif, T. Novel LC–MS/MS method for estimation of niacin with negligible matrix effect and its application to the BE study. J. Pharm. Investig. 2017, 47, 241–248. [Google Scholar] [CrossRef]
- Adib, N.A.M.; Mandal, U.K.; Mohamed, F.; Chatterjee, B. Fast and simple gas chromatographic method for simultaneous estimation of camphor, menthol and methyl salicylate in analgesic ointment: Application in stability study. J. Pharm. Investig. 2017, 47, 275–285. [Google Scholar] [CrossRef]
- Landim, L.P.; Feitoza, G.S.; da Costa, J.G. Development and validation of a HPLC method for the quantification of three flavonoids in a crude extract of Dimorphandra gardneriana. Rev. Bras. Farmacogn. 2013, 23, 58–64. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Part | Usage | Material | Reference |
|---|---|---|---|
| Leaf | Diabetes | Water extract | [15] |
| Fruit | Anti-obesity | 6,8-Diprenylgenistein | [14] |
| Fruit | Neuroprotective | Cudraisoflavones B–K, 5,7,3′,4′-tetrahydroxy-6,8-diprenylisoflavone warangalone, auriculasin, erythrinin B, gancaonin B, gancaonin A, osajin, euchrenone b8, euchrenone b9, alpinumisoflavone, 40-O-methyl-alpinumisoflavone, 5,7,40-trihydroxy-6,8-diprenylisoflavone, erysenegalensein E, anagyroidisoflavone A, euchrenone b10, senegalensin, eryvarin B, 5,7-dihydroxy-6-(2″-hydroxy-3″-methylbut-3″-enyl)-4′-methoxylisoflavone, 3′,5-dihydroxy-4′-methoxyl-2″,2″-dimethylpyrano(6″,5″-h)isoflavone, derrone, 4′-O-methyl-200-hydroxydihydroalpinumisoflavone, isoerysenegalensein E, biochanin A, lupiwighteone, 5,3′,4′-trihydroxy-6″,6″-dimethylpyrano-(2″,3″,7,6)isoflavone, erythrinin C, and flemiphilippinin G | [8] |
| Fruit | Lipase inhibition | Genistein, orobol, 7,4′-dimethoxy-5-hydroxyisoflavone, genistin, oroboside 3′-O-methylorobol-7-glucoside, sphaerobioside, wighteone, gancaonin A 4′,5,7-trihydroxy isoflavonone, 5,7,3′,4′-tetrahydroxy-6-8-diprenylisoflavone, alpinumisoflavone, 4′-O-methylalpinumisoflavone, 5,3′,4′-trihydroxy-6′′,6′′-dimethylpyrano-(2′′,3′′;7,6)isoflavone, Scandenone, derrone, derrone-4′-O-methylether, isochandalone, ulexin B, ulexone B, (+)-dihydrokaempferol, (+)-taxifolin, (2R,3R)-7-(β-glucopyranosyloxy)-2,3-dihydro-3,5-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, astragalin, hirsutrin, populnin, nicotiflorin, rutin | [11] |
| Fruit | Anti-allergy | 5,7,3′,4′-Tetrahydroxy-6,8-diprenylisoflavone | [12] |
| Fruit | Dermatitis | Water extract (rutin) | [12] |
| Fruit | Monoamine oxidase inhibition | Gancaonin A, 4′-O-methylalpinumisoflavone, alpinumisoflavone | [13] |
| Root bark | Anti-athersclerotic | Catecholic xanthones | [2] |
| Root bark | Anti-inflammatory | Cudratricusxanthone A | [3] |
| Root bark | Antioxidant | Cudranian 1, cudranian 2, kaempferol-7-O-β-d-glucopyranoside, quercetin-7-O-β-d-glucopyranoside, aromadendrin | [4] |
| Root bark | Neuroprotective | 5,7-Dihydroxychromone, demethylsuberosin, 3-O-methylcudraxanthone G | [5] |
| Root bark | Hepatoprotective | Cudratricusxanthone A, cudraxanthone L, cudratricusxanthone E, macluraxanthone B | [6] |
| Root bark | Antioxidant Anti-cancer | 1,3,7-Trihydroxy-4-(1,1-dimethyl-2-propenyl)-5,6-(2,2-dimethylchromeno)-xanthone catecholic xanthones | [7] |
| Analyte | Retention Time (min) | R2 | Linear Range (μg/mL) | LOQ (μg/mL) | LOD (μg/mL) |
|---|---|---|---|---|---|
| Chlorogenic acid | 6.0 | 0.9999 | 6.25–100 | 6.25 | 0.88 |
| Rutin | 11.8 | 0.9999 | 6.25–100 | 6.25 | 0.32 |
| Kaempferol | 18.1 | 0.9999 | 6.25–100 | 6.25 | 0.05 |
| Analyte | Conc. (μg/mL) | Intraday (n = 3) | Interday (n = 3) | ||
|---|---|---|---|---|---|
| RSD (%) a | Accuracy (%) | RSD (%) | Accuracy (%) | ||
| Chlorogenic acid | 12.5 | 1.7 | 105.6 | 6.1 | 102.6 |
| 25 | 3.4 | 106.9 | 4.0 | 99.2 | |
| 50 | 0.3 | 106.2 | 4.1 | 102.5 | |
| Rutin | 12.5 | 0.5 | 106.6 | 2.8 | 108.3 |
| 25 | 1.3 | 103.6 | 2.3 | 105.3 | |
| 50 | 0.2 | 107.1 | 1.0 | 107.0 | |
| Kaempferol | 12.5 | 2.8 | 107.6 | 3.0 | 107.7 |
| 25 | 2.5 | 103.7 | 2.4 | 104.3 | |
| 50 | 1.0 | 107.2 | 1.0 | 106.0 | |
| Analyte | Added (μg/mL) | Recovery (%; mean ± SD) | RSD (%) a |
|---|---|---|---|
| Chlorogenic acid | 12.5 | 103.6 ± 1.7 | 1.7 |
| 25 | 101.0 ± 1.6 | 1.6 | |
| 50 | 101.4 ± 1.4 | 1.4 | |
| Rutin | 12.5 | 104.0 ± 1.6 | 1.5 |
| 25 | 99.2 ± 0.1 | 0.1 | |
| 50 | 101.4 ± 0.6 | 0.6 | |
| Kaempferol | 12.5 | 104.4 ± 1.2 | 1.2 |
| 25 | 99.2 ± 0.2 | 0.2 | |
| 50 | 101.3 ± 0.2 | 0.2 |
| Extract | Reducing Power (Ascorbic Acid Eq. μg/100 μg Extract) | Total Phenolic Content (Gallic Acid Eq. mg/g) |
|---|---|---|
| Water | 26.3 ± 0.6 | 85.6 ± 0.9 |
| 20% EtOH Ex | 24.9 ± 0.6 | 81.3 ± 1.4 |
| 40% EtOH Ex | 26.7 ± 0.3 | 86.9 ± 2.1 |
| 60% EtOH Ex | 27.5 ± 0.3 | 93.2 ± 2.4 |
| 80% EtOH Ex | 28.3 ± 0.5 | 99.2 ± 1.2 |
| 100% EtOH Ex | 25.8 ± 0.3 | 92.8 ± 4.1 |
| Parameter | Condition | ||
|---|---|---|---|
| Column | Zorbax extended C18 (C18; 4.6 mm × 150 mm, 5 μm) | ||
| Flow rate | 0.8 mL/min | ||
| Injection volume | 10 μL | ||
| UV detection | 340 nm | ||
| Run time | 24 min | ||
| Gradient | Time (min) | % A 1 | % B 2 |
| 0 | 10 | 90 | |
| 5 | 10 | 90 | |
| 18 | 50 | 50 | |
| 20 | 100 | 0 | |
| 21 | 10 | 90 | |
| 25 | 10 | 90 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.-H.; Ki, S.H.; Park, D.-H.; Moon, H.-S.; Lee, C.-D.; Yoon, I.-S.; Cho, S.-S. Quantitative Analysis, Extraction Optimization, and Biological Evaluation of Cudrania tricuspidata Leaf and Fruit Extracts. Molecules 2017, 22, 1489. https://doi.org/10.3390/molecules22091489
Song S-H, Ki SH, Park D-H, Moon H-S, Lee C-D, Yoon I-S, Cho S-S. Quantitative Analysis, Extraction Optimization, and Biological Evaluation of Cudrania tricuspidata Leaf and Fruit Extracts. Molecules. 2017; 22(9):1489. https://doi.org/10.3390/molecules22091489
Chicago/Turabian StyleSong, Seung-Hui, Sung Hwan Ki, Dae-Hun Park, Hong-Seop Moon, Chang-Dai Lee, In-Soo Yoon, and Seung-Sik Cho. 2017. "Quantitative Analysis, Extraction Optimization, and Biological Evaluation of Cudrania tricuspidata Leaf and Fruit Extracts" Molecules 22, no. 9: 1489. https://doi.org/10.3390/molecules22091489
APA StyleSong, S.-H., Ki, S. H., Park, D.-H., Moon, H.-S., Lee, C.-D., Yoon, I.-S., & Cho, S.-S. (2017). Quantitative Analysis, Extraction Optimization, and Biological Evaluation of Cudrania tricuspidata Leaf and Fruit Extracts. Molecules, 22(9), 1489. https://doi.org/10.3390/molecules22091489








