Structure Identification and In Vitro Anticancer Activity of Lathyrol-3-phenylacetate-5,15-diacetate
Abstract
:1. Introduction
2. Results
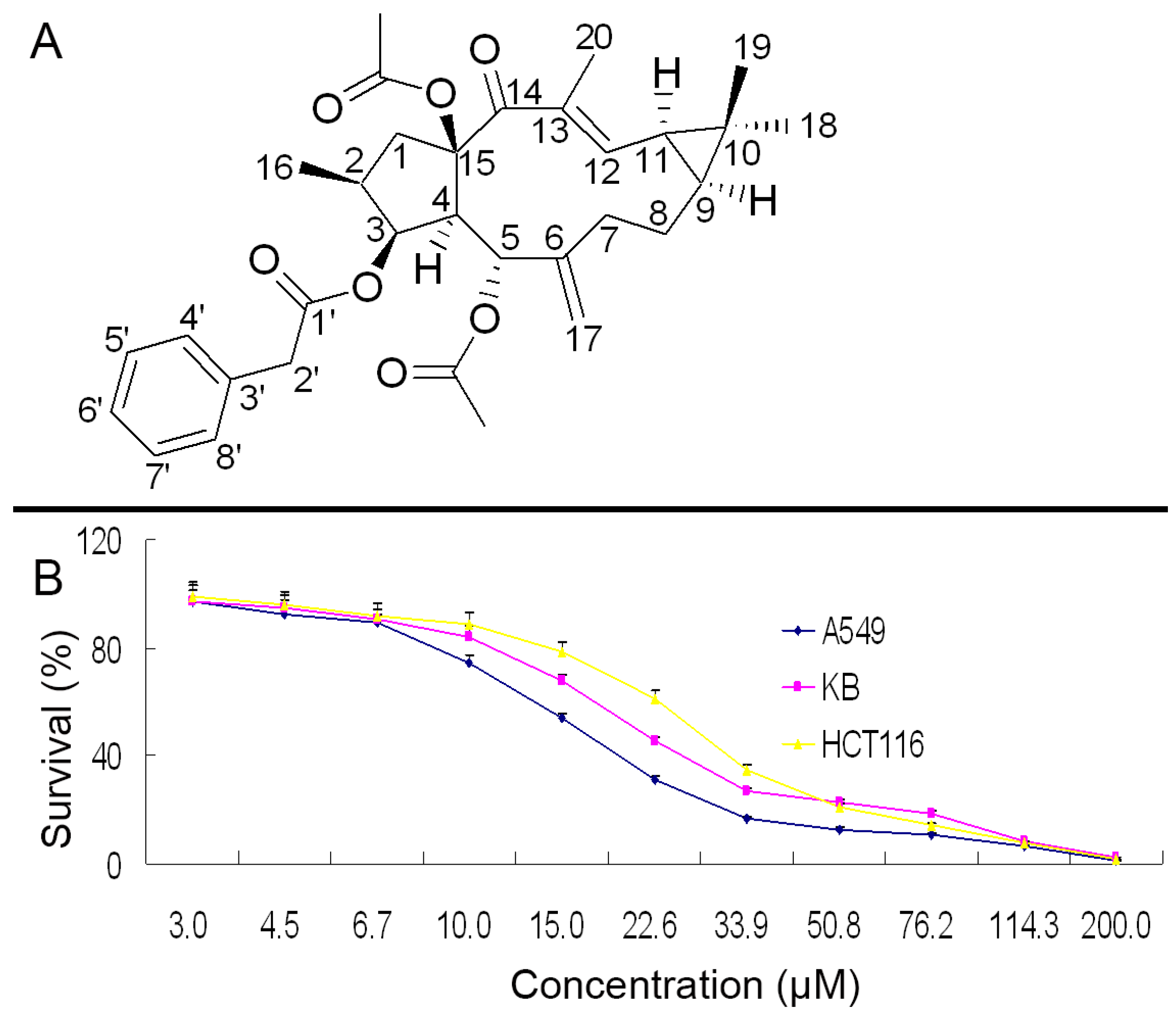
2.1. Structure Identification and NMR Signal Assignments
2.2. Growth Inhibition in A549, KB and HCT116 Cells
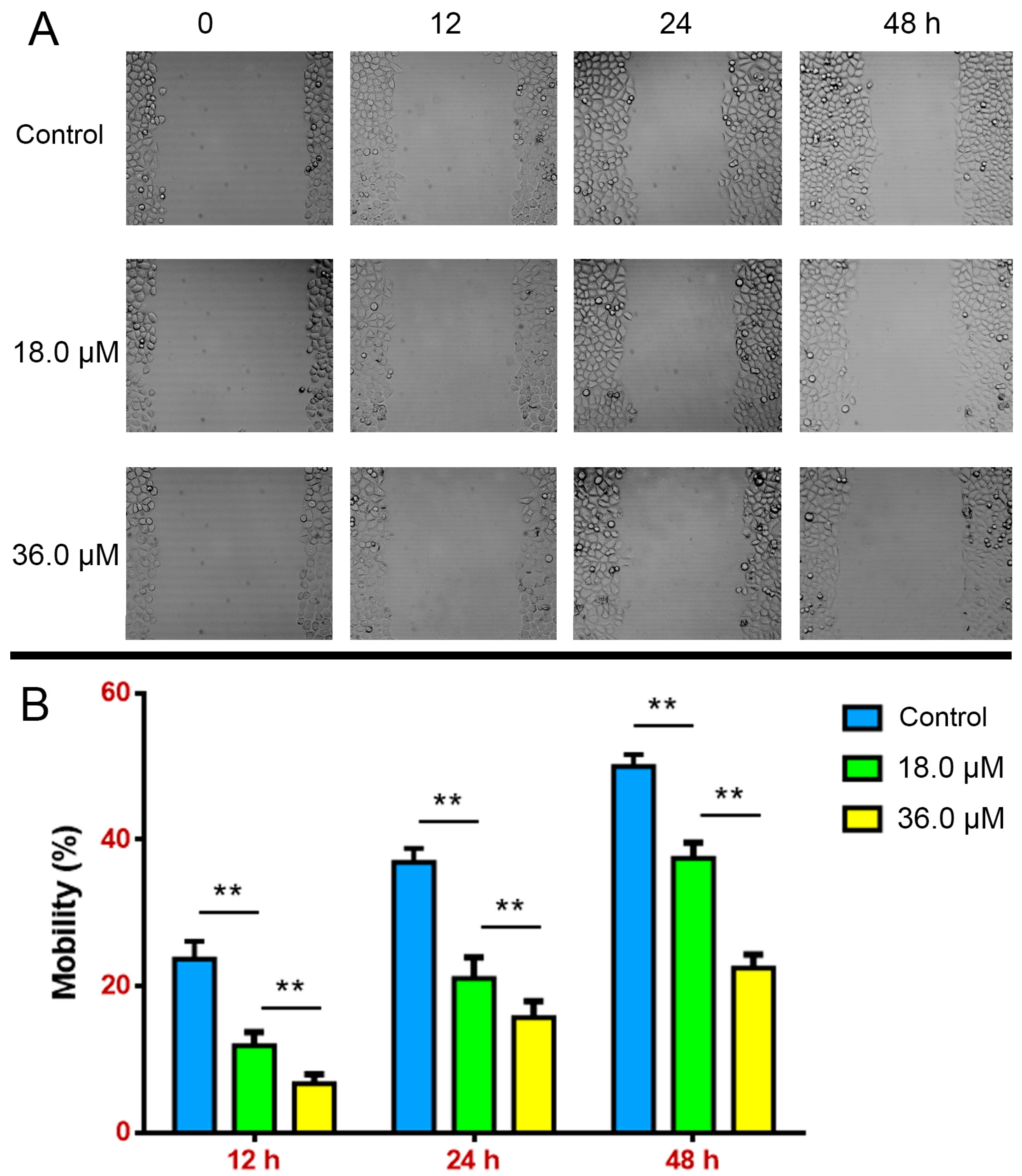
2.3. Suppressed Wound Healing in A549 Cells
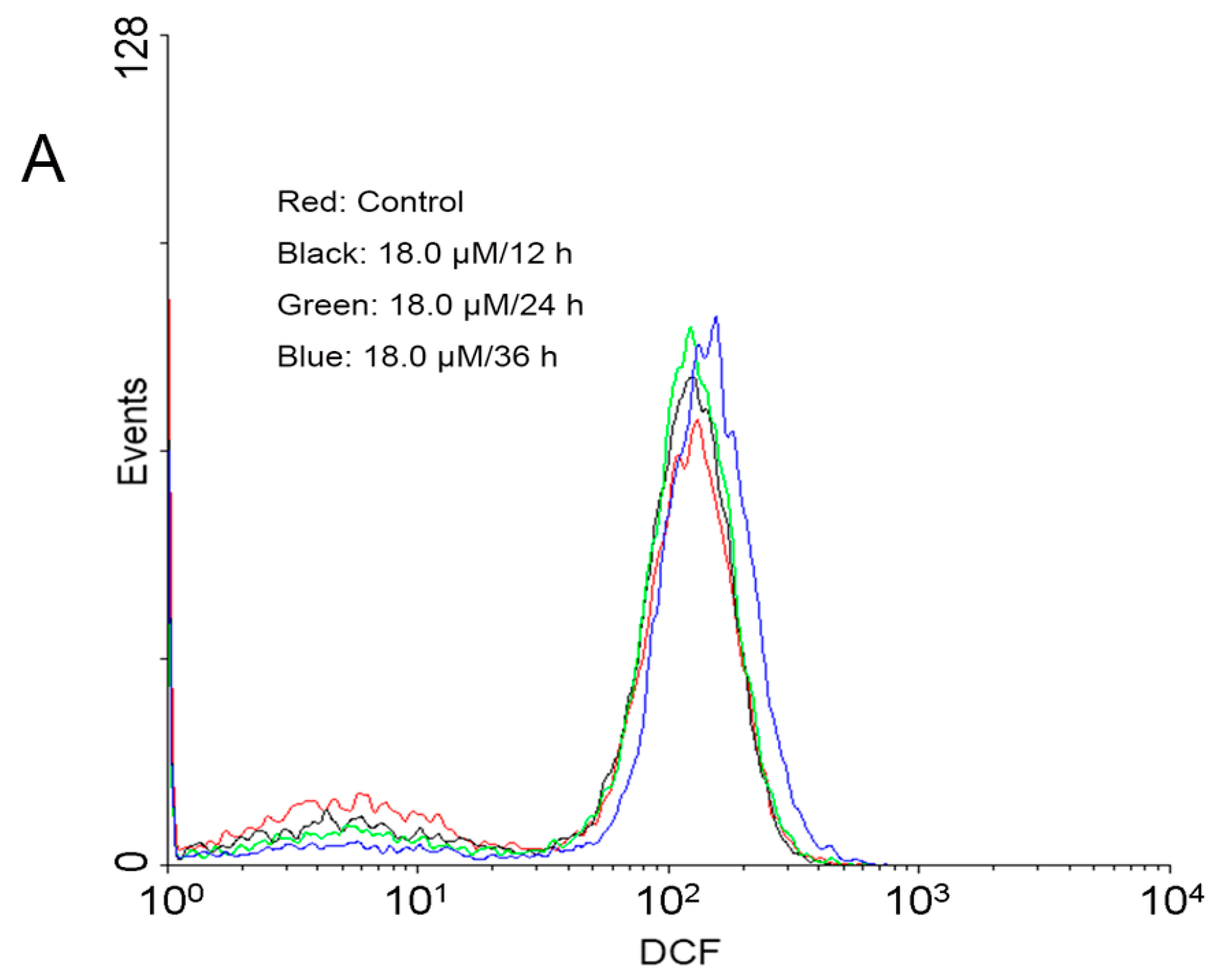
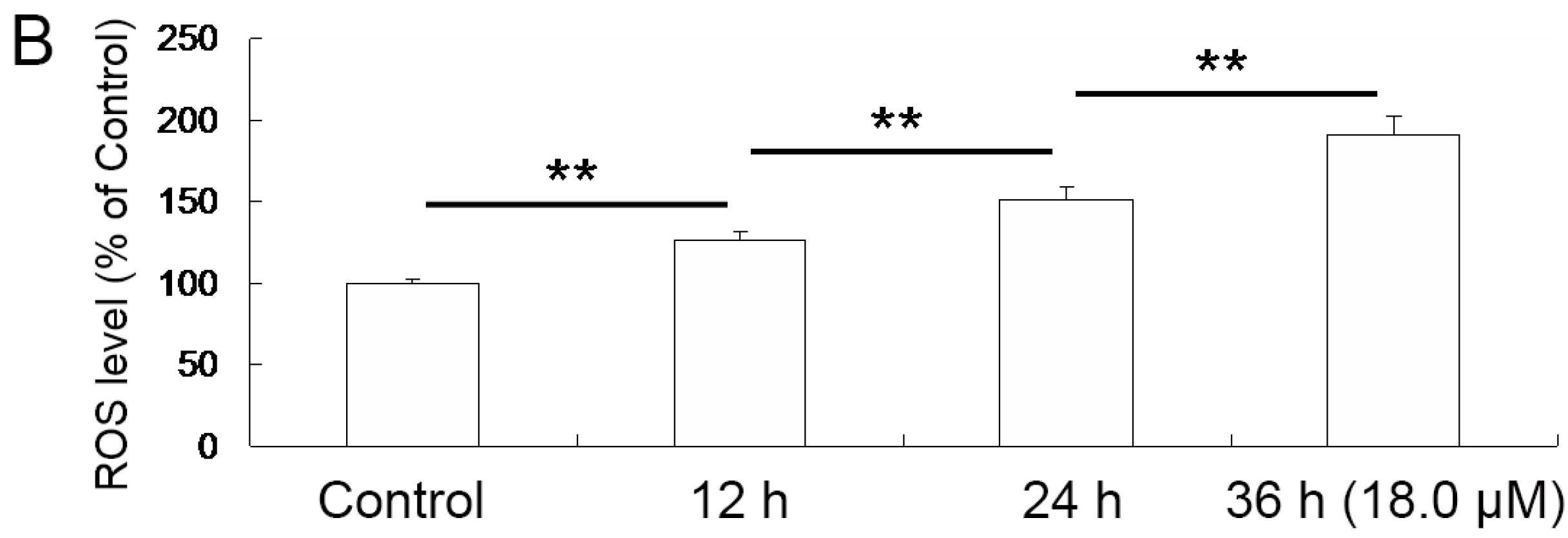
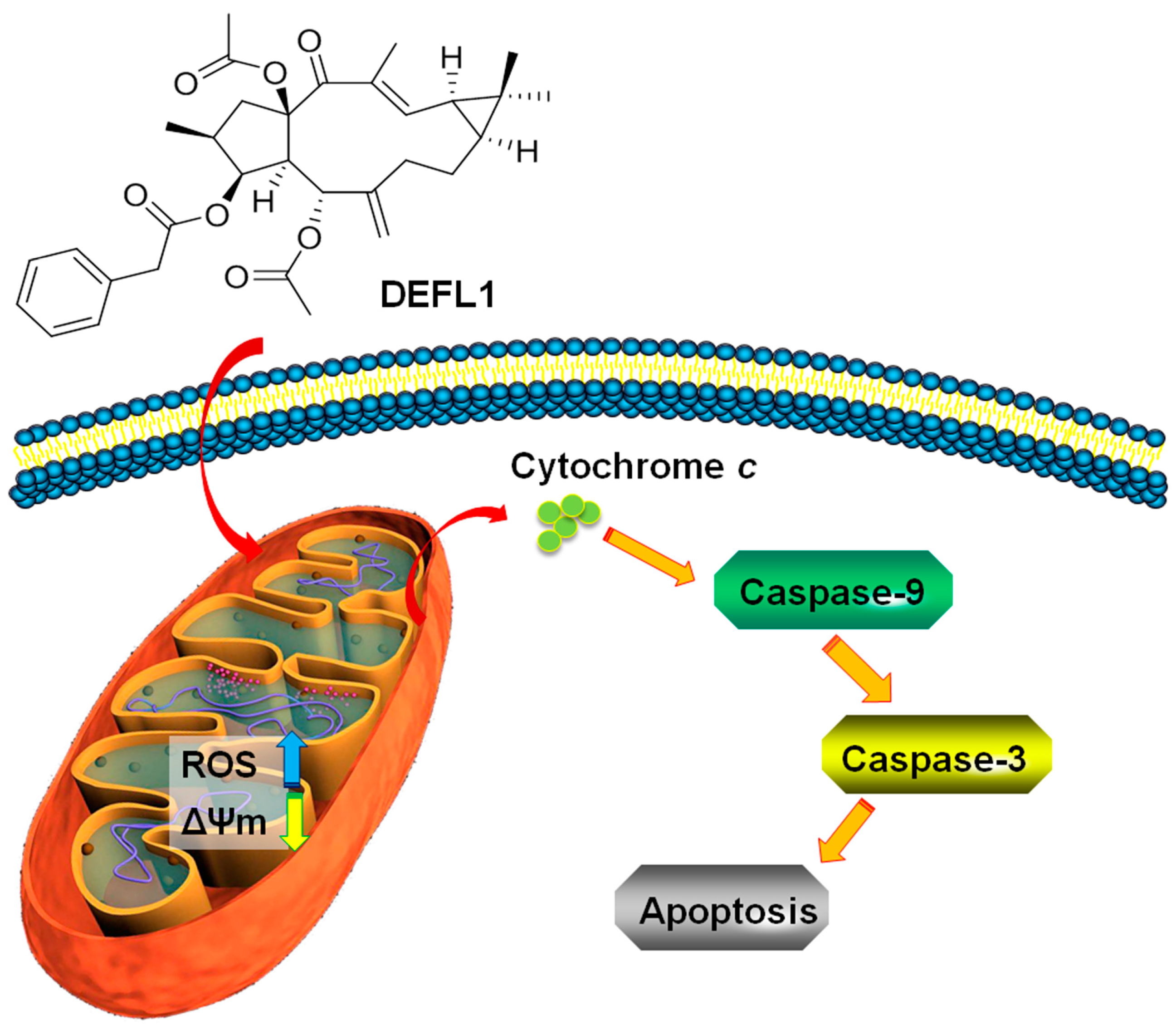
2.4. Increase of Intracellular ROS Levels in A549 Cells
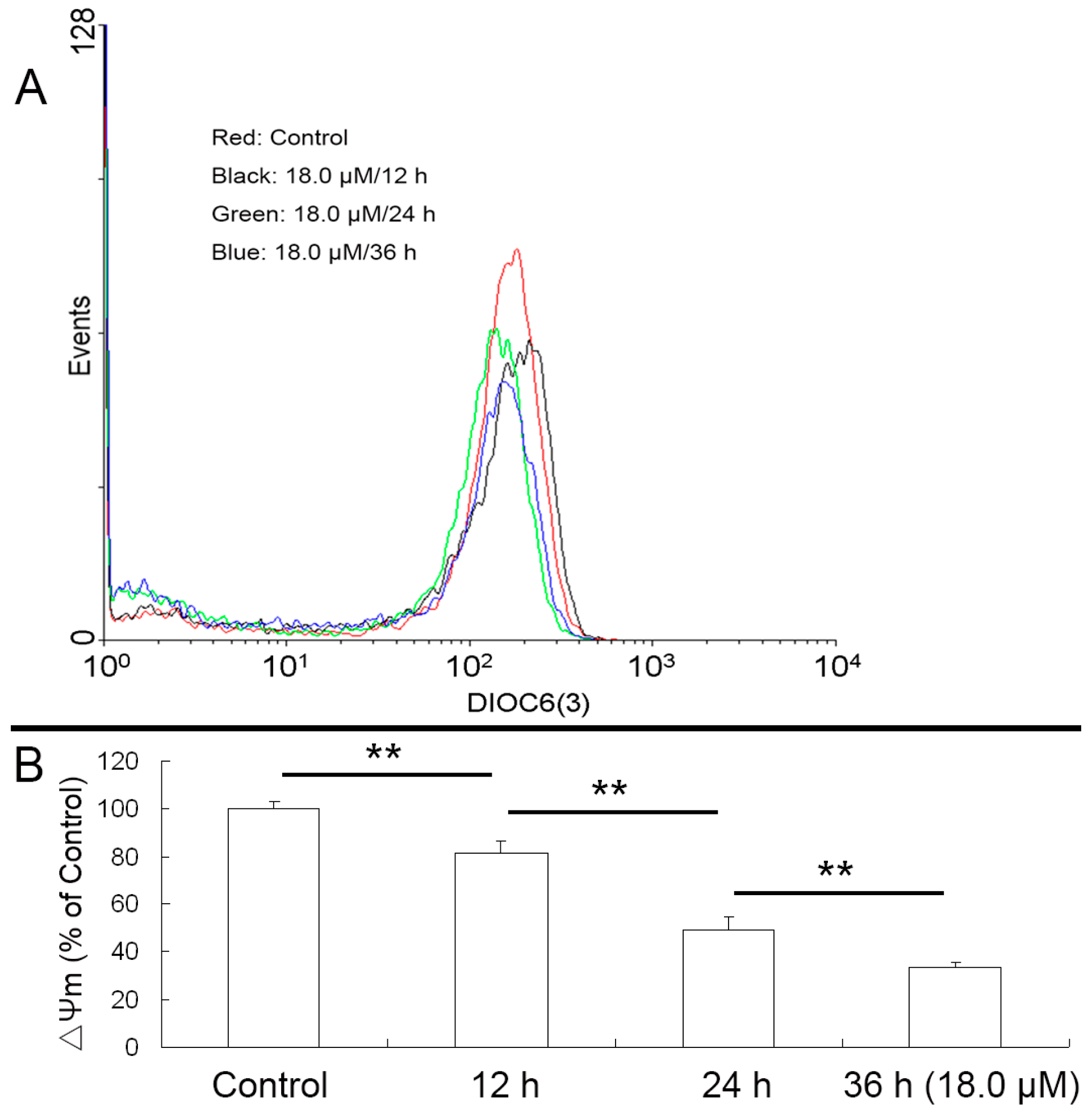
2.5. Reduced Mitochondrial Membrane Potential (ΔΨm) of A549 Cells
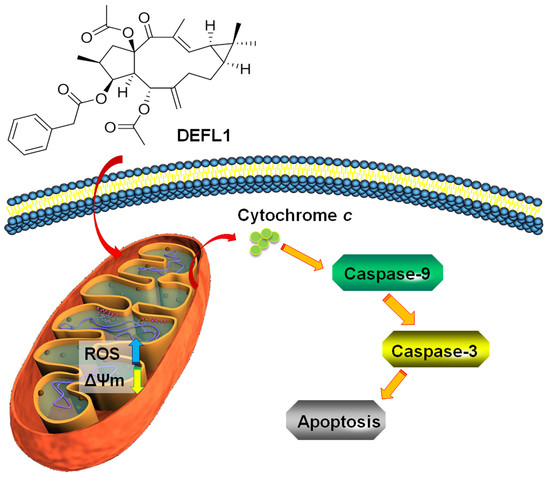
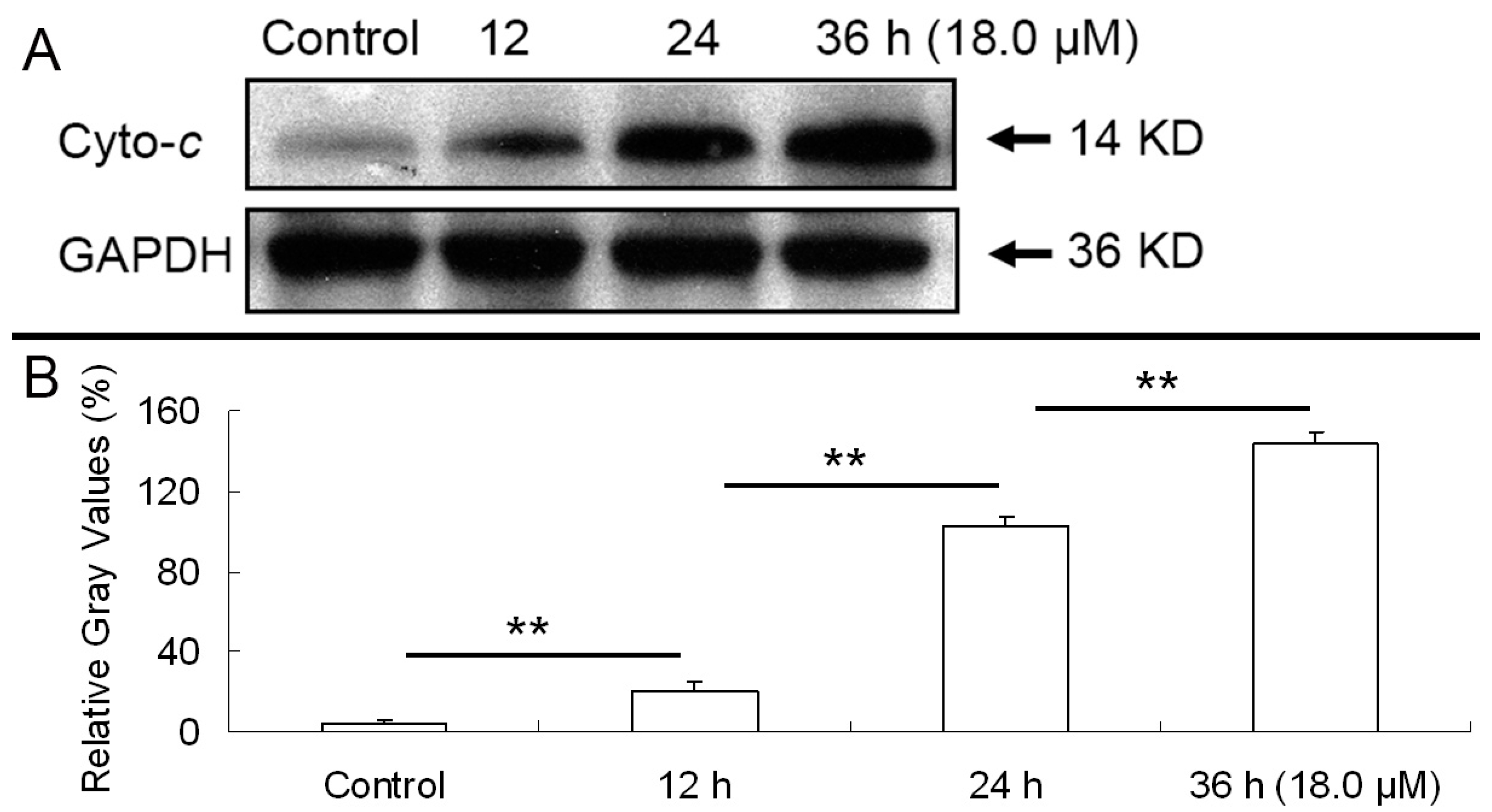
2.6. Release of Cytochrome C
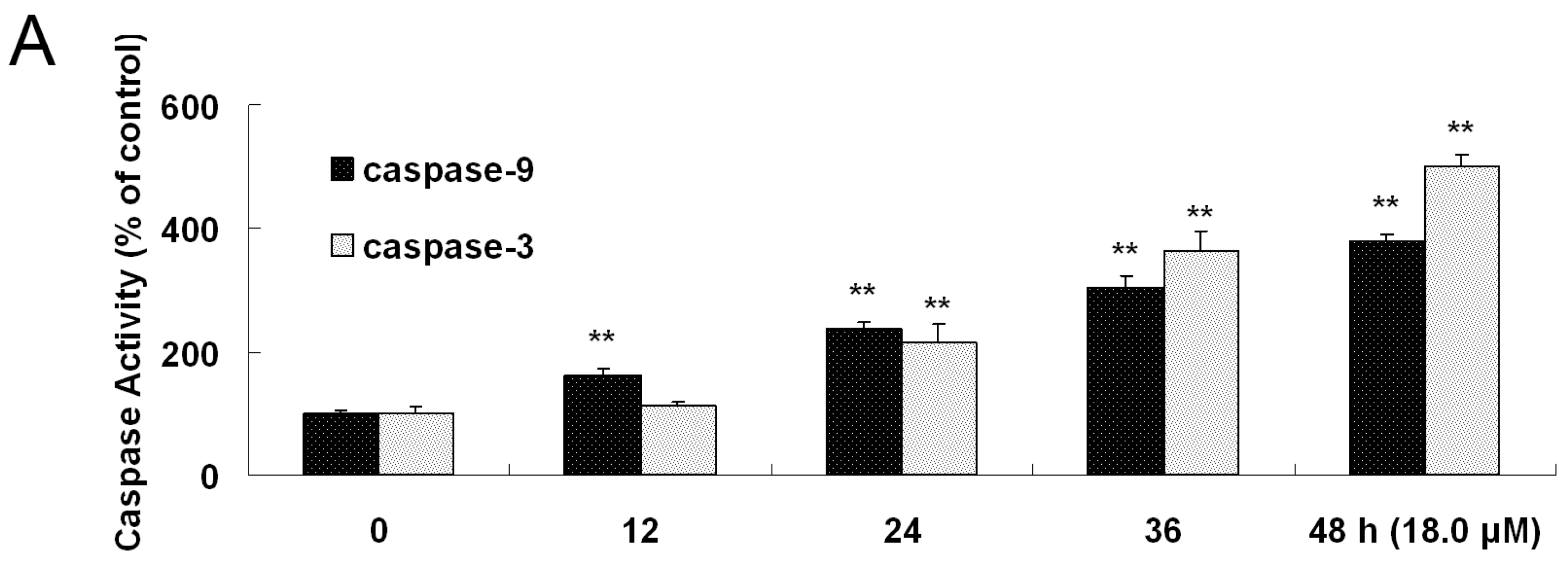
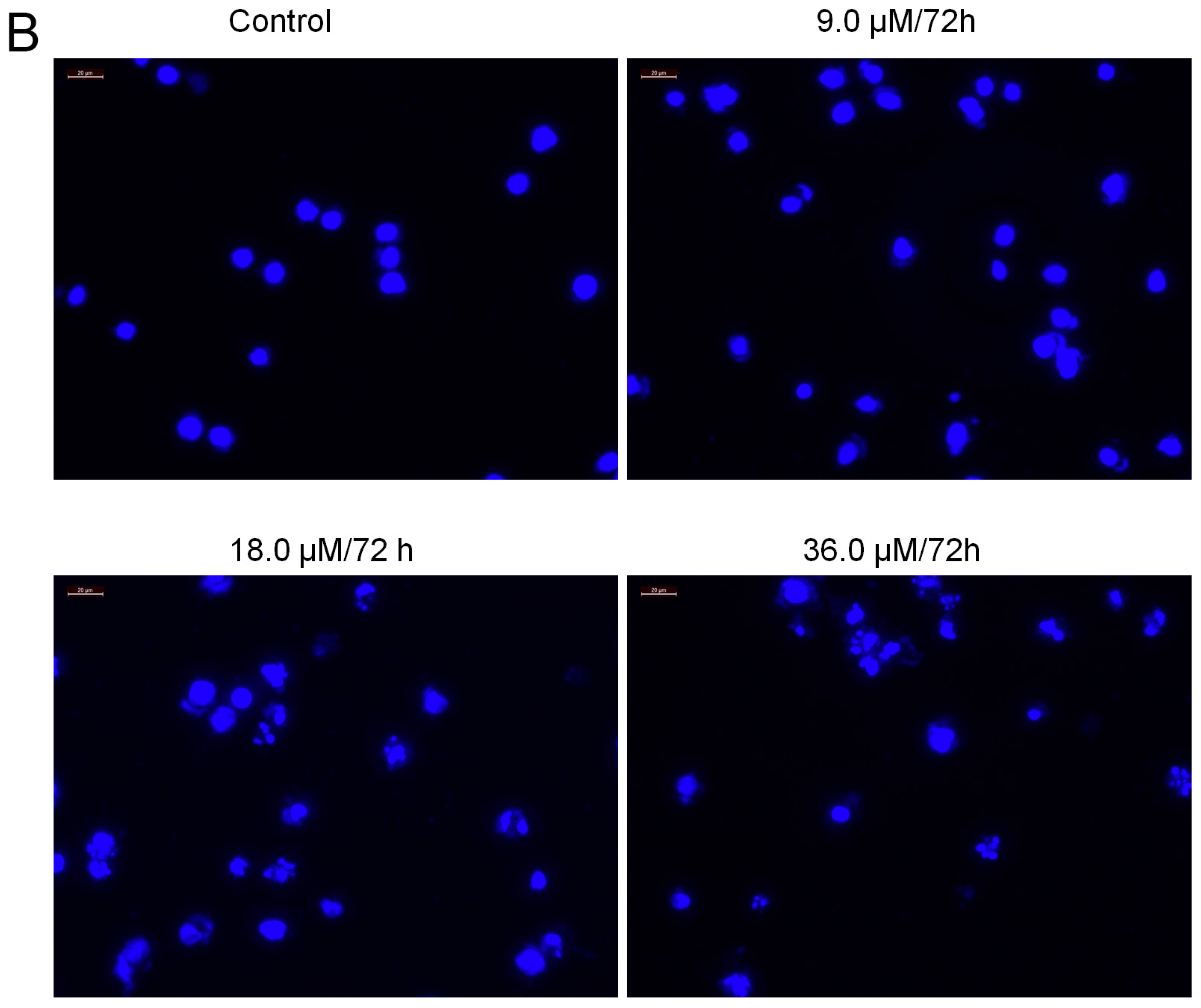
2.7. Increased Activities of Caspase-9, -3 and Apoptosis Confirmation by Hoechst 33258 Staining
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Plants, Extraction, Isolation and Characterization of DEFL1
4.3. Cell Lines and Culture
4.4. Cell Viability Assay
4.5. Wound Healing Assay
4.6. Measurement of ROS Generation
4.7. Determination of Mitochondrial Potential (ΔΨm)
4.8. Subcellular Isolation for Western Blot Analysis of Cytosolic Cytochrome c
4.9. Measurement of Caspase-9 and -3 Activities
4.10. Hoechst 33258 Staining
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, Z.; Li, Z.; Li, Z.J.; Cheng, K.; Du, Y.; Fu, H.; Khuri, F.R. Cables1 controls p21/Cip1 protein stability by antagonizing proteasome subunit alpha type 3. Oncogene 2015, 34, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Park, H.R.; Du, Y.; Li, Z.; Cheng, K.; Sun, S.Y.; Li, Z.; Fu, H.; Khuri, F.R. Cables1 complex couples survival signaling to the cell death machinery. Cancer Res. 2015, 75, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Peng, X.X.; Kim, I.W.; Shukla, S.; Si, Q.S.; Robey, R.W.; Bates, S.E.; Shen, T.; Ashby, C.R., Jr.; Fu, L.W.; et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007, 67, 11012–11020. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Bi, H.; Yan, Y.; Huang, W.; Zhang, G.; Zhang, G.; Tang, S.; Liu, Y.; Zhang, L.; Ma, J.; et al. Parthenolide suppresses non-small cell lung cancer GLC-82 cells growth via B-Raf/MAPK/Erk pathway. Oncotarget 2017, 8, 23436–23447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.W.; Cheng, K.J.; Mei, X.L.; Qiu, J.G.; Zhang, W.J.; Xue, Y.Q.; Qin, W.M.; Yang, Y.; Zheng, D.W.; Chen, Y.; et al. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget 2015, 6, 32790–32804. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tiwari, A.K.; Shukla, S.; Robey, R.W.; Singh, S.; Kim, I.W.; Bates, S.E.; Peng, X.; Abraham, I.; Ambudkar, S.V.; et al. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011, 71, 3029–3041. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Liang, Y.J.; Chen, Z.S.; Wang, X.W.; Wang, X.H.; Ding, Y.; Chen, L.M.; Yang, X.P.; Fu, L.W. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol. Ther. 2006, 5, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updat. 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Ambudka, S.V. The pharmacological impact of ATP-binding cassette drug transporters on vemurafenib-based therapy. Acta Pharm. Sin. B 2014, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fu, L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B 2015, 5, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.W.; Chen, M.W.; Cheng, K.J.; Yu, P.Z.; Wei, X.; Shi, Z. Therapeutic potential of steroidal alkaloids in cancer and other diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm. Sin. B 2015, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Aldonza, M.B.; Hong, J.Y.; Alinsug, M.V.; Song, J.; Lee, S.K. Multiplicity of acquired cross-resistance in paclitaxel-resistant cancer cells is associated with feedback control of TUBB3 via FOXO3a-mediated ABCB1 regulation. Oncotarget 2016, 7, 34395–34419. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Zhang, Y.B.; Zhou, Q.L.; Yang, Y.F.; Yang, X.W. Simultaneous determination of eight ginsenosides in rat plasma by liquid chromatography-electrospray ionization tandem mass spectrometry: Application to their pharmacokinetics. Molecules 2015, 20, 21597–21608. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Dong, W.; Li, Z.; Deng, M.; Lu, R. Lathyrane diterpenes from Euphorbia lathyris as modulators of multidrug resistance and their crystal structures. Bioorg. Med. Chem. 2009, 17, 4786–4792. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, G.; Huang, J.; Zhang, C.; Zhang, L.; Zhang, K.; Li, P.; Lin, R.; Wang, J. Lathyrane-type diterpenoids from the seeds of Euphorbia lathyris. Phytochemistry 2014, 104, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.; Varga, A.; Cherepnev, G.; Radics, R.; Molnar, J.; Ferreira, M.J. Apoptosis induction and modulation of P-glycoprotein mediated multidrug resistance by new macrocyclic lathyrane-type diterpenoids. Bioorg. Med. Chem. 2007, 15, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Belloro, E.; Tron, G.C.; Jakupovic, J.; Ballero, M. Diterpenoids from euphorbia pithyusa subsp. cupanii. J. Nat. Prod. 1999, 62, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Tang, S.; Zhang, C.; Chen, H.; Huang, W.; Liu, Y.; Zhang, J. Euphorbia factor L2 induces apoptosis in A549 cells through the mitochondrial pathway. Acta Pharm. Sin. B 2017, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liang, Y.J.; Chen, H.B.; Zheng, L.S.; Mi, Y.J.; Wang, F.; Zhao, X.Q.; Wang, X.K.; Zhang, H.; Fu, L.W. Structure identification of Euphorbia factor L3 and its induction of apoptosis through the mitochondrial pathway. Molecules 2011, 16, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Lin, M.T.; Yi, T.; Tang, Y.N.; Fan, L.L.; He, X.C.; Zhao, Z.Z.; Chen, H.B. Apoptosis sensitization by Euphorbia factor L1 in ABCB1-mediated multidrug resistant K562/ADR cells. Molecules 2013, 18, 12793–12808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Mi, Y.J.; Chen, S.P.; Wang, F.; Liang, Y.J.; Zheng, L.S.; Shi, C.J.; Tao, L.Y.; Chen, L.M.; Chen, H.B.; et al. Euphorbia factor L1 reverses ABCB1-mediated multidrug resistance involving interaction with ABCB1 independent of ABCB1 downregualtion. J. Cell. Biochem. 2011, 112, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, X.; Wang, F.; Wang, X.; Yang, K.; Xu, M.; To, K.K.; Li, Q.; Fu, L. Effect of ceritinib (LDK378) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo. Oncotarget 2015, 6, 44643–44659. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, C.; Roca, M.S.; Noto, A.; Bruzzese, F.; Moccia, T.; Vitagliano, C.; Di Gennaro, E.; Ciliberto, G.; Roscilli, G.; Aurisicchio, L.; et al. Synergistic antitumor activity of histone deacetylase inhibitors and anti-ErbB3 antibody in NSCLC primary cultures via modulation of ErbB receptors expression. Oncotarget 2016, 7, 19559–19574. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, S.H.; Lee, J.S.; Nam, B.; Seong, T.W.; Son, J.; Jang, H.; Hong, K.M.; Lee, C.; Kim, S.Y. Aldehyde dehydrogenase inhibition combined with phenformin treatment reversed NSCLC through ATP depletion. Oncotarget 2016, 7, 49397–49410. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Li, S.J.; Li, D.J. Hsa-miR-134 suppresses non-small cell lung cancer (NSCLC) development through down-regulation of CCND1. Oncotarget 2016, 7, 35960–35978. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.; Huang, L.; Liu, T.; Huang, Y.; Zhao, J.; Wang, X.; Yang, K.; Ma, S.; Huang, L.; et al. Cetuximab enhanced the efficacy of chemotherapeutic agent in ABCB1/P-glycoprotein-overexpressing cancer cells. Oncotarget 2015, 6, 40850–40865. [Google Scholar] [CrossRef] [PubMed]
- Corte, C.M.D.; Ciaramella, V.; Mauro, C.D.; Castellone, M.D.; Papaccio, F.; Fasano, M.; Sasso, F.C.; Martinelli, E.; Troiani, T.; De Vita, F.; et al. Metformin increases antitumor activity of MEK inhibitors through GLI1 downregulation in LKB1 positive human NSCLC cancer cells. Oncotarget 2016, 7, 4265–4278. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Y.; Zhou, W.; Li, S.; Peng, J.; Shi, Z.; Hu, J.; Liu, Y.C.; Ding, H.; Lin, Y.; et al. Identifying novel selective non-nucleoside DNA methyltransferase 1 inhibitors through docking-based virtual screening. J. Med. Chem. 2014, 57, 9028–9041. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. A new golden age of natural products drug discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, J.; Liu, H.; Liang, S.; Lin, M.; Gu, Y.; Liu, T.; Wang, D.; Ge, H.; Mo, S.L. The antitumor effect of tanshinone IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on the human non-small cell lung cancer A549 cell line. Acta Pharm. Sin. B 2015, 5, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, A.; Fang, G.; Liu, H.; Zhang, M.; Tang, S.; Xiong, B.; Ma, L.; Geng, M.; Shen, J. Tetrahydroisoquinolines as novel histone deacetylase inhibitors for treatment of cancer. Acta Pharm. Sin. B 2016, 6, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, L.; Cao, Y.; Yao, W.; Tang, Y.; Ding, A. An ingenol derived from Euphorbia kansui induces hepatocyte cytotoxicity by triggering G0/G1 cell cycle arrest and regulating the mitochondrial apoptosis pathway in vitro. Molecules 2016, 21, 813. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, N.; Ravinder, R.M.; Ramesh, C.; Ramu, R.; Prabhakar, A.; Jagadeesh, B.; Das, B. New lathyrane and podocarpane diterpenoids from Jatropha curcas. Chem. Pharm. Bull. (Tokyo) 2004, 52, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.J.; Ferreira, M.J.; Molnar, J.; Fernandes, M.X. QSAR studies of macrocyclic diterpenes with P-glycoprotein inhibitory activity. Eur. J. Pharm. Sci. 2013, 48, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, N.; Mohammed, K.A.; Lee, H.; Kaye, F.; Nasreen, N. MicroRNA-302b targets Mcl-1 and inhibits cell proliferation and induces apoptosis in malignant pleural mesothelioma cells. Am. J. Cancer Res. 2016, 6, 1996–2009. [Google Scholar] [PubMed]
- Liu, X.; Lv, Z.; Zou, J.; Liu, X.; Ma, J.; Wang, J.; Sa, N.; Jing, P.; Xu, W. Afatinib down-regulates MCL-1 expression through the PERK-eIF2alpha-ATF4 axis and leads to apoptosis in head and neck squamous cell carcinoma. Am. J. Cancer Res. 2016, 6, 1708–1719. [Google Scholar] [PubMed]
- Huang, W.; Zhang, S.; Yang, Z.; Feng, B. Screening novel SAHA derivatives as anti-lung carcinoma agents: Synthesis, biological evaluation, docking studies and further mechanism research between apoptosis and autophagy. Anticancer Agents Med. Chem. 2015, 15, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Liu, J.; Guo, L.; Wu, D.; Matsumoto, K.; Huang, L. PRAS40 deregulates apoptosis in Ewing sarcoma family tumors by enhancing the insulin receptor/Akt and mTOR signaling pathways. Am. J. Cancer Res. 2016, 6, 486–497. [Google Scholar] [PubMed]
- Su, J.; Zhou, X.; Wang, L.; Yin, X.; Wang, Z. Curcumin inhibits cell growth and invasion and induces apoptosis through down-regulation of Skp2 in pancreatic cancer cells. Am. J. Cancer Res. 2016, 6, 1949–1962. [Google Scholar] [PubMed]
- Wu, X.; Li, L.; Li, Y.; Liu, Z. MiR-153 promotes breast cancer cell apoptosis by targeting HECTD3. Am. J. Cancer Res. 2016, 6, 1563–1571. [Google Scholar] [PubMed]
- Cho, Y.; Yoon, J.H.; Yoo, J.J.; Lee, M.; Lee, D.H.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Kim, Y.J.; Kim, C.Y. Fucoidan protects hepatocytes from apoptosis and inhibits invasion of hepatocellular carcinoma by up-regulating p42/44 MAPK-dependent NDRG-1/CAP43. Acta Pharm. Sin. B 2015, 5, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Mao, G.; Wang, G.; Chen, Y.; Wu, X.; Wang, Z. DZNep inhibits the proliferation of colon cancer HCT116 cells by inducing senescence and apoptosis. Acta Pharm. Sin. B 2015, 5, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yu, Z.; Liang, N.; Chi, X.; Li, X.; Jiang, M.; Fang, J.; Cui, H.; Lai, W.; Zhou, Y.; et al. The mitochondrial and death receptor pathways involved in the thymocytes apoptosis induced by aflatoxin B1. Oncotarget 2016, 7, 12222–12234. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Lu, N.; Zhou, Y.; Ni, T.; Dai, Y.; Li, Z.; Guo, Q.; Wei, L. Oroxylin A modulates mitochondrial function and apoptosis in human colon cancer cells by inducing mitochondrial translocation of wild-type p53. Oncotarget 2016, 7, 17009–17020. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.L.; Lin, P.Y.; Lin, H.C.; Chen, Y.L. The apoptotic effect of ursolic acid on SK-Hep-1 cells is regulated by the PI3K/Akt, p38 and JNK MAPK signaling pathways. Molecules 2016, 21, 460. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Jia, D.Z.; Liang, Y.J.; Yan, S.L.; Ding, Y.; Chen, L.M.; Shi, Z.; Zeng, M.S.; Liu, G.F.; Fu, L.W. Lgf-YL-9 induces apoptosis in human epidermoid carcinoma KB cells and multidrug resistant KBv200 cells via reactive oxygen species-independent mitochondrial pathway. Cancer Lett. 2007, 249, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Hanikoglu, F.; Cort, A.; Ozben, H.; Hanikoglu, A.; Ozben, T. Epoxomicin sensitizes resistant osteosarcoma cells to Trail induced apoptosis. Anticancer Agents Med. Chem. 2015, 15, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, Y.; Li, H.; Zhang, W.; Liu, H.; Sun, Z.; Liu, T.; Li, S. Two trichothecene mycotoxins from myrothecium roridum induce apoptosis of HepG-2 cells via caspase activation and disruption of mitochondrial membrane potential. Molecules 2016, 21, 781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Tao, L.Y.; Liang, Y.J.; Chen, L.M.; Mi, Y.J.; Zheng, L.S.; Wang, F.; She, Z.G.; Lin, Y.C.; To, K.K.; et al. Anthracenedione derivatives as anticancer agents isolated from secondary metabolites of the mangrove endophytic fungi. Mar. Drugs 2010, 8, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.W.; Lin, Y.C.; She, Z.G.; Lin, M.T.; Chen, P.X.; Zhang, J.Y. Anticancer activity and mechanism investigation of beauvericin isolated from secondary metabolites of the mangrove endophytic fungi. Anticancer Agents Med. Chem. 2015, 15, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.; Shah, B.A.; Batra, N.; Kumar, A.; Guru, S.K.; Bhushan, S.; Malik, F.A.; Joshi, A.; Singh, J. Multiple pharmacological properties of a novel parthenin analog P16 as evident by its cytostatic and antiangiogenic potential against pancreatic adenocarcinoma PANC-1 cells. Anticancer Agents Med. Chem. 2016, 16, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wu, H.Y.; Xia, X.K.; Liang, Y.J.; Yan, Y.Y.; She, Z.G.; Lin, Y.C.; Fu, L.W. Anthracenedione derivative 1403P-3 induces apoptosis in KB and KBv200 cells via reactive oxygen species-independent mitochondrial pathway and death receptor pathway. Cancer Biol. Ther. 2007, 6, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, S.; Bu, W.; Wei, M.Y.; Li, W.W.; Yao, M.N.; Ma, Z.Y.; Lu, C.T.; Li, H.H.; Hu, N.P.; et al. Synthesis of a novel adamantyl nitroxide derivative with potent anti-hepatoma activity in vitro and in vivo. Am. J. Cancer Res. 2016, 6, 1271–1286. [Google Scholar] [PubMed]
- Wang, F.; Lin, J.; Hou, W.; Huang, M.Y.; Sun, P.H.; Chen, W.M. 5-Benzylidene-3,4-dihalo-furan-2-one derivatives inhibit human leukemia cancer cells through suppression of NF-kappaB and GSK-3beta. Anticancer Agents Med. Chem. 2015, 15, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.K.; He, J.H.; Xu, J.H.; Ye, S.; Wang, F.; Zhang, H.; Huang, Z.C.; To, K.K.; Fu, L.W. Afatinib enhances the efficacy of conventional chemotherapeutic agents by eradicating cancer stem-like cells. Cancer Res. 2014, 74, 4431–4445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Lin, M.T.; Zhou, M.J.; Yi, T.; Tang, Y.N.; Tang, S.L.; Yang, Z.J.; Zhao, Z.Z.; Chen, H.B. Combinational treatment of curcumin and quercetin against gastric cancer MGC-803 cells in vitro. Molecules 2015, 20, 11524–11534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Yi, T.; Liu, J.; Zhao, Z.Z.; Chen, H.B. Quercetin induces apoptosis via the mitochondrial pathway in KB and KBv200 cells. J. Agric. Food Chem. 2013, 61, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Lin, M.T.; Tung, H.Y.; Tang, S.L.; Yi, T.; Zhang, Y.Z.; Tang, Y.N.; Zhao, Z.Z.; Chen, H.B. Bruceine D induces apoptosis in human chronic myeloid leukemia K562 cells via mitochondrial pathway. Am. J. Cancer Res. 2016, 6, 819–826. [Google Scholar] [PubMed]
Sample Availability: Sample of the lathyrol-3-phenylacetate-5,15-diacetate are available from the authors. |
| Position | 13C | 1H | HMBC (H→C) | |
|---|---|---|---|---|
| 1 | 48.3 | 3.40 (1H, dd, J = 8, 14 Hz) 1.44 (1H, J = 14, 11 Hz) | 2, 3, 4, 14, 15 | |
| 2 | 37.4 | 2.01 (1H, m) | 1, 3, 4, 15, 16 | |
| 3 | 80.6 | 5.60 (1H, t, J = 3 Hz) | 1, 1’, 15 | |
| 4 | 52.2 | 2.79 (1H, dd, J = 3.6, 10 Hz) | 5, 6, 14 | |
| 5 | 65.9 | 6.12 (1H, d, J = 10.4 Hz) | 4, 6, 7, 15, 5-CO | |
| 6 | 144.4 | |||
| 7 | 35.0 | 2.11 (1H, m), 2.20 (1H, m) | 5, 6, 8, 9, 17 | |
| 8 | 21.7 | 2.01 (1H, m), 1.71 (1H, m) | 6, 9 | |
| 9 | 35.3 | 1.1 (1H , m) | 18, 19 | |
| 10 | 25.2 | |||
| 11 | 29.0 | 1.40 (1H, dd, J = 8.4, 11.6 Hz) | 9, 10, 12, 13 | |
| 12 | 146.7 | 6.54 (1H, d, 11.2 Hz) | 9, 14, 20 | |
| 13 | 134.1 | |||
| 14 | 196.8 | |||
| 15 | 92.3 | |||
| 16 | 13.7 | 0.73 (3H, d, J = 6.4 Hz) | 1, 2, 3 | |
| 17 | 115.6 | 5.01 (1H, s), 4.74 (1H, s) | 5, 6, 7 | |
| 18 | 28.4 | 1.19 (3H, s) | 9, 10, 11, 19 | |
| 19 | 16.8 | 1.18 (3H, s) | 9, 10, 11, 18 | |
| 20 | 12.4 | 1.70 (3H, s) | 12, 13, 14 | |
| 5-COCH3 | CH3 | 21.3 | 1.96 (3H, s) | 5-CO |
| CO | 170.7 | |||
| 15-COCH3 | CH3 | 22.0 | 2.20 (3H, s) | 15-CO |
| CO | 169.8 | |||
| 3-OPhAc | 1′ | 171.3 | ||
| 2’ | 41.6 | 3.64 (1H, d, J = 15 Hz), 3.62 (1H, d, J = 15 Hz) | 1′, 3′, 4′, 8′ | |
| 3′ | 134.0 | |||
| 6′ | 127.1 | 7.25 (1H, m) | 4′, 8′ | |
| 4′, 8′ | 129.5 | 7.31 (2H, m) | 2’, 3′ | |
| 5′, 7′ | 128.5 | 7.33 (2H, m) | 3′ | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-y.; Huang, W.-j.; Sun, H.-m.; Liu, Y.; Zhao, X.-q.; Tang, S.-l.; Sun, M.-n.; Wang, S.; Li, J.-j.; Zhang, L.-l.; et al. Structure Identification and In Vitro Anticancer Activity of Lathyrol-3-phenylacetate-5,15-diacetate. Molecules 2017, 22, 1412. https://doi.org/10.3390/molecules22091412
Zhang J-y, Huang W-j, Sun H-m, Liu Y, Zhao X-q, Tang S-l, Sun M-n, Wang S, Li J-j, Zhang L-l, et al. Structure Identification and In Vitro Anticancer Activity of Lathyrol-3-phenylacetate-5,15-diacetate. Molecules. 2017; 22(9):1412. https://doi.org/10.3390/molecules22091412
Chicago/Turabian StyleZhang, Jian-ye, Wen-jing Huang, Hong-mei Sun, Yun Liu, Xiao-qin Zhao, Si-li Tang, Ming-na Sun, Sheng Wang, Jia-jun Li, Ling-ling Zhang, and et al. 2017. "Structure Identification and In Vitro Anticancer Activity of Lathyrol-3-phenylacetate-5,15-diacetate" Molecules 22, no. 9: 1412. https://doi.org/10.3390/molecules22091412
APA StyleZhang, J.-y., Huang, W.-j., Sun, H.-m., Liu, Y., Zhao, X.-q., Tang, S.-l., Sun, M.-n., Wang, S., Li, J.-j., Zhang, L.-l., Zhou, J.-h., Pan, Q.-r., & Chen, H.-b. (2017). Structure Identification and In Vitro Anticancer Activity of Lathyrol-3-phenylacetate-5,15-diacetate. Molecules, 22(9), 1412. https://doi.org/10.3390/molecules22091412