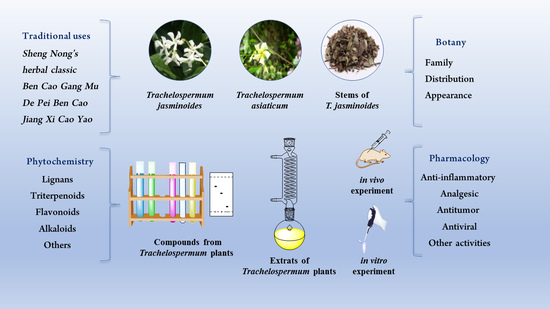
Phytochemistry, Pharmacology and Traditional Uses of Plants from the Genus Trachelospermum L.
Abstract
:1. Introduction
2. Botany
3. Phytochemistry
3.1. Lignans
3.2. Triterpenoids
3.3. Flavonoids
3.4. Alkaloids
3.5. Other Compounds
4. Traditional Uses
5. Pharmacology
5.1. Anti-inflammatory and Analgesic Activity
5.2. Antitumor Activity
5.3. Antiviral Activity
5.4. Antibacterial Activity
5.5. Other Biological Activities
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoo, H.H.; Park, J.H.; Kwon, S.W. An anti-estrogenic lignan glycoside, tracheloside, from seeds of Carthamus tinctorius. Biosci. Biotech. Biochem. 2006, 70, 2783–2785. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, I.; Hisada, S.; Nishibe, S. The triterpenoids of Trachelospermum asiaticum var. intermedium. Phytochemistry 1970, 9, 2241. [Google Scholar] [CrossRef]
- Sheu, M.J.; Chou, P.Y.; Cheng, H.C.; Wu, C.H.; Huang, G.J.; Wang, B.S.; Chen, J.S.; Chien, Y.C.; Huang, M.H. Analgesic and anti-inflammatory activities of a water extract of Trachelospermum jasminoides (Apocynaceae). J. Ethnopharmacol. 2009, 126, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Yamagishi, M.; Okazaki, K.; Son, H.Y.; Imazawa, T.; Nishikawa, A.; Iwata, T.; Yamauchi, Y.; Kasai, M.; Tsutsumi, K.; et al. Lack of significant inhibitory effects of a plant lignan tracheloside on 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP)-induced mammary carcinogenesis in female Sprague-Dawley rats. Cancer Let. 2003, 200, 133–139. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lu, A.M.; D‘Arcy, W.G. Flora of China. Volume 17: Verbenaceae through Solanaceae; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1994; pp. 301–304. [Google Scholar]
- Watanabe, H.; Takano, T. Components of Trachelospermum asiaticum var. inter-medium. II. Structure of tracheloside. (1). Yakugaku Zasshi 1958, 78, 882–885. [Google Scholar] [CrossRef]
- Inagaki, I.; Hisada, S.; Nishibe, S. Constituents of Trachelospermum asiaticum intermedium. Chem. Pharm. Bull. 1968, 16, 2307–2308. [Google Scholar] [CrossRef]
- Nishibe, S.; Hisada, S.; Inagaki, I. The ether-soluble lignans of Trachelospermum asiaticum var. intermedium. Phytochemistry 1971, 10, 2231–2232. [Google Scholar] [CrossRef]
- Nishibe, S.; Hisada, S.; Inagaki, I. Lignan diglucosides from Trachelospermum asiaticum. Phytochemistry 1972, 11, 3084–3085. [Google Scholar] [CrossRef]
- Nishibe, S.; Hisada, S.; Inagaki, I. Lignans of Trachelospermum asiaticum var. intermedium. V. Isolation of nortrachelogenin-4,4′-di-O-β-d-glucopyranoside. Chem. Pharm. Bull. 1973, 21, 1114–1117. [Google Scholar] [CrossRef]
- Nishibe, S.; Hisada, S.; Inagaki, I. Lignans of Trachelospermum asiaticum var intermedium. IV. Matairesinol-4,4′-di-O-β-d-glucopyranoside from Trachelospermum asiaticum var intermedium. Can. J. Chem. 1973, 51, 1050–1052. [Google Scholar] [CrossRef]
- Nishibe, S.; Hisada, S.; Inagaki, I. Lignans of Trachelospermum asiaticum var intermedium. III. Isolation of new lignan glycoside, arctigenin-4′-β-gentiobioside. Chem. Pharm. Bull. 1973, 21, 639–642. [Google Scholar] [CrossRef]
- Inagaki, I.; Sakushima, A.; Nishibe, S.; Hisada, S. Flavones and flavone glucosides from the leaves of Trachelospermum asiaticum. Phytochemistry 1973, 12, 1498. [Google Scholar] [CrossRef]
- Nishibe, S.; Okabe, K.; Hisada, S. Isolation of phenolic compounds and spectroscopic analysis of a new lignan from Trachelospermum asiaticum var. intermedium. Chem. Pharm. Bull. 1981, 29, 2078–2082. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Lignans from Trachelospermum asiaticum (Tracheolospermum. II). Chem. Pharm. Bull. 1986, 34, 4340–4345. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Tanegosides A, B and C, lignan glycosides from Trachelospermum liukiuense. Chem. Pharm. Bull. 1990, 38, 2143–2145. [Google Scholar] [CrossRef]
- Nishibe, S.; Fujimoto, T.; Nose, M.; Takeda, T.; Ogihara, Y.; Xu, Y. Lignans from Trachelospermum axillare. Phytochemistry 1993, 32, 1579–1581. [Google Scholar] [CrossRef]
- Tan, X.Q.; Chen, H.S.; Liu, R.H.; Tan, C.H.; Xu, C.L.; Xuan, W.D.; Zhang, W.D. Lignans from Trachelospermum jasminoides. Planta Med. 2005, 71, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Kousar, F.; Noomrio, M.H.; Talpur, M.M.A.; Ahmad, V.U.; Arfan, M. Two new lignan glycosides from Trachelospermum lucidum (D. Don) Schum. J. Asian Nat. Prod. Res. 2008, 10, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Yu, N.J.; Li, Y.S.; Fu, L.; Zhao, Y.M. Novel lignans from the stems and leaves of Trachelospermum jasminoides. Chin. Chem. Lett. 2011, 22, 1075–1077. [Google Scholar] [CrossRef]
- Rao, M.A.; Rao, E.V. Crystalline components from the leaves and twigs of Trachelospermum fragrans. Planta Med. 1977, 32, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Yamauchi, T. Teikaside A, a pregnane glycoside of Trachelospermum asiaticum. Chem. Pharm. Bull. 1981, 29, 416–420. [Google Scholar] [CrossRef]
- Lin, Y.L.; Liu, K.C. Studies on the constituents of Trachelosperum jasminoides (Lindi.) Lem. Kuo Li Chung-Kuo I Yao Yen Chiu So Yen Chiu Pao Kao 1981, 7, 80–85. [Google Scholar]
- Abe, F.; Yamauchi, T. Trachelospermum. IV. Glycosides of 19α-hydroxyoleanane-type triterpenoids from Trachelospermum asiaticum. Chem. Pharm. Bull. 1987, 35, 1833–1838. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Trachelospermum. III. Trachelosperosides, glycosides of 19α-hydroxyursane-type triterpenoids from Trachelospermum asiaticum. Chem. Pharm. Bull. 1987, 35, 1748–1754. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Studies on Trachelospermum. Part VI. Pregnane glycosides of teikasides B and C series, from Trachelospermum asiaticum. Chem. Pharm. Bull. 1988, 36, 4330–4336. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Studies on Trachelospermum. Part V. Pregnane glycosides from Trachelospermum asiaticum. Chem. Pharm. Bull. 1988, 36, 621–626. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Studies on Trachelospermum. Part VII. Pregnane and pregnane glycosides from Trachelospermum liukiuense. Chem. Pharm. Bull. 1989, 37, 33–35. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Kousar, F.; Khan, A.; Zubair, M.; Iqbal, S.; Farooq, U.; Rasool, N.; Arfan, N.; Nawaz, S.A.; Choudhary, M.I. Isolation of a new lipoxygenase active saponin and a new triterpenoid from the leaves of Trachelospermum lucidum. Z. Naturforsch. B 2005, 60, 1287–1290. [Google Scholar]
- Tan, X.; Chen, H.; Zhou, H.; Zhang, Y. Triterpenoids from canes with leaves of Trachelospermum jasminoides. Zhongcaoyao. 2006, 37, 171–174. [Google Scholar]
- Zhang, J.; Yin, Z.Q.; Liang, J.Y.; Diao, Y.J. Chemical constituents from petroleum ether extract of Trachelospermum jasminoids. Chin. Tradit. Pat. Med. 2012, 34, 1939–1942. [Google Scholar]
- Sakushima, A.; Hisada, S.; Nishibe, S.; Inagaki, I. Constituents of the leaves of Trachelospermum asiaticum var intermedium. II. Isolation and structure of a new flavone glycoside. Yakugaku Zasshi 1973, 93, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Sakushima, A.; Hisada, S.; Agata, I.; Nishibe, S. Studies on the constituents of Apocynaceae plants. Flavonoids from Trachelospermum jasminoides var pubescens and T. difforme. Shoyakugaku Zasshi 1982, 36, 82–87. [Google Scholar]
- Nishibe, S.; Sakushima, A.; Noro, T.; Fukushima, T. Studies on the Chinese drug Luoshiteng (I). Xanthine oxidase inhibitors from the leaf part of Luoshiteng originating from Trachelospermum jasminoides. Shoyakugaku Zasshi 1987, 41, 116–120. [Google Scholar]
- Sakushima, A.; Nishibe, S. Taxifolin 3-arabinoside from Trachelospermum jasminoides var. pubescens. Phytochemistry 1988, 27, 948–950. [Google Scholar] [CrossRef]
- Lin, S.J.; Yang, T.H.; Hsu, C.T. Some apigenin and luteolin glycosides from Trachelospermum gracilipes. Zhonghua Yaoxue Zazhi. 1992, 44, 395–405. [Google Scholar]
- Nishibe, S.; Noguchi, Y.; Yingmei, H. Flavonoids from leaves of Trachelospermum axillare (Natural Medicine Note). Shoyakugaku Zasshi 2000, 54, 272. [Google Scholar]
- Hosoi, S.; Shimizu, E.; Ohno, K.; Yokosawa, R.; Kuninaga, S.; Coskun, M.; Sakushima, A. Structural studies of zoospore attractants from Trachelospermum jasminoides var. pubescens: Taxifolin 3-O-glycosides. Phytochem. Anal. 2006, 17, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhao, Y.M.; Wang, J.H.; Yu, N.J. Study on the flavonoid constituents of the stem and leaves of Trachelospermum jasminoides. Pharm. J. Chin. People’s Lib. Army 2008, 24, 299–301. [Google Scholar]
- Hosoi, S.; Shimizu, E.; Tanaka, T.; Sakai, E.; Yamada, M.; Sakushima, A. Main phenolic compounds from the flower of Trachelospermum asiaticum var. intermedium (Apocynaceae). J. Nat. Med. 2008, 62, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Guo, L.; Chen, H.; Wu, L.; Kong, F. Study on the flavonoids constituents of Trachelospermum jasminoides. J. Chin. Med. Mater. 2010, 33, 58–60. [Google Scholar]
- Zhang, J.; Yin, Z.Q.; Liang, J.Y. A new isoflavonoid glycoside from the aerial parts of Trachelospermum jasminoides. Chin. J. Nat. Med. 2013, 11, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Attaur, R.; Fatima, T.; Mehrun, N.; Ijaz, N.; Crank, G.; Wasti, S. Indole alkaloids from Trachelospermum jasminoides. Planta Med. 1987, 53, 57–59. [Google Scholar]
- Attaur, R.; Fatima, T.; Crank, G.; Wasti, S. Alkaloids from Trachelospermum jasminoides. Planta Med. 1988, 54, 364. [Google Scholar]
- Nishibe, S.; Hisada, S.; Inagaki, I. Isolation of 5-hydroxymethylfurfural from Trachelospermum asiaticum var intermedium. Chem. Pharm. Bull. 1973, 21, 1155–1157. [Google Scholar] [CrossRef]
- Nishibe, S.; Hisada, S.; Inagaki, I. Cyclitol of several Trachelospermum species. Yakugaku Zasshi 1973, 93, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Q.; Guo, L.J.; Qiu, Y.H.; Chen, H.S.; Tan, C.H. Chemical constituents of Trachelospermum jasminoides. Nat. Prod. Res. 2010, 24, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Miyazawa, M. Potent α-glucosidase inhibitors from Safflower (Carthamus tinctorius L.) seed. Phytother. Res. 2012, 26, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Cunico, M.M.; Lopes, A.R.; Cocco, L.C.; Yamamoto, C.I.; Plocharski, R.C.B.; Miguel, M.D.; Junior, A.G.; Auer, C.G.; Miguel, O.G. Phytochemical and antibacterial evaluation of essential oils from Ottonia martiana Miq. (Piperaceae). J. Braz. Chem. Soc. 2007, 18, 184–188. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part I; Chemical Industry Press: Beijing, China, 2015; pp. 269–270. ISBN 9787506773379.
- Li, R.W.; Lin, G.D.; Myers, S.P.; Leach, D.N. Anti-inflammatory activity of Chinese medicinal vine plants. J. Ethnopharmacol. 2003, 85, 61–67. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, J.M.; Jun, S.H.; Ha, C.G.; Lee, S.H.; Kim, N.W.; Lee, J.H.; Ko, N.Y.; Mun, S.H.; Park, S.H.; et al. In vitro and in vivo anti-inflammatory action of the ethanol extract of Trachelospermi caulis. J. Pharm. Pharmacol. 2007, 59, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.N.; Choi, Y.H.; Lee, J.M.; Noh, I.C.; Park, J.W.; Choi, W.S.; Choi, J.H. Anti-inflammatory effects of beta-sitosterol-beta-d-glucoside from Trachelospermum jasminoides (Apocynaceae) in lipopolysaccharide-stimulated RAW 264.7 murine macrophages. Nat. Prod. Res. 2012, 26, 2340–2343. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, A.R.; Lee, J.M.; Kim, S.N.; Choi, J.H.; Kim, D.K.; Kim, J.H.; Kim, B.; Her, E.; Yang, Y.M.; et al. A mixture of Trachelospermi caulis and Moutan cortex radicis extracts suppresses collagen-induced arthritis in mice by inhibiting NF-kappaB and AP-1. J. Pharm. Pharmacol. 2012, 64, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R.; Dong, C.; Jiao, L.; Xu, L.; Liu, J.; Wang, Z.; Ying, Q.L.M.; Fong, H.; Lao, L. Topical treatment with Tong-Luo-San-Jie gel alleviates bone cancer pain in rats. J. Ethnopharmacol. 2012, 143, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R.; Dong, C.; Jiao, L.; Xu, L.; Liu, J.; Wang, Z.; Lao, L. Transient receptor potential channel and interleukin-17A involvement in LTTL gel inhibition of bone cancer pain in a rat model. Integr. Cancer Ther. 2015, 14, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Wang, Z.X.; Yang, Y.; Wang, L.; Sun, R.F.; Zhao, Y.M.; Yu, N.J. Active components with inhibitory activities on IFN-γ/STAT1 and IL-6/STAT3 signaling pathways from Caulis Trachelospermi. Molecules 2014, 19, 11560–11571. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Kato, M.; Dibwe, D.F.; Li, F.; Miyoshi, C.; Esumi, H.; Kadota, S.; Tezuka, Y. Antiausterity activity of arctigenin enantiomers: importance of (2R,3R)-absolute configuration. Nat. Prod. Commun. 2014, 9, 79–82. [Google Scholar] [PubMed]
- Yu, Y.B.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Park, J.C. Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Arch. Pharm. Res. 2007, 30, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Ishitsuka, K.; Kamikawa, M.; Matsuda, M.; Tsuchihashi, R.; Okawa, M.; Okabe, H.; Tamura, K.; Kinjo, J. Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (III). J. Nat. Med. 2013, 67, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.J.; Jin, Y.S.; Chen, H.S.; Xu, H.S.; Ren, H.; Zhu, S.Y.; Tang, H.L.; Wang, Y.; Zhao, P.; Qi, Z.T. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J. Gen. Virol. 2016, 97, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Bae, M.J.; Jung, S.Y.; See, H.J.; Kim, Y.T.; Do, J.R.; Back, S.Y.; Choi, S.W.; Shon, D.H. Enhancing Effect of Trachelogenin from Trachelospermi caulis Extract on Intestinal Barrier Function. Biol. Pharm. Bull. 2015, 38, 1707–1713. [Google Scholar] [CrossRef] [PubMed]

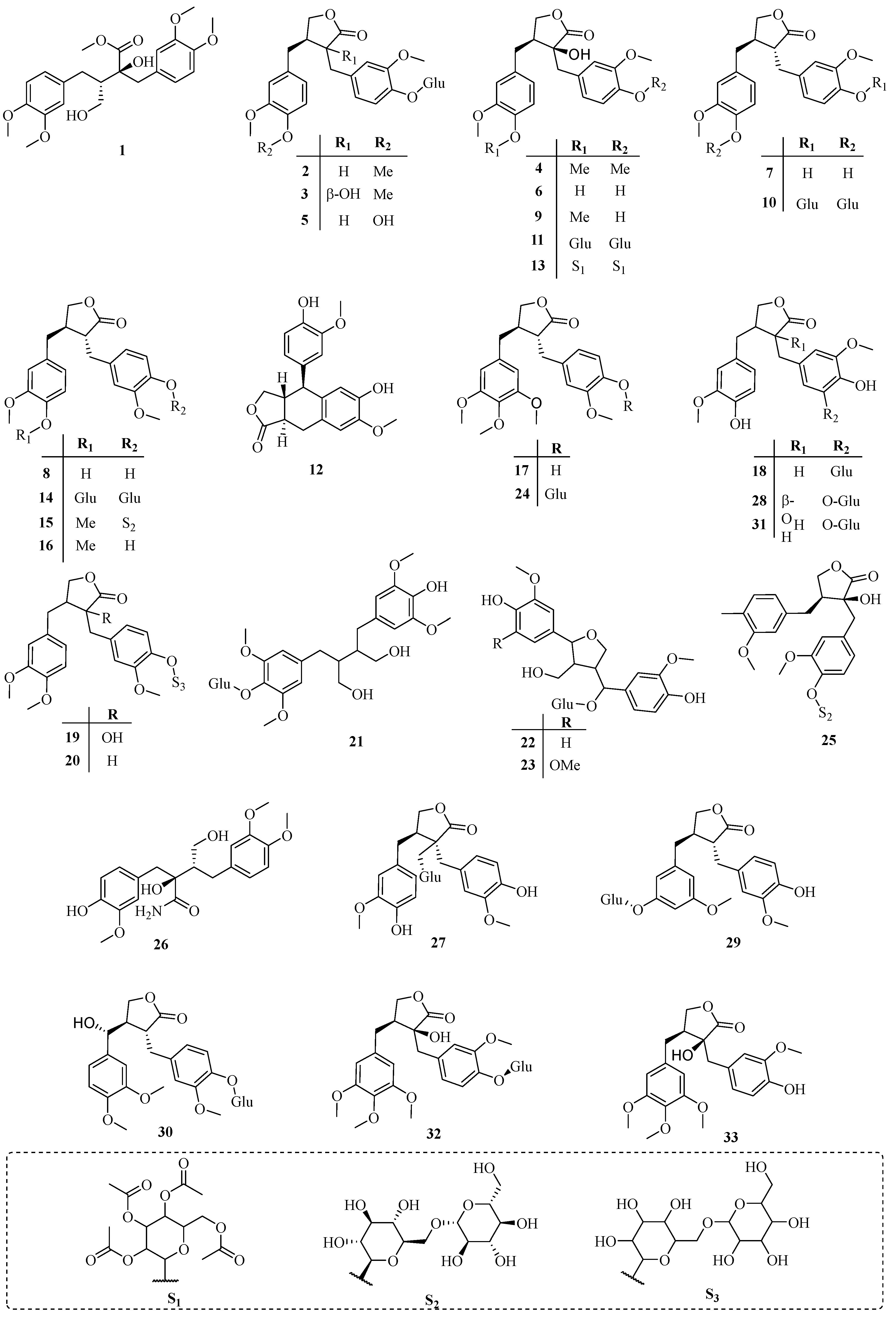


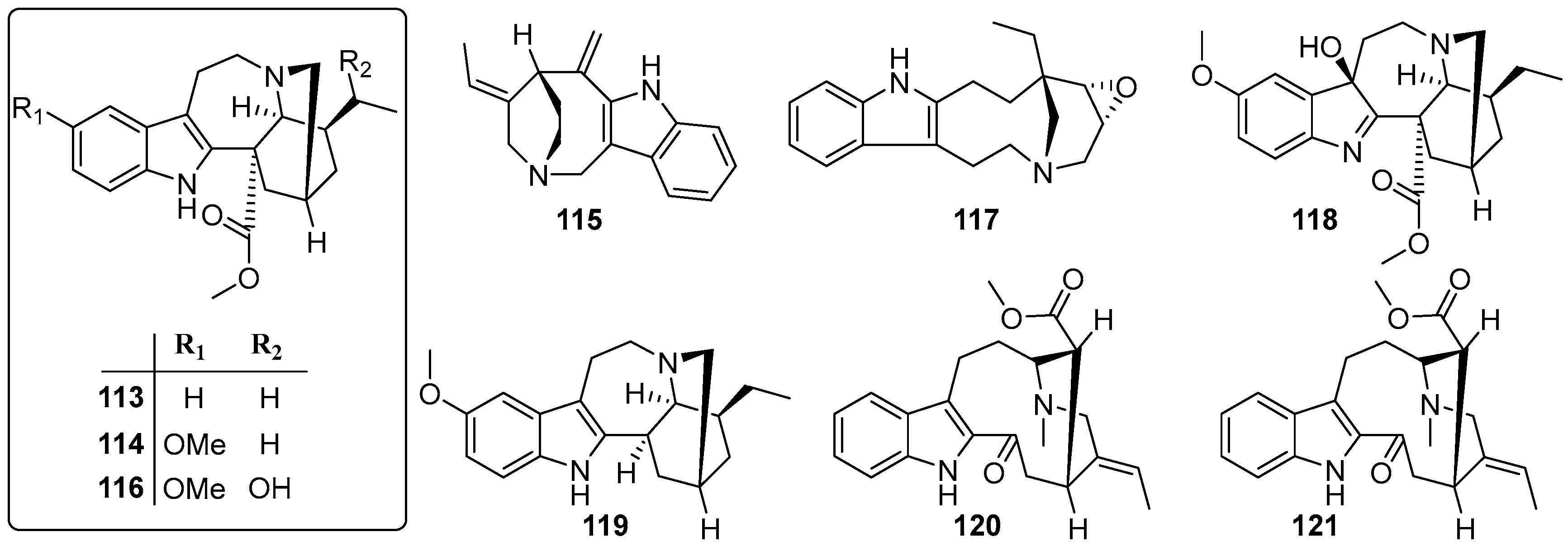
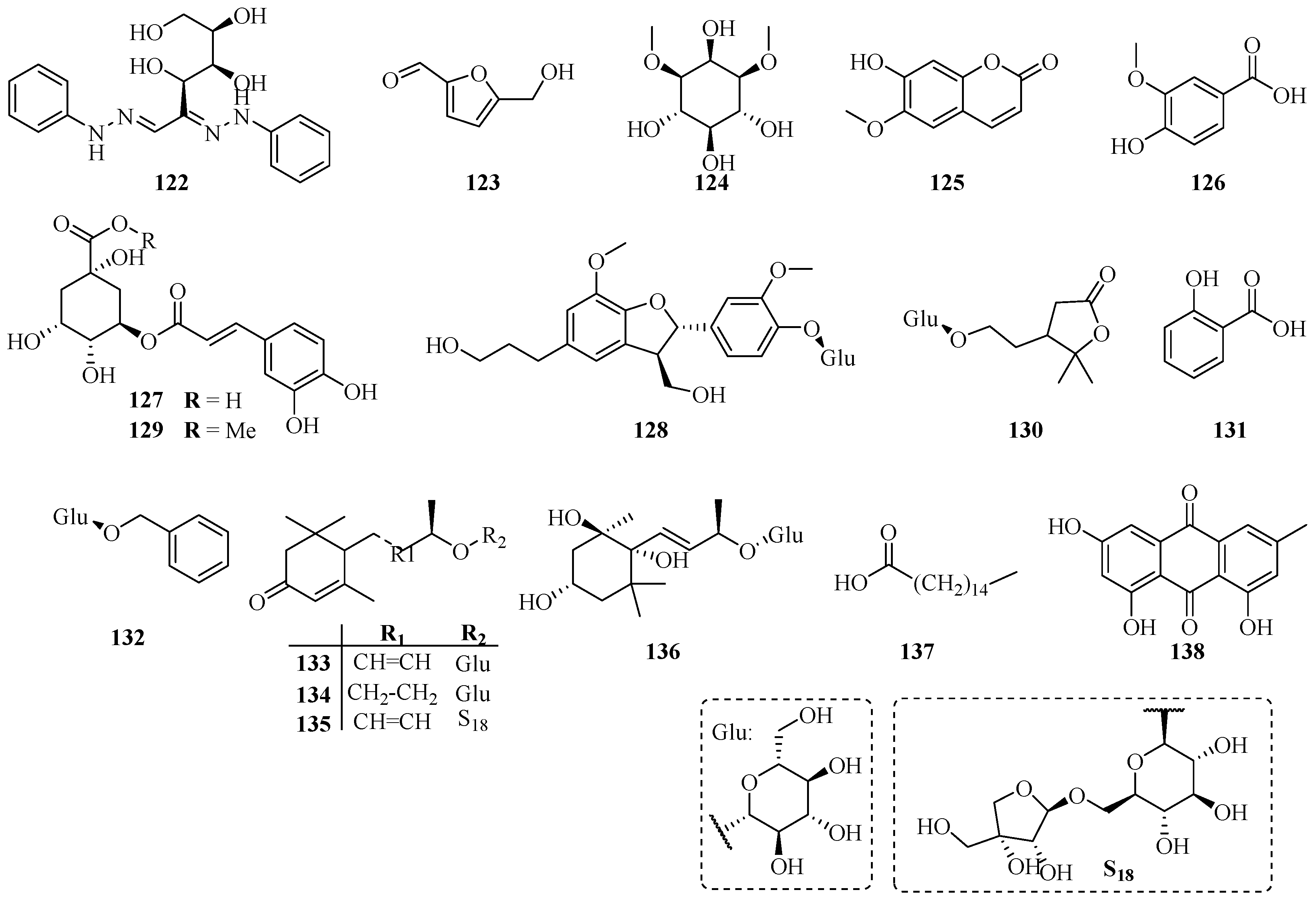

| No. | Name | CAS | Source | Ref. |
|---|---|---|---|---|
| Lignans | ||||
| 1 | Methyl methyltrachelogenate | 42320-76-3 | A | [6] |
| 2 | Arctiin | 20362-31-6 | A | [7] |
| 3 | Tracheloside | 33464-71-0 | A | [7] |
| 4 | Methyltrachelogenin | 33464-73-2 | A | [7] |
| 5 | Matairesinoside | 23202-85-9 | A | [2] |
| 6 | Nortrachelogenin | 34444-37-6 | A | [8] |
| 7 | Matairesinol | 580-72-3 | A | [8] |
| 8 | Maculatin | 25488-59-9 | A | [8] |
| 9 | Trachelogenin | 34209-69-3 | A | [8] |
| 10 | Matairesinol 4,4′-di-O-β-d-glucopyranoside | 38976-08-8 | A | [9] |
| 11 | Nortrachelogenin 4,4′-di-O-β-d-glucopyranoside | 38976-09-9 | A | [9] |
| 12 | conidendrin | 518-55-8 | A | [10] |
| 13 | Nortrachelogenin-4,4′-di-O-β-d-glucopyranoside octaacetate | 43179-85-7 | A | [10] |
| 14 | 2(3H)-Furanone,3,4-bis[[4-(β-d-glucopyranosyloxy)-3-methoxyphenyl]methyl]dihydro-, (3R-trans)- | 41948-08-7 | A | [11] |
| 15 | Arctigenin 4′-O-β-gentiobioside | 41682-24-0 | A | [12] |
| 16 | Arctigenin | 7770-78-7 | A | [13] |
| 17 | Traxillagenin | 79288-73-6 | A | [14] |
| 18 | Trachelosiaside | 106647-12-5 | A | [15] |
| 19 | Trachelogenin 4′-O-β-gentiobioside | 106647-13-6 | A | [15] |
| 20 | 2(3H)-Furanone,3-[[4-[(6-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-3-methoxyphenyl]methyl]dihydro-4-[(4-hydroxy-3-methoxy-phenyl)methyl]-, (3R-trans)- | 106647-14-7 | A | [15] |
| 21 | β-d-Glucopyranoside, 4-[4-(4-hydroxy-3,5-dimethoxyphenyl)-2,3-bis(hydroxymethyl)butyl]-2,6-dimethoxyphenyl | 106647-15-8 | A | [15] |
| 22 | Tanegoside A | 131653-21-9 | C | [16] |
| 23 | Tanegoside C | 131653-22-0 | C | [16] |
| 24 | Traxillaside | 149415-62-3 | D | [17] |
| 25 | 2(3H)-Furanone,3-[[4-[(6-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-3-methoxyphenyl]methyl]dihydro-3-hydroxy-4-[(3-methoxy-4-methylphenyl)methyl]-, (3S,4S)- | 858127-40-9 | B | [18] |
| 26 | Benzenebutanamide,α-hydroxy-α-[(4-hydroxy-3-methoxyphenyl)methyl]-β-(hydroxymethyl)-3,4-dimethoxy-, (αS,βS)- | 132472-34-5 | B | [18] |
| 27 | (3S,4S)-3-(β-d-Glucopyranosyloxy)dihydro-3,4-bis[(4-hydroxy-3-methoxyphenyl)methyl]-2(3H)-furanone | 858127-38-5 | B | [18] |
| 28 | (3S,4S)-3-[(3-β-d-Glucopyranosyl-4-hydroxy-5-methoxyphenyl)methyl]dihydro-3-hydroxy-4-[(4-hydroxy-3-methoxyphenyl)methyl]-2(3H)-furanone | 858127-39-6 | B | [18] |
| 29 | 2(3H)-Furanone,4-[[3-(β-d-glucopyranosyloxy)-5-methoxyphenyl]methyl]dihydro-3-[(3-hydroxy-4-methoxyphenyl)methyl]-, (3R,4R)- | 1069136-59-9 | C | [19] |
| 30 | 2(3H)-Furanone,4-[(R)-(3,4-dimethoxyphenyl)hydroxymethyl]-3-[[4-(β-d-glucopyranosyloxy)-3-methoxyphenyl]methyl]dihydro-, (3R,4R)- | 1069136-60-2 | C | [19] |
| 31 | 2(3H)-Furanone,3-[[3-(β-d-glucopyranosyloxy)-4-hydroxy-5-methoxyphenyl]methyl]dihydro-4-[(3-hydroxy-4-methoxyphenyl)methyl]-, (3R,4R)- | 1069136-62-4 | C | [19] |
| 32 | 2(3H)-Furanone,3-[[4-(β-d-glucopyranosyloxy)-3-methoxyphenyl]methyl]dihydro-3-hydroxy-4-[(3,4,5-trimethoxyphenyl)methyl]-, (3S,4S)- | 1321810-65-4 | B | [20] |
| 33 | (3S,4S)-Dihydro-3-hydroxy-3-[(4-hydroxy-3-methoxyphenyl)methyl]-4-[(3,4,5-trimethoxyphenyl)methyl]-2(3H)-furanone | 1321810-66-5 | B | [20] |
| Triterpenoids | ||||
| 34 | β-Sitosterol | 83-46-5 | A | [2] |
| 35 | Eleutheroside A | 474-58-8 | A | [2] |
| 36 | β-Amyrin | 559-70-6 | A | [2] |
| 37 | β-Amyrin acetate | 1616-93-9 | A | [2] |
| 38 | Ursolic acid | 77-52-1 | F | [21] |
| 39 | Teikaside A | 77369-82-5 | A | [22] |
| 40 | Stigmasterol | 83-48-7 | B | [23] |
| 41 | Campesterol | 474-62-4 | B | [23] |
| 42 | Fagarasterol | 545-47-1 | B | [23] |
| 43 | Lupenyl acetate | 1617-68-1 | B | [23] |
| 44 | Arjunglucoside I | 62319-70-4 | A | [24] |
| 45 | 3-O-β-d-Glucopyranosyl-2α,3β,19α,23-tetrahydroxyolean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester | 82843-99-0 | A | [24] |
| 46 | Trachelosperoside F 2 | 109742-49-6 | A | [24] |
| 47 | Trachelosperoside E 1 | 109742-50-9 | A | [24] |
| 48 | Trachelosperoside D 1 | 109742-52-1 | A | [24] |
| 49 | Trachelosperoside D 2 | 109742-51-0 | A | [24] |
| 50 | Arjungenin 23,28-bis-O-glucopyranoside | 109792-80-5 | A | [24] |
| 51 | Olean-12-en-28-oic acid, 2,3,19,23-tetrahydroxy-,2-O-β-d-xylopyranosyl-β-d-glucopyranosyl ester, (2α,3β,4α,19α)- | 109792-81-6 | A | [24] |
| 52 | Suavissimoside F 1 | 95645-51-5 | A | [25] |
| 53 | Urs-12-ene-23,28-dioic acid,3-(β-d-glucopyranosyloxy)-2,19-dihydroxy-, 28-β-d-glucopyranosyl ester, (2α,3β,4α)- | 109825-38-9 | A | [25] |
| 54 | 3-O-β-d-Glucopyranosyl-2α,3β,19α,23-tetrahydroxyurs-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester | 82843-98-9 | A | [25] |
| 55 | Trachelosperoside A 1 | 109750-36-9 | A | [25] |
| 56 | Trachelosperoside B 1 | 109742-56-5 | A | [25] |
| 57 | Trachelosperoside B 2 | 109742-55-4 | A | [25] |
| 58 | Trachelosperoside C 1 | 109742-54-3 | A | [25] |
| 59 | Trachelosperoside C 2 | 109744-39-0 | A | [25] |
| 60 | Teikaside C 0 | 120727-46-0 | A | [23] |
| 61 | Teikaside C IIa | 120727-47-1 | A | [26] |
| 62 | Teikaside C IIIa | 120768-72-1 | A | [26] |
| 63 | Teikaside C IVa | 120727-49-3 | A | [26] |
| 64 | Teikaside C IIc | 120727-48-2 | A | [26] |
| 65 | Teikaside A-Ia | 114892-50-1 | A | [27] |
| 66 | Teikaside A-Ib | 114892-51-2 | A | [27] |
| 67 | Teikaside A-IIa | 114912-34-4 | A | [27] |
| 68 | Teikaside A-IIc | 114892-53-4 | A | [27] |
| 69 | Teikaside A-IIIb | 114892-54-5 | A | [27] |
| 70 | Deoxycortone | 64-85-7 | C | [28] |
| 71 | β-d-Galactopyranoside, (3β,5α,20S)-17,20-dihydroxypregn-6-en-3-yl 6-deoxy-3-O-methyl- | 77369-84-7 | C | [28] |
| 72 | Leucioside | 898801-61-1 | E | [29] |
| 73 | Olean-11-en-3-ol, acetate, (3β)- | 898798-42-0 | E | [29] |
| 74 | Quinovic acid 3-O-β-d-glucoside | 79955-41-2 | B | [30] |
| 75 | Trachelosperogenin B | 109742-53-2 | B | [30] |
| 76 | Quinovic acid 3-O-β-d-glucopyranoside 27-O-β-d-glucopyranosyl ester | 117751-62-9 | B | [30] |
| 77 | Cincholic acid 3-O-β-d-glucopyranoside 27-O-β-d-glucopyranosyl ester | 1004519-37-2 | B | [30] |
| 78 | Trachelosperoside F | 1004519-89-4 | B | [30] |
| 79 | Cycloeucalenol | 469-39-6 | B | [31] |
| 80 | α-Amyrin | 638-95-9 | B | [31] |
| 81 | α-Amyrenyl acetate | 863-76-3 | B | [31] |
| 82 | β-Sitostenone | 1058-61-3 | B | [31] |
| Flavonoids | ||||
| 83 | Luteolin | 491-70-3 | A | [13] |
| 84 | Apigenin | 520-36-5 | A | [13] |
| 85 | Apigenin 7-glucoside | 578-74-5 | A | [13] |
| 86 | Luteolin 7-O-glucopyranoside | 5373-11-5 | A | [13] |
| 87 | Luteolin 4′-O-β-d-glucopyranoside | 6920-38-3 | A | [13] |
| 88 | Rhoifolin | 17306-46-6 | A | [32] |
| 89 | Luteolin 7-β-neohesperidoside | 25694-72-8 | A | [32] |
| 90 | Apigenin 7-O-β-gentiobioside | 50826-94-3 | A | [32] |
| 91 | Quercetin | 117-39-5 | F | [21] |
| 92 | Quercetin 3-β-galactoside | 482-36-0 | F | [21] |
| 93 | Quercimelin | 522-12-3 | F | [21] |
| 94 | Taxifolin 3-O-rhamnoside | 29838-67-3 | F | [21] |
| 95 | Eldrin | 153-18-4 | G | [33] |
| 96 | Quercetin 3-O-β-d-glucofuranoside | 21637-25-2 | G | [33] |
| 97 | Vicenin | 23666-13-9 | B | [33] |
| 98 | Luteolin 7-O-β-gentiobioside | 70855-41-3 | B | [33] |
| 99 | Kaempferol | 520-18-3 | B | [34] |
| 100 | Taxifolin | 480-18-2 | B | [35] |
| 101 | Taxifolin 3-O-β-d-glucopyranoside | 27297-45-6 | B | [35] |
| 102 | Quercetin O-arabinoside | 30370-87-7 | B | [35] |
| 103 | Apigenin-7-O-β-d-rutinoside | 552-57-8 | H | [36] |
| 104 | Quercetin 3-O-β-d-glucoside | 482-35-9 | D | [37] |
| 105 | Taxifolin 3-O-β-d-arabinopyranoside | 209005-26-5 | B | [38] |
| 106 | 4H-1-Benzopyran-4-one,3-(β-d-arabinopyranosyloxy)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-5,7-dihydroxy-, (2S,3S)- | 901123-12-4 | B | [38] |
| 107 | Chrysoeriol | 491-71-4 | B | [39] |
| 108 | Daidzin | 552-66-9 | B | [39] |
| 109 | Afzelin | 482-39-3 | A | [40] |
| 110 | Naringin | 10236-47-2 | B | [41] |
| 111 | 5-(β-d-Glucopyranosyloxy)-3-[4-(β-d-glucopyranosyloxy)-3-methoxyphenyl]-7-methoxy-4H-1-benzopyran-4-one | 1620385-26-3 | B | [42] |
| 112 | Luteolin-4′-O-rutinoside | 150460-69-8 | B | [42] |
| Alkaloids | ||||
| 113 | Coronaridine | 467-77-6 | B | [43] |
| 114 | Voacangine | 510-22-5 | B | [43] |
| 115 | Apparicine | 3463-93-2 | B | [43] |
| 116 | 19-epi-Voacangarine | 6883-77-8 | B | [43] |
| 117 | Conoflorine | 15266-46-3 | B | [43] |
| 118 | Voacangine-7-hydroxyindolenine | 3464-63-9 | B | [44] |
| 119 | Ibogaine | 83-74-9 | B | [44] |
| 120 | Vobasine | 2134-83-0 | B | [44] |
| 121 | Tabernaemontanine | 2134-98-7 | B | [44] |
| Others | ||||
| 122 | Glucosazone | 4746-10-5 | A | [6] |
| 123 | 5-Hydroxymethylfuraldehyde | 67-47-0 | A | [45] |
| 124 | Dambonitol | 523-94-4 | A | [46] |
| 125 | Scopoletine | 92-61-5 | A | [14] |
| 126 | Vanillic acid | 121-34-6 | A | [14] |
| 127 | Chlorogenic acid | 327-97-9 | B | [35] |
| 128 | Dihydrodehydrodiconiferyl alcohol 4-O-β-d-glucopyranoside | 131723-83-6 | C | [16] |
| 129 | Methyl chlorogenate | 29708-87-0 | A | [40] |
| 130 | Trachelinoside | 1251939-00-0 | B | [47] |
| 131 | Salicylic acid | 69-72-7 | B | [47] |
| 132 | Benzyl glucopyranoside | 4304-12-5 | B | [47] |
| 133 | Roseoside A | 54835-70-0 | B | [47] |
| 134 | Icariside B5 | 114226-08-3 | B | [47] |
| 135 | 2-Cyclohexen-1-one,4-[(1E,3R)-3-[(6-O-d-apio-β-d-furanosyl-β-d-glucopyranosyl)oxy]-1-butenyl]-3,5,5-trimethyl-, (4R)- | 143363-62-6 | B | [47] |
| 136 | Actinidioionoside | 540528-05-0 | B | [47] |
| 137 | Palmitic acid | 57-10-3 | B | [31] |
| 138 | Emodin | 518-82-1 | B | [31] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; He, X.; Zhao, Y.; Sun, Y.; Chen, X.; Cun, Y.; Huang, L.; Bai, Y.; Zheng, X. Phytochemistry, Pharmacology and Traditional Uses of Plants from the Genus Trachelospermum L. Molecules 2017, 22, 1406. https://doi.org/10.3390/molecules22091406
Zhao Z, He X, Zhao Y, Sun Y, Chen X, Cun Y, Huang L, Bai Y, Zheng X. Phytochemistry, Pharmacology and Traditional Uses of Plants from the Genus Trachelospermum L. Molecules. 2017; 22(9):1406. https://doi.org/10.3390/molecules22091406
Chicago/Turabian StyleZhao, Zefeng, Xirui He, Yuhui Zhao, Ying Sun, Xufei Chen, Ye Cun, Linhong Huang, Yajun Bai, and Xiaohui Zheng. 2017. "Phytochemistry, Pharmacology and Traditional Uses of Plants from the Genus Trachelospermum L." Molecules 22, no. 9: 1406. https://doi.org/10.3390/molecules22091406
APA StyleZhao, Z., He, X., Zhao, Y., Sun, Y., Chen, X., Cun, Y., Huang, L., Bai, Y., & Zheng, X. (2017). Phytochemistry, Pharmacology and Traditional Uses of Plants from the Genus Trachelospermum L. Molecules, 22(9), 1406. https://doi.org/10.3390/molecules22091406