Phenolic compounds are metabolites possessing different structure and function, and they possess an aromatic ring generally containing one or more hydroxyl groups [
48]. Based on their chemical structure, they are a highly diverse group ranging from simple molecules such as phenolic acids to complex polymers such as tannins and lignin [
43]. The common bean contains a great amount of polyphenols. These are bioactive compounds widely known because of their antioxidant properties, therefore they have a very important role for decreasing the risk of cardiovascular diseases, diabetes, some types of cancer, Alzheimer’s and Parkinson’s diseases. The antioxidant properties of these compounds lies on their ability to neutralize free radicals and the chelation of transition metals, thus they counteract the initiation and propagation of oxidative processes [
49].
González de Mejía et al. [
51], observed that the major amounts of polyphenols compounds were located in the seed coat of the “Flor de Mayo” variety of the Mexican common bean and it represents 11% of the total seed. These authors reported that the obtained methanolic extract displayed anti-mutagenic activity against 1-nitropyrene and benzopyrene. A phenolic compound content of 145 mg/g was measured by Cardador-Martínez et al. [
9] in a methanolic extract obtained from the bean seed coat of the “Flor de Mayo FM-38” variety and they also found an anti-mutagenic activity against aflatoxin B1. Furthermore, Espinosa-Alonso et al. [
14] studied 62 Mexican lines of the common wild bean and they reported that total phenolic compound content was 0.90–2.11 mg gallic acid equivalent (GAE)/g of bean flour. In this study, the Mexican “Negro Jamapa” and “Frijol Pinto” varieties were also analyzed. The overall phenol concentrations were 1.41 and 1.98 mg GAE/g bean flour, respectively. The results measured on the Mexican bean lines were similar to those observed for the Vaccinium wild berries, one of the most important polyphenol source in fruits (0.81–1.70 mg GAE/g). In yet another study, Almanza–Aguilera et al. [
12] analyzed 16 genotypes of the black Mexican bean and reported a total phenol range of 503.2–1062 mg/100 g of raw beans and 210–711 mg/100 g of cooked beans. They demonstrated a high content of phenolic compounds on the raw seed that were invariably reduced after the cooking (50%) and frying (64%) processes, although this decrease was different among genotypes. In some cultivars, such as that of “Negro Guanajuato” the decrease induced by cooking was minimal, whereas others such as “Negro Otomí” and “Negro San Luis” contained less phenolic compounds after being fried. However, other varieties as “Negro 8025” exhibited a higher concentration of phenolic compounds after the latter treatment. Boateng et al. [
52] also reported a higher phenolic content of beans possessing a darker seed coatin comparison with those characterized by light colors. These results were confirmed by the study conducted by Orak et al. [
53], in which the phenolic content of 10 varieties of the white-colored bean from Turkey was assessed resulting in decreased levels in comparison with those characterized by red and black colors.
Other factors affecting the phenolic compound content of the common bean are the storage conditions for the seeds. Mujica et al. [
54] reported a significant decrease of both phenolic compounds content and antioxidant activity on Mexican common bean samples stored at 37 °C and 75% RH for 120 days when compared to those stored at 5 °C and 34% RH for 120 d. Herrera et al. [
55] identified a significant effect of location, genotype and humidity treatment on the overall soluble phenolic compounds level in eight bean genotypes from the Jalisco race (most of them pink-colored mottled with cream-colored dots matching the “Flor de Mayo Anita”, “Flor de Mayo Noura”, “Flor de Junio Marcela” and “Flor de Junio Bajío” varieties), the Durango race (light cream-colored seeds with brown- or beige-colored dots of the “Pinto Zapata” and “Pinto Saltillo” varieties) and the yellow-colored Nueva Granada race (“Azufrado 26” and “Azufrado Noroeste”). They were grown in two contrasting regions (Celaya, Guanajuato and Ahome, Sinaloa, both in Mexico). The authors mention that those varieties from the Jalisco race produced with irrigation and terminal hydric stress exhibited higher total soluble phenols when compared to the Durango and Nueva Granada races.
4.2.1. Flavonoids
Flavonoids contained on the common bean are phenolic compounds that have been reported to act as inhibitors of tumor growth and some cancer types. These, along with phenolic acids and tannins, confer to this food a superior antioxidant capacity [
9,
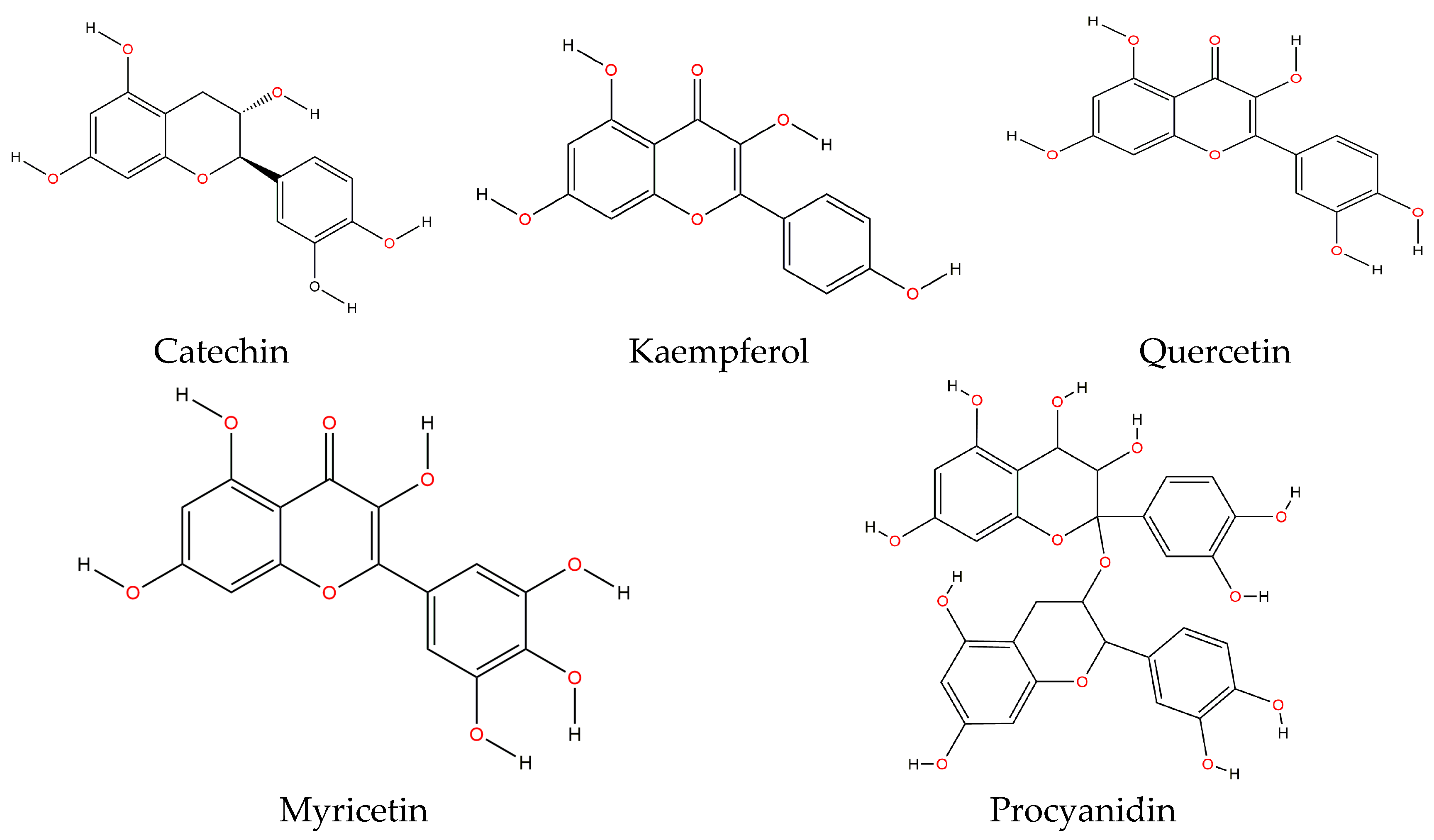
49]. Flavonoids share a common structure consisting of two aromatic rings that are linked through three carbons, forming an oxygenated heterocycle. These are classified in six subclasses, depending on their heterocycle: flavonols, flavones, isoflavones, flavanones, anthocyanidins and flavanols (catechin and proanthocyanidin or condensed tannins). The main flavonoids contained in both raw and cooked bean are catechin, kaempferol, quercetin, myricetin and procyanidin [
49] (
Figure 2).
Flavonoids biological activity depends on the type of phytochemical constituents and the complexity of their structure and the composition of the flavonoids mixture, since it has been well established that phytochemical mixtures on fruits and legumes may provide protecting benefits to health, mainly through a synergic effect between them [
56].
The consumption of these flavonoids has been inversely correlated with lung cancer and the risk of cardiovascular diseases (CVD). The putative mechanism of action involves the modulation of detoxifying enzymes and the inhibition of cell proliferation, although its most recognized effect is their antioxidant capacity [
57]. It has been also found that flavonoids prevent platelet aggregation and induce muscle relaxation and, along with proteoglycans, they display an inhibitory effect of allergy symptoms. Additionally, flavonoids such as procyanidin B1 and resveratrol may enhance brain capacity and longevity [
22]. Conversely, quercetin has showed a wide range of biologic activities, including anti-carcinogenic, anti-inflammatory, antiviral activities as well as decreased lipid peroxidation, platelet aggregation and capillary permeability. Moreover, this flavonoid displayed anti-inflammatory and immune properties in vitro (cells) and in vivo (animals). However, the studies conducted on humans did not completely support these results. The effect of quercetin as immune reinforcement on humans needs to be studied in detail before implementing a wide application in the future [
58].
A number of epidemiological studies have also shown that diet flavonoids are linked to a low incidence of degenerative diseases such as CVD, type 2 diabetes, dementia and cancer. For example, flavonols showed a protective effect against type 2 diabetes in a cohort study [
59], and anthocyanidins, flavan-3-ols, flavones, and flavonols were individually associated with a decreased mortality rate caused by CVD in the Cancer Prevention Study II Nutrition Cohort [
60]. Nevertheless, a considerable knowledge gap still exists in this field. Other studies have reported inconsistent associations [
61,
62,
63], but the underlying mechanisms have not been fully clarified [
64]. Despite research which has shown that flavonoids have beneficial effects on health, there is no dietary reference intake (DRI) for this compound. In 1998, phenols, polyphenols, and flavonoids were excluded from the DRI panel’s consideration due to lack of food composition data and knowledge of actual intake amounts and limited information on their absorption and metabolism. The DRI committee report concluded that although these components “may be important dietary constituents, insufficient data are available at this time” [
65]. Thus, many more studies are required along with long-term trials in order to establish diet recommendations for these compounds [
65]. One of the main difficulties is the ability to safely estimate a safe consumption for these bioactive compounds. This, along with the elucidation of their main food source, is the first step in order to evidence a correlation between flavonoids and disease [
66]. In this regard, some researchers made an effort to build and to analyze a database reporting the flavonoid content on several foods as well as the consumed amounts by some groups at different locations. For example, Jun et al. [
66] estimated the overall and individual consumption of flavonoids in Korean adults and they identified the main sources of this compound. Thus, they found that the average consumption of total flavonoids per day in this particular group was 318 mg/day and they were comprised by proanthocyanidins (22.3%), flavonols (20.3%), isoflavones (18.1%), flavan-3-ols (16.2%), anthocyanidins (11.6%), flavanones (11.3%) and flavones (0.3%). Moreover, the food groups that contributed the most to this consumption were fruits (54.4%), vegetables (20.5%), legumes and their products (16.2%) as well as beverages and alcohol (3.1%). Furthermore, it was found that the main food contributing flavonoids on the diet were apples (21.9%), tangerines (12.5%), tofu (11.5%), onions (9.6%) and grapes (9.0%). In a similar study, Sebastián et al. [
67] reported that the average daily consumption of total flavonoids by American adults was 251 mg/day; a lower value when compared to that consumed by Koreans, and flavan-3-ol represented the 81% of the intake. They found that the highest consumption of total flavonoids was observed for non-Hispanic white Americans (275 mg/day), followed by non-Hispanic Black (176 mg/day) and finally the Hispanic population (139 mg/day). The main source of these bioactive compounds was tea (80%). Peterson et al. [
68] reported that the highest consumption of total flavonoids per d was in the United Kingdom (1017 mg/day), followed by Australia (775 mg/day) and they pointed out that the imprecision for estimating bioactive compound consumption, such as flavonoids, is challenging. The imprecisions may have originated from the variability of these compounds on food caused by the different culture and processing conditions, the quantification methods in the laboratory, incomplete food composition charts as well as the lack of a suitable instrument for diet evaluation.
Other studies were conducted to evaluate the effect of oral supplementation of quercetin in healthy individuals. For example, Egert et al. [
69] investigated the effects of an oral supplementation of quercetin at three different doses on plasma concentrations of quercetin, parameters of oxidant/antioxidant status, inflammation, and metabolism. To this end, 35 healthy volunteers were randomly assigned to take 50, 100, or 150 mg/day (group Q50–Q150) quercetin for two weeks. Fasting blood samples were collected at the beginning and end of the supplementation period. Compared with baseline, quercetin supplementation significantly increased plasma concentrations of quercetin by 178% (Q50), 359% (Q100), and 570% (Q150). These authors reported that daily supplementation of healthy humans with graded concentrations of quercetin for two weeks dose-dependently increased plasma quercetin concentrations but did not affect antioxidant status, oxidized LDL, inflammation, or metabolism. However, to date there is no DRI for quercetin [
65].
Table 3 shows the flavonoid profile for the Mexican black bean of the “Negro San Luis” variety. It exhibited a total flavonoid content of 765.50 mg/100 g of sample. In this variety, the most important flavonoids were quercetin 4-
O-galactoside, myricetin 3-
O-glucoside and kaempferol 3-
O-glucoside [
45]. Quercetin is also in skins of fruits, leafy vegetables, and berries, as well as in black tea, red wine, and various fruit juices [
70]. Hertog et al. [
71] reported quercetin content in onion of 284–486 mg/kg, in broccoli of 30 mg/kg, pear 6.4 mg/kg, and in different apple varieties of 21–72 mg/kg. These values are lower than those reported in raw black bean “Negro San Luis” variety [
45] (
Table 3).
Other studies also showed the presence of quercetin and kaempferol in several Mexican bean varieties, both wild-type and domesticated. In these samples, quercetin was within the following ranges: 6.9–23.5 μg/g of cooked bean and 4.3–12.0 μg/g of raw bean. On the other hand, kaempferol was within the ranges 13.8–209.4 μg/g of raw bean and 7.1–123.2 μg/g of cooked bean. The study observed that black bean displayed the highest quercetin content, whereas kaempferol was the highest in bayo beans [
57]. A study conducted by Espinosa-Alonso et al. [
14] on 63 Mexican wild-type bean lines reported both kaempferol and quercetin as being the main flavonoids.
Condensed Tannins
The color of the bean seed coat is attributed to the presence and the amount of polyphenols such as flavonols glucosides, condensed tannins and anthocyanins. Their function is to provide protection against pathogens. These compounds display antioxidant, anti-mutagenic, anti-carcinogenic properties and also as free radical scavengers [
14]. According to Guzmán-Maldonado et al. [
72], the seed coat contains most of the tannins in beans, whereas their concentration is low in cotyledons.
Tannins are polymeric flavonoids that comprise a small part of the widely diverse group of phenolic compounds produced by vegetables as secondary metabolites. Along with oxalates and phytates, they are considered as anti-nutritionals because they affect nutrient bioavailability for the consumer. However, they are also considered as nutritionally important as they are antioxidants and potential anti-carcinogenic [
64,
73]. Condensed tannins or proanthocyanidins constitute a group of polymers and oligomers of polyhydroxy-flavon-3-ol linked through carbon-carbon bonds among the flavanol subunits. Their multiple phenolic hydroxyl groups allow their binding to proteins, metallic ions and other macromolecules as polysaccharides in order to form complexes [
65,
74]. They are considered bioactive compounds because their antioxidant, anti-carcinogenic and anti-mutagenic properties have been demonstrated [
17]. Juárez-López and Aparicio-Fernández [
13] reported a tannin content of 10.65 mg catechin equivalents (CE)/g on the “Flor de Junio” variety of the Mexican bean, a seed displaying pink spots on its seed coat. They also reported 2.15 mg CE/g on the “Peruano” variety of the Mexican bean that is characterized by a light yellow color. This suggested that the color on seed coat is directly correlated with the content of these compounds. These authors reported that thermal treatment affected the amount of condensed tannins on both studied varieties. This may be caused by the destruction of phenolic compounds or by changes in their structure or solubility. Iniestra-González et al. [
16] reported a condensed tannin content of 10.5 and 10.56 mg CE/g in the Mexican bean varieties “Flor de Mayo Bajío” and “Flor de Mayo M38”, respectively. These share features with the “Flor de Junio” variety. González de Mejía et al. [
17] reported higher values for these compounds on the Jalisco races of the common bean: “Flor de Mayo Criollo” (29 mg CE/g), “Flor de Mayo M-38” (38 mg CE/g) and “Flor de Junio Marcela” (32.9 mg CE/g), as well on the Durango races: “Bayo Victoria” (16.8 mg CE/g) and “Pinto Villa” (19.9 mg CE/g). They were cultured on five different sites within the semi-arid region in Mexico. Almanza-Aguilera et al. [
12] reported that the condensed tannins content considerably decreased after the cooking (94%) and frying (95%) processes in 16 Mexican genotypes. This shows that seed maceration eliminates of most of these compounds. It is recommended to use the water resulting from this process for cooking purposes in order to preserve them on the final product. These authors found that bean cultivar “Negro Durango” possessed the highest content of condensed tannins after its cooking. The observed value was 132 mg CE/100 g, followed by “Negro Guanajuato” with 68.8 mg CE/100 g. After the frying process, “Negro 8025” contained the highest amount of this bioactive compound with a 74.9 mg CE/100 g value. A study carried out by Reynoso et al. [
18] showed that the bean variety “Flor de Junio Marcela” possessed a high tannin content (698.4 mg CE/100 g) when compared to other cultivars of this grain. This content is 3.5-times higher regarding the “Pinto Zapata” bean (197.9 mg CE/100 g), nine-times higher when compared to the “Flor de Mayo Anita” variety (75.6 mg CE/100 g) and over thousand-times regarding “Blanco Tlaxcala” (0.60 mg CE/100 g). Guzman-Maldonado et al. [
63,
74] reported tannin content within the 6.9–32.4 mg CE/g range in 19 common bean varieties grown in the states of Aguascalientes and Durango (Mexico). These authors mention that there is an undetected amount of condensed tannins; consequently, the actual content of these compounds is underestimated. In their study, they found 9.9–66.4% of undetected tannins in the Aguascalientes varieties and 4.1–69.7% in those from Durango. Another study conducted by Espinosa-Alonso et al. [
14] on 62 Mexican wild-type bean lines reported that the amount of condensed tannins is 9.49–35.70 mg CE/g of flour. This study also analyzed the content of condensed tannins in the “Negro Jamapa” and “Frijol Pinto” varieties and the following values were found: 30.86 and 21.37 mg CE/g of flour, respectively.
Anthocyanins
Anthocyanins are classified as phenolic compounds, particularly as flavonoids. They provide pigments to vegetables and they are widely distributed in nature. They confer color to the bean seed coat on the red, black and pink-colored varieties. Juárez-López and Aparicio-Fernández [
13] assessed the amount of anthocyanins on the Mexican variety “Flor de Junio” and they found 0.43 mg equivalents to cyaniding 3-glucoside (EC3G)/g of raw bean. They reported a significant effect caused by processing: a decrease correlated with the intensity of the thermal treatment. Other studies have found three types of anthocyanins in the black bean: delphinidin 3-glucoside (56%), petunidin 3-glucoside (26%) and malvidin 3-glucoside (18%) [
66,
75]. Salinas-Moreno et al. [
67,
76] also reported the former anthocyanidins as predominant in 15 Mexican varieties of the Jalisco, Mesoamerica and Recombined races. This suggests that these compounds possess a high antioxidant activity and they prevent diseases such as cancer, atherosclerosis and inflammation. Conversely, Díaz et al. [
75] reported low anthocyanin content in the Mexican genotypes of the yellow-colored common bean or their combinations when compared to the high content on the red-colored beans. This observation indicates that the level of these bioactive compounds is higher in dark-colored beans in comparison to those light-colored. Reynoso et al. [
18] reported an anthocyanin content of 3.75 mg EC3G/100 g in bean cultivar “Flor de Junio Marcela”. This content was higher when compared to that found on other cultivars such as “Pinto Zapata” (1.77 mg EC3G/100 g), “Flor de Mayo Anita” (0.0 mg EC3G/100 g) and “Blanco Tlaxcala” (0.09 mg EC3G/100 g). Espinosa-Alonso et al. [
14] studied 62 Mexican wild-type bean lines and they identified delphinidin, petunidin, cyanidin, malvidin, pelargonidin and peonidin as the main anthocyanins on the analyzed samples. They reported total anthocyanidin content within the 0.01–1.85 mg EC3G/g range in all of the studied varieties. Martínez et al. [
15] evaluated the anthocyanin content on the “Negro Jamapa” and “Mayocoba” varieties of the Mexican bean and they only found the previously mentioned compounds on the former. The main anthocyanins were delfinidin (47%), petunidin (22%) and malvidin (13.53%). Additionally, they measured a higher content of total pigments in the “Negro Jamapa” peels (13.53%) regarding “Mayocoba” (6.7%). They also observed that anthocyanidins, similarly to flavonoids, were degraded by thermal treatments at high temperatures and/or prolonged thermic contact. After a pre-cooking process the levels contained on its raw form decreased from 20% up to 80% after the cooking and canning processes. This effect was pronounced on the cultivar “Negro Jamapa”; thus, it was identified as the most sensitive towards thermal treatments.
Other sources of anthocyanins are strawberry (7–66 mg/100 g), cherry (3–230 mg/100 g), blackberry (28–366 mg/100 g), and red raspberry (28–116 mg/100 g) [
77]. These results are higher than those obtained in Mexican bean cultivar “Flor de Junio Marcela” [
18].
Although anthocyanins offer great health benefits, dietary reference intakes do not currently exist for anthocyanins and many other dietary bioactive compounds in the United States, Canada, or the European Union. China has currently defined a specific proposed level of 50 mg/day for anthocyanins, while the Joint FAO/WHO Expert Committee on Food Additives has established an acceptable daily intake of 2.5 mg/kg per d for anthocyanins from grape-skin extracts but not for anthocyanins in general. After a request from the European Commission to the European Food Safety Authority, the Scientific Panel on Food Additives and Nutrient Sources Added to Food was asked to provide a scientific opinion re-evaluating the safety of anthocyanins. The panel concluded that the currently available toxicologic database was inadequate to establish a numerically acceptable daily intake for anthocyanins [
78].
Isoflavonoids
Isoflavonoids are a subgroup of flavonoids, are phytoestrogens that exhibit pseudohormonal properties as a result of their functional and structural similarity to the natural estrogen 17β-estradiol and may interact with estrogen receptors. Isoflavone content is variable among plant species and may even vary among genotypes of the same species. In addition, isoflavone content may also be affected by external factors related to crop location, such as temperature, fertilization levels, occurrence of pests and diseases, time since harvest, farming practices, and processing and food preparation methods [
79].
The primary isoflavones in soybeans are genistein (4′,5,7-trihydroxyisoflavone) and daidzein (4′,7-dihydroxyisoflavone) and their respective b-glycosides, genistin and daidzin (sugars are attached at the 7 position of the A ring) [
80].
Common bean is not recognized as an isoflavone source, the soybean is the legume that exhibits the highest content of these compounds [
57]. However, some studies have shown the presence of these compounds in common bean. Lima et al. [
79] reported the presence of nonglycosylated forms of isoflavonoids daidzein and genistein in 16 genotypes of Brazilian common bean germplasm. These authors found that grains of the black type showed the highest concentrations of isoflavonoids and were the only ones to exhibit daidzein. However, the isoflavonoid content obtained in the bean genotypes evaluated was very low compared to that obtained in soybean grains.
Diaz-Batalla et al. [
57] analyzed ten cultivated and four wild varieties of Mexican common bean seeds, and reported that the presence of daidzein and genistein in raw and cooked bean seed flour was not confirmed by the UV spectra. However, during the germination of the seven common beans seed samples (five cultivated and two wild), there was daidzein and genistein genesis, and the values for daidzein ranged from 8.2 to 129.1 μg/g, those for genistein from 2.6 to 9.7 μg/g. Similar results were obtained by Guajardo-Flores et al. [
45], in Mexican black bean “Negro San Luis” variety, these authors reported that Genistein was detected only after three d of germination, in concentrations that ranged from 0.12 to 0.31 mg/100 g. Higher results were reported for soybean (600 μg/g and 950 μg/g respectively) [
81].
Daidzein, exhibits anticancer effects; e.g., it inhibited the growth of HL-60 cells implanted in the subrenal capsules of mice. However, genistein has attracted most of the interest. There are literally hundreds of in vitro studies showing that genistein inhibits the growth of a wide range of both hormone-dependent and hormone independent cancer cells with an IC50 between <5 and 40 mM (2–10 mg/mL), including breast, prostate, colon, and skin cells. Also, in vitro, genistein inhibits the metastatic activity of both breast and prostate cancer cells independent of the effects on cell growth [
2]. The effect of isoflavones is not exclusively hormonal; genistein is a specific inhibitor of protein tyrosine kinases and DNA topoisomerases I and II and arrests cell grow by interfering wth the signal transduction pathways. Additionally, phytoestrogens exhibit antioxidant activity [
57].
4.2.2. Phenolic Acids
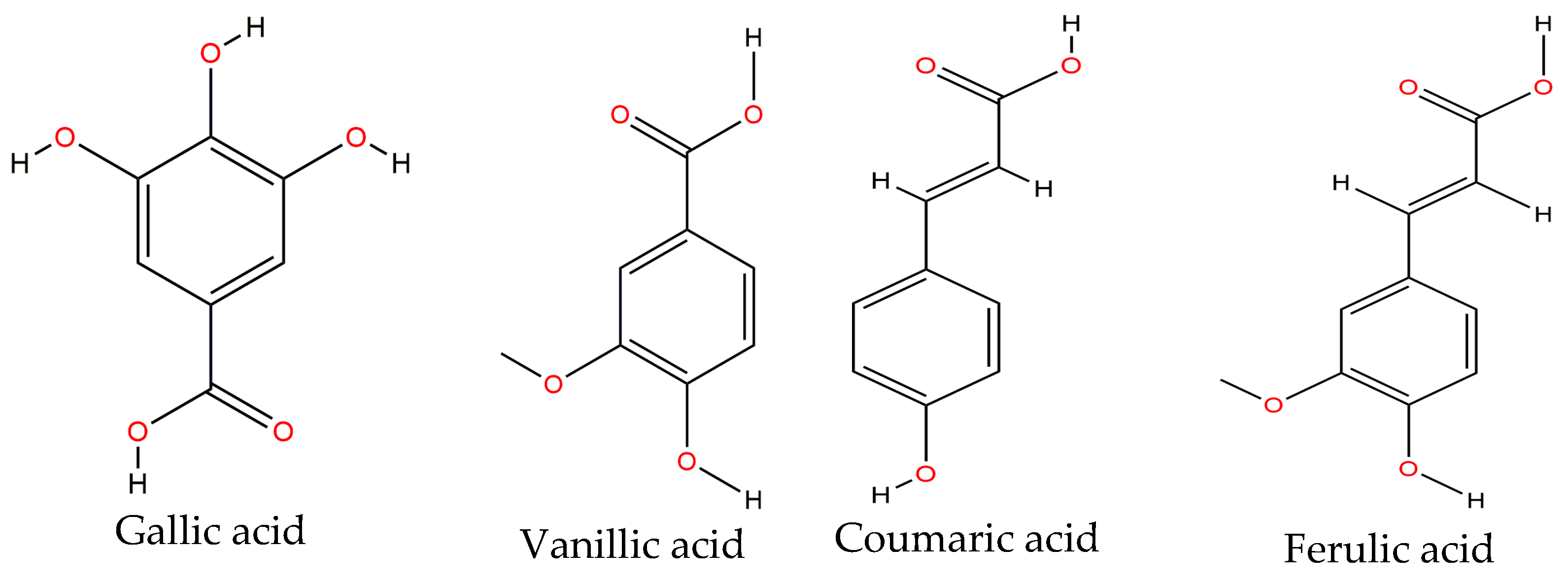
Phenolic acids are of great importance in vegetables as they are precursors of other more complex phenolic compounds. These may be classified in two types: those derived from benzoic acid (e.g.,
p-hydroxybenzoic, vanillic and gallic acids) and those derived from cinnamic acid (e.g., ferulic, p-coumaric, and caffeic acids) [
57]. Gallic, vanillic, coumaric, sinapic, ferulic and chlorogenic acids (
Figure 3) are mainly found on the common bean, either raw or cooked. A study conducted by Espinosa-Alonso et al. [
14] showed that ferulic acid is the main phenolic acid on 62 lines of the wild-type Mexican bean.
Some studies have shown that cooking does not affect the content of these phenolic acids [
49], although some authors have reported otherwise [
82]. The effect of a thermal treatment has been also reported for some varieties of the common Mexican bean. Díaz-Batalla et al. [
57] reported decreased levels of p-hydroxybenzoic acid in the raw form when compared to the cooked form. The quantified values were 5.7–13.8 μg/g and 4.5–8.6 μg/g, respectively. The decrease after processing changed from 17.9 to 44.5%. Furthermore, vanillic acid changed from 5.2–16.6 μg/g in raw bean to 3.5–12.1 μg/g in its cooked form. Thus, the decrease caused by cooking was 12.5 to 36.9%. Coumaric acid was also reported on these samples within the 3.2–6.8 μg/g and 1.7–4.7 μg/g ranges for the raw and cooked forms, respectively, that represented a 26.3–66.3% decrease. Ferulic acid changed from 17.0–36.0 μg/g in raw bean to 11.9–27.9 μg/g in cooked bean, implying a decrease from 15.5% to 36.5%. Martínez et al. [
15] also quantified gallic, p-hydroxybenzoic, vanillic, ferulic and coumaric acids on the “Jamapa” and “Mayocoba” varieties of the black bean in their raw and cooked forms. They reported that thermal processing only affected gallic and p-hydroxybenzoic acids, whereas the rest remained constant.
Regarding their biologic activity, it has been found that phenolic acids display anthelmintic activity, they prevent sickle red cells and suppress hepatic fibrosis in chronic liver disease [
49]. Some studies have reported that gallic acid possesses bacteriostatic properties, it counteracts melanoma onset and it functions as an antioxidant and it has been also proposed for the treatment of brain tumors. On the other hand, chlorogenic acid protects against neurotoxins by decreasing apoptosis induced by the beta-amyloid peptide. It also displays of anticholinesterase, radical scavenger and anti-amnesic activities. Ferulic acid also displays beneficial effects on health as antioxidant, anti-inflammatory and inmunostimulat. It also promotes the degradation of the recombinant beta-amyloid peptide. Caffeic acid also displays a neuroprotective effect against beta-induced toxicity as it inhibits calcium efflux and Tau phosphorylation. It also protects neurons against oxidative stress-induced cytotoxicity and, along with coumaric acid, it may induce neuroprotective effects against Parkinson disease in a similar manner as previously observed with flavonoids, catechin and quercetin [
83].