A New Approach to Synthesize of 4-Phenacylideneflavene Derivatives and to Evaluate Their Cytotoxic Effects on HepG2 Cell Line
Abstract
:1. Introduction
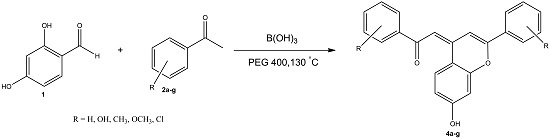
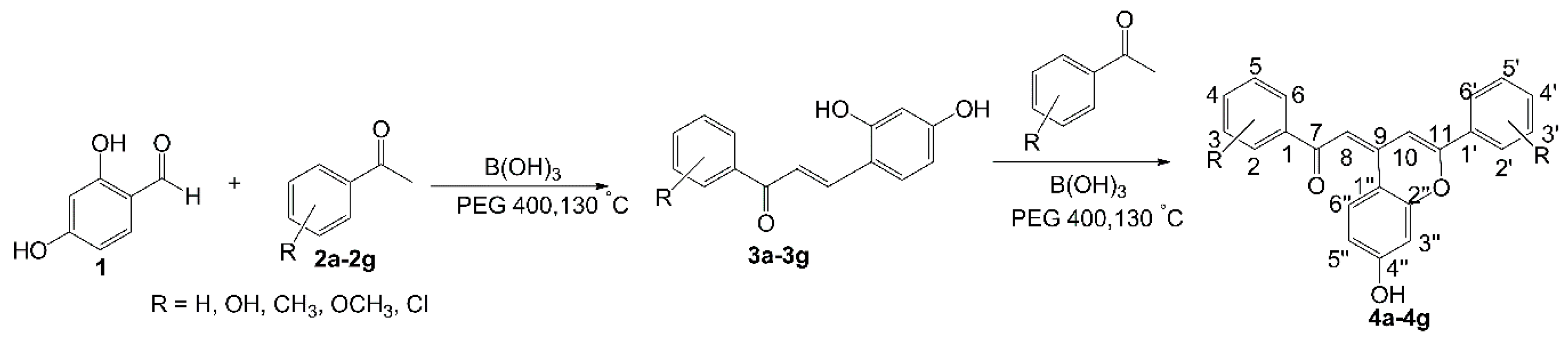
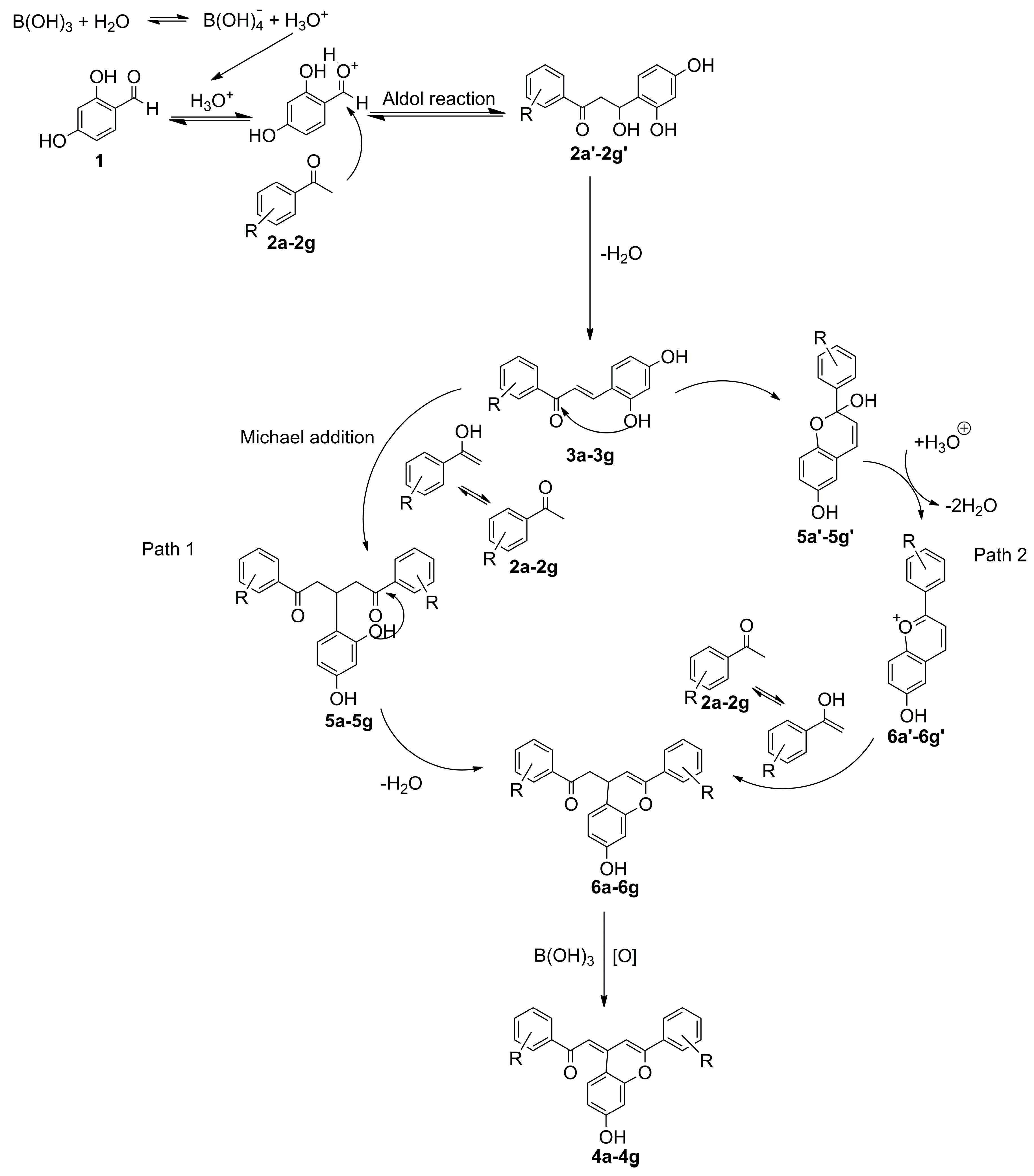
2. Results and Discussion
2.1. Chemistry
2.2. Cytotoxic Effects on HepG2 Cell Line
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Compounds 4a–4g
3.3. HPLC Analysis of the Products
3.4. Biological Evaluation
3.4.1. Cell Culture
3.4.2. Growth Inhibition Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Korobkova, E.A. Flavonoids and mechanisms of their anticancer action. Chem. Biol. Interface 2013, 3, 346–365. [Google Scholar]
- Ritter, M.; Martins, R.M.; Dias, D.; Pereira, C.M.P. Recent advances on the synthesis of chalcones with antimicrobial activities: A brief review. Lett. Org. Chem. 2014, 11, 498–508. [Google Scholar] [CrossRef]
- Yasuda, M.; Kawabata, K.; Miyashita, M.; Okumura, M.; Yamamoto, N.; Takahashi, M.; Ashida, H.; Ohigashi, H. Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J. Agric. Food Chem. 2014, 62, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Casano, G.; Dumetre, A.; Pannecouque, C.; Hutter, S.; Azas, N.; Robin, M. Anti-HIV and antiplasmodial activity of original flavonoid derivatives. Bioorgan. Med. Chem. 2010, 18, 6012–6023. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Das, D.K.; Khan, A.T. Bromodimethylsulfonium bromide: An efficient catalyst for one-pot synthesis of 4-phenacylidene flavene derivatives. Tetrahedron Lett. 2015, 56, 2412–2415. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Agarwal, S.; Kumar, M.; Kumar, B.; Sridhar, B. A simple and efficient access to new functionalized 4-phenacylideneflavenes. Adv. Synth. Catal. 2012, 354, 1129–1140. [Google Scholar] [CrossRef]
- Fichtner, C.; Remennikov, G.; Mayr, H. Kinetics of the reactions of flavylium ions with π-nucleophiles. Eur. J. Org. Chem. 2001, 20, 4451–4456. [Google Scholar] [CrossRef]
- Vanallan, J.A.; Reynolds, G.A.; Regan, T.H. Formation of 4-phenacylideneflavene from 4-phenacylflavene. J. Org. Chem. 1967, 32, 1897–1899. [Google Scholar] [CrossRef]
- Hill, D.W. Reactions of o-hydroxybenzylidenediacetophenones. Part I. Reaction with acids. J. Chem. Soc. 1934, 9, 1255–1258. [Google Scholar] [CrossRef]
- Hwang, H.T.; Varma, A. Effect of boric acid on thermal dehydrogenation of ammonia borane: Mechanistic studies. Int. J. Hydrogen Energy 2013, 38, 1925–1931. [Google Scholar] [CrossRef]
- Chaudhuri, M.K.; Hussain, S.; Kantam, M.L.; Neelima, B. Boric acid: A novel and safe catalyst for aza-Michael reactions in water. Tetrahedron Lett. 2005, 46, 8329–8331. [Google Scholar] [CrossRef]
- Tu, S.; Fang, F.; Miao, C.; Jiang, H.; Feng, Y.; Shi, Y.; Wang, X. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using boric acid as catalyst. Tetrahedron Lett. 2003, 44, 6153–6155. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.; Datta, A.; Butcher, R.J. Highly efficient one-pot, three-component Mannich reaction catalysed by boric acid and glycerol in water with major ‘syn’ diastereoselectivity. Tetrahedron Lett. 2009, 50, 4246–4250. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Sorres, J.; Tran, M.Q.; Ermolenko, L.A. Boric acid: A highly efficient catalyst for transamidation of carboxamides with amines. Org. Lett. 2012, 14, 3202–3205. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4a–4g are available from the authors. |
| Products | R | HepG2 (IC50 µM) |
|---|---|---|
| 4a | H | 20–50 |
| 4b | 2-OH | 20–50 |
| 4c | 3-OH | >50 |
| 4d | 4-OH | >50 |
| 4e | 4-CH3 | ≈12.5 a |
| 4f | 4-OCH3 | 20–50 |
| 4g | 4-Cl | >50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Xu, Y.; Zhang, Y.; Zheng, Z. A New Approach to Synthesize of 4-Phenacylideneflavene Derivatives and to Evaluate Their Cytotoxic Effects on HepG2 Cell Line. Molecules 2017, 22, 1296. https://doi.org/10.3390/molecules22081296
Chen H, Xu Y, Zhang Y, Zheng Z. A New Approach to Synthesize of 4-Phenacylideneflavene Derivatives and to Evaluate Their Cytotoxic Effects on HepG2 Cell Line. Molecules. 2017; 22(8):1296. https://doi.org/10.3390/molecules22081296
Chicago/Turabian StyleChen, Hongbin, Yang Xu, Yinan Zhang, and Zongping Zheng. 2017. "A New Approach to Synthesize of 4-Phenacylideneflavene Derivatives and to Evaluate Their Cytotoxic Effects on HepG2 Cell Line" Molecules 22, no. 8: 1296. https://doi.org/10.3390/molecules22081296
APA StyleChen, H., Xu, Y., Zhang, Y., & Zheng, Z. (2017). A New Approach to Synthesize of 4-Phenacylideneflavene Derivatives and to Evaluate Their Cytotoxic Effects on HepG2 Cell Line. Molecules, 22(8), 1296. https://doi.org/10.3390/molecules22081296