Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement
Abstract
1. Introduction
2. Results and Discussion
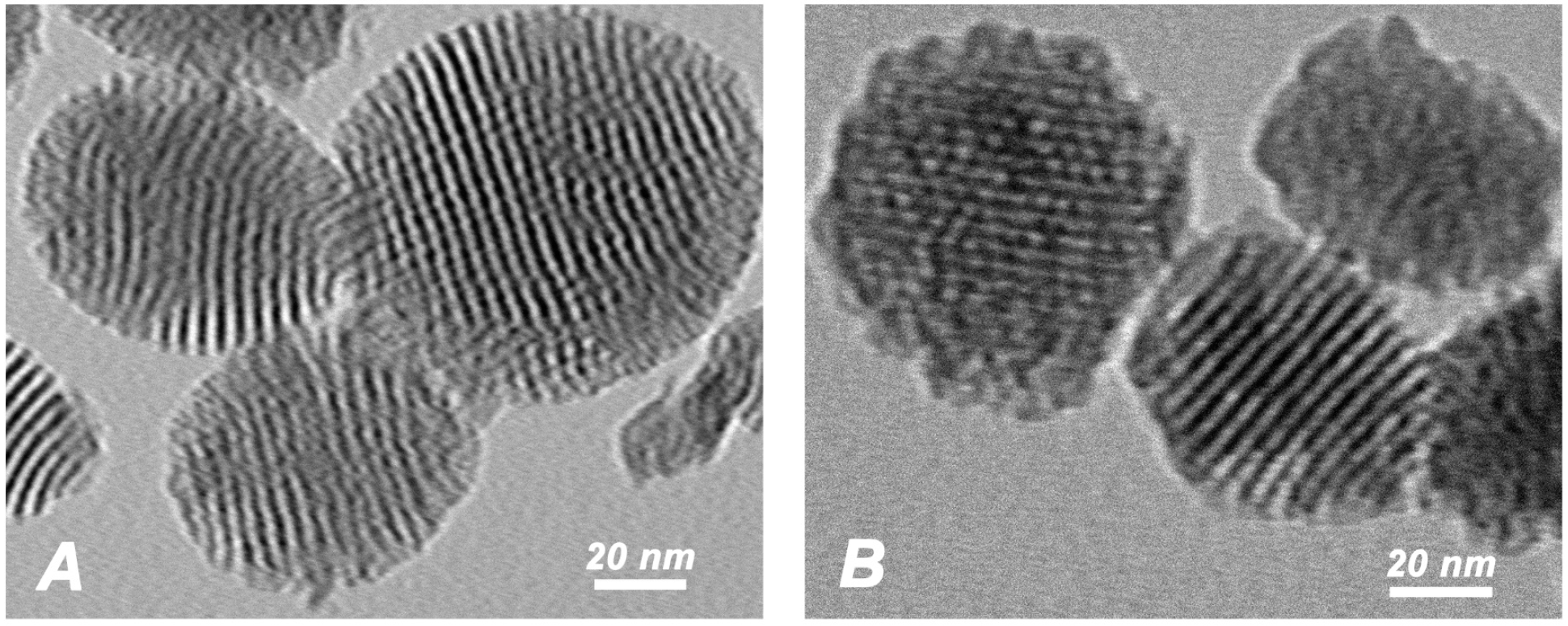
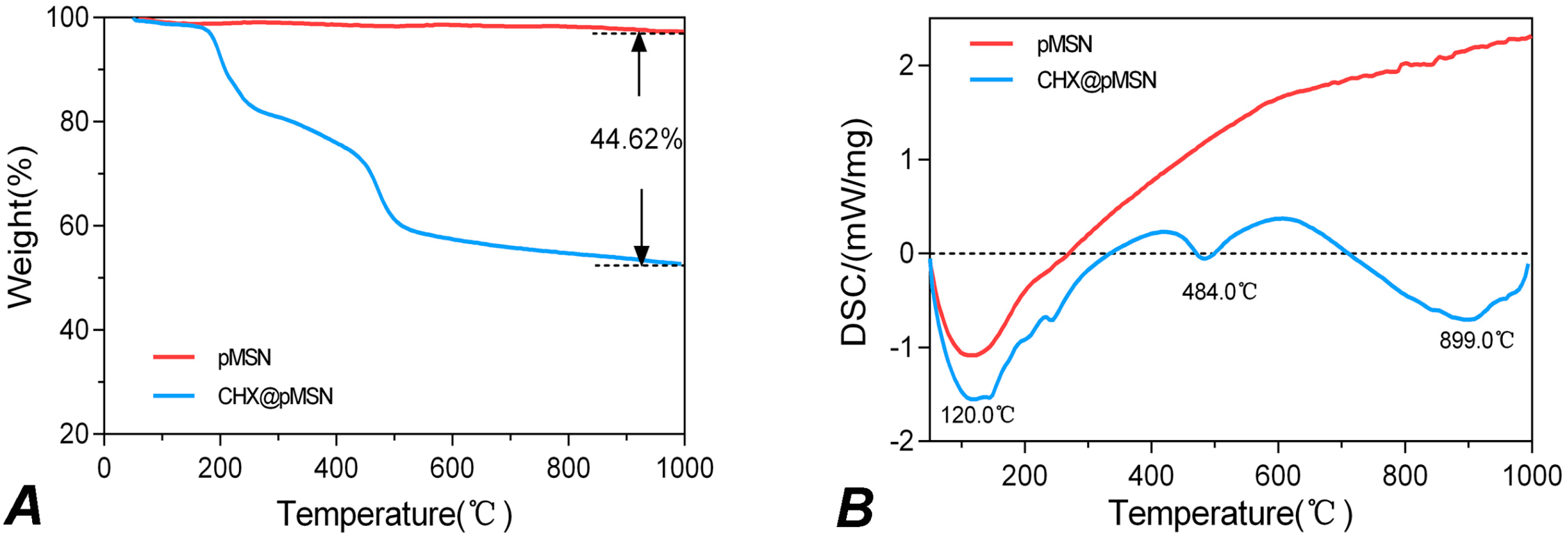
2.1. Characterization of pMSN and CHX@pMSN
2.2. Anti-Biofilm Property
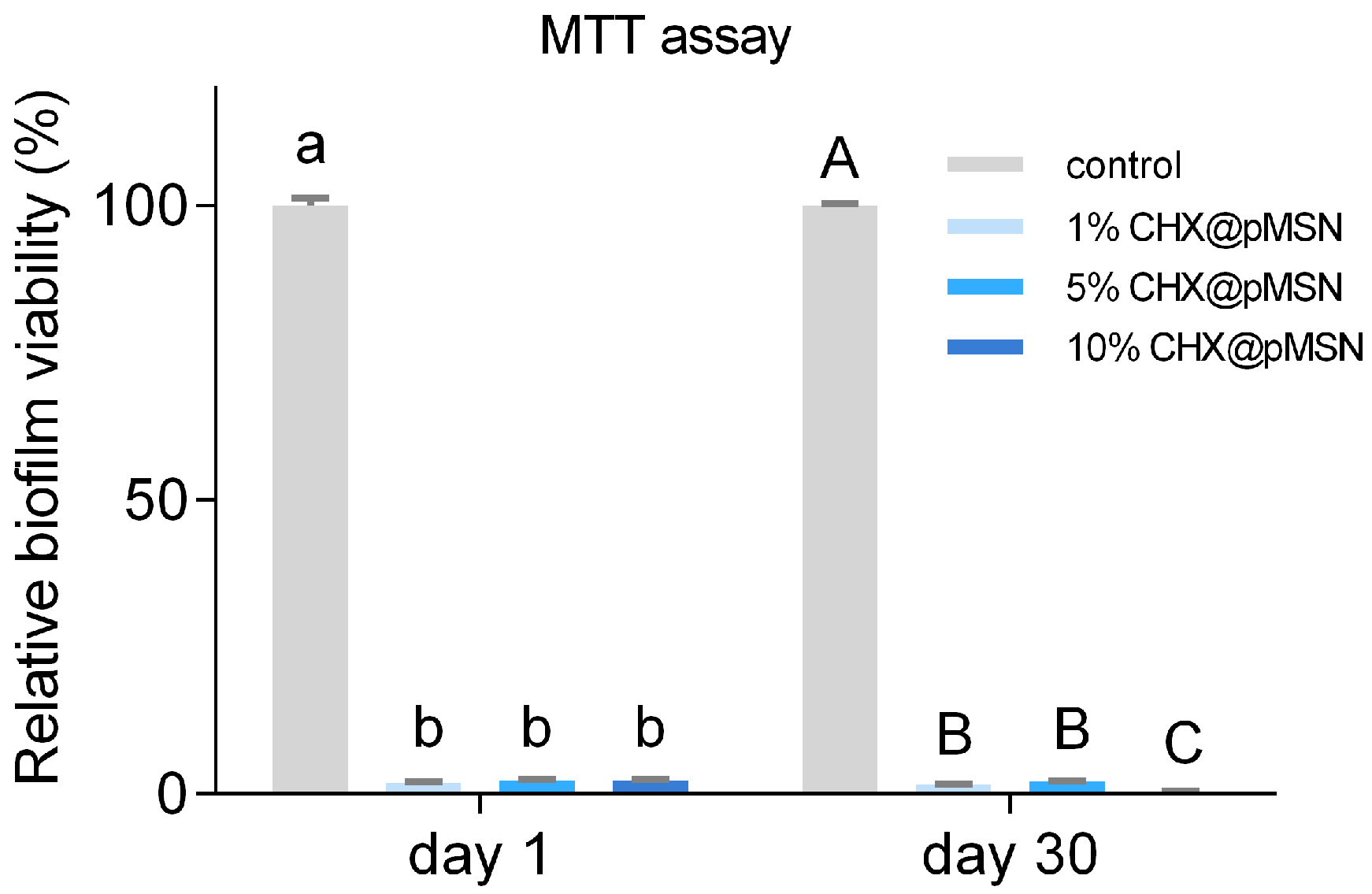
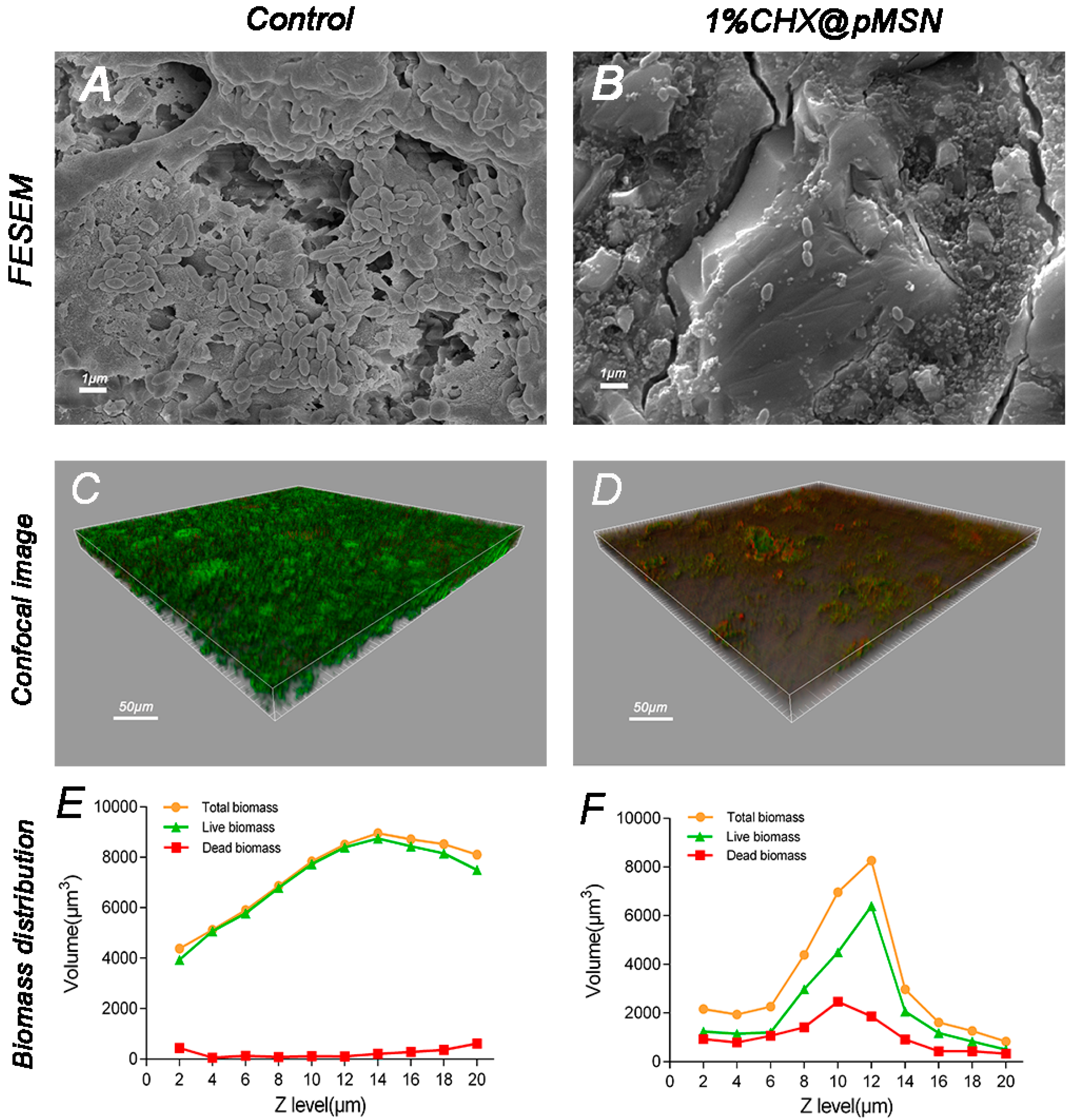
2.2.1. MTT Assay and FESEM Observation
2.2.2. CLSM Observation
2.3. Mechanical and Physical Properties
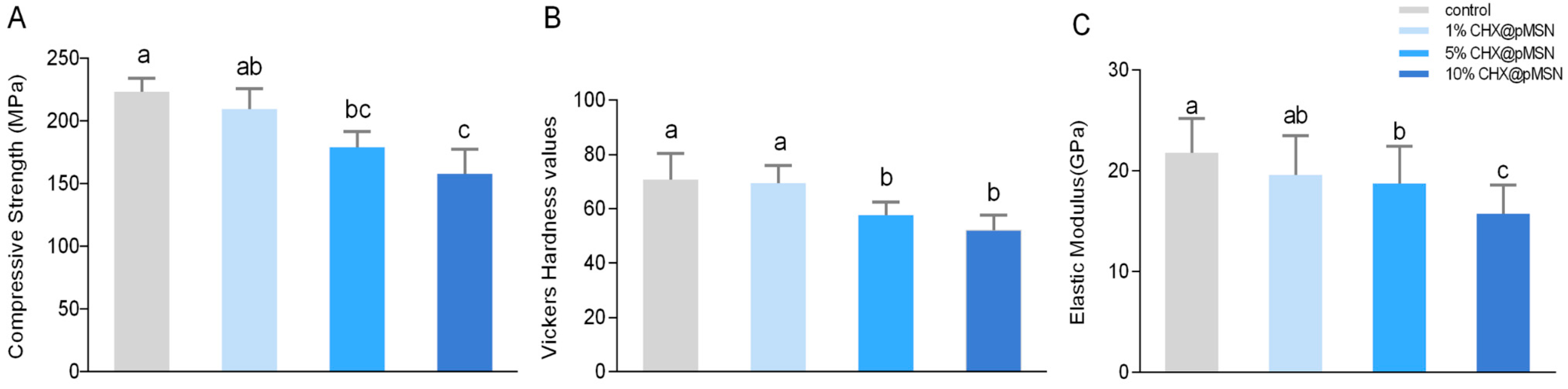
2.3.1. Compressive Strength, Surface Vickers Hardness, and Elastic Modulus
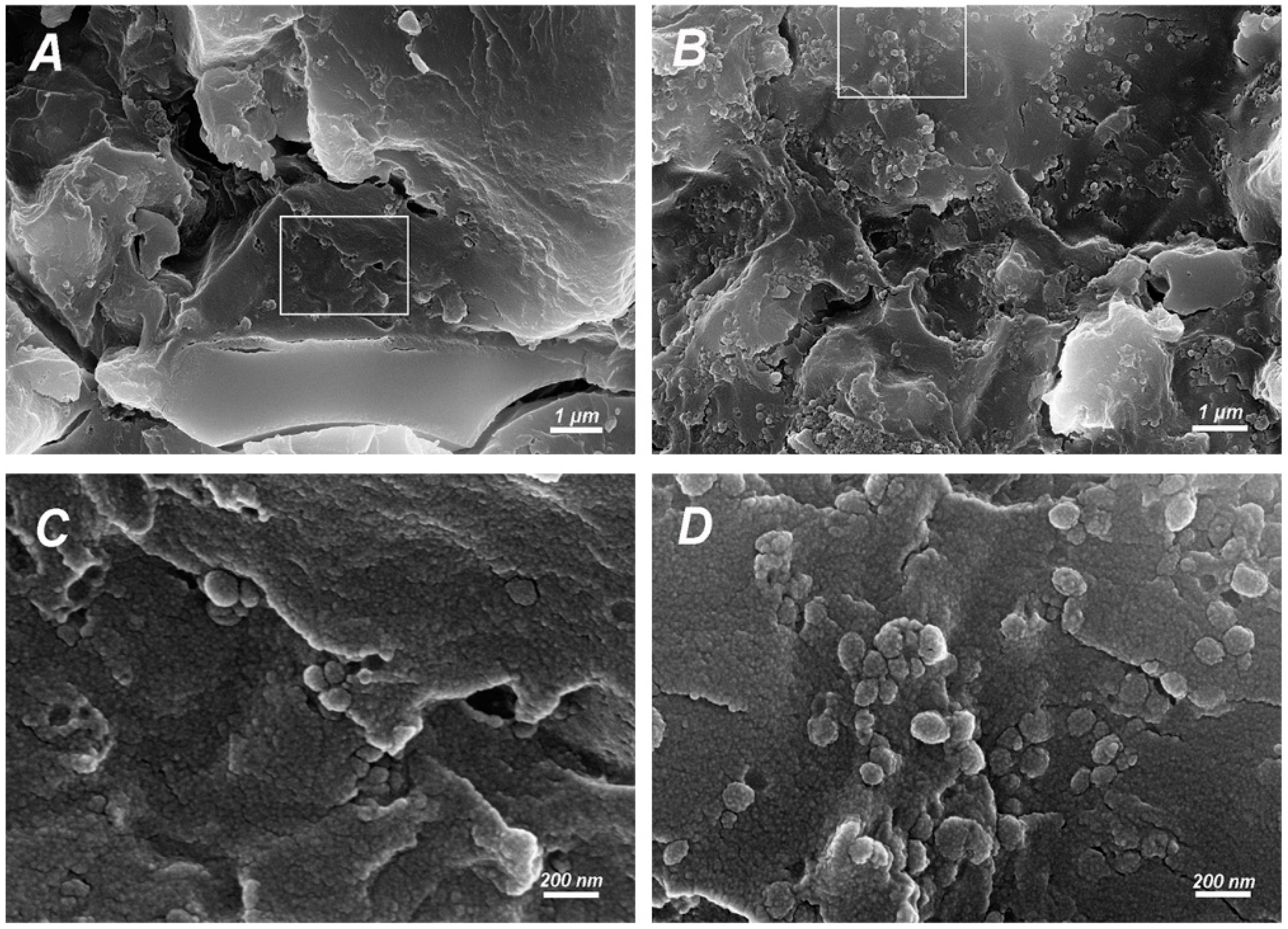
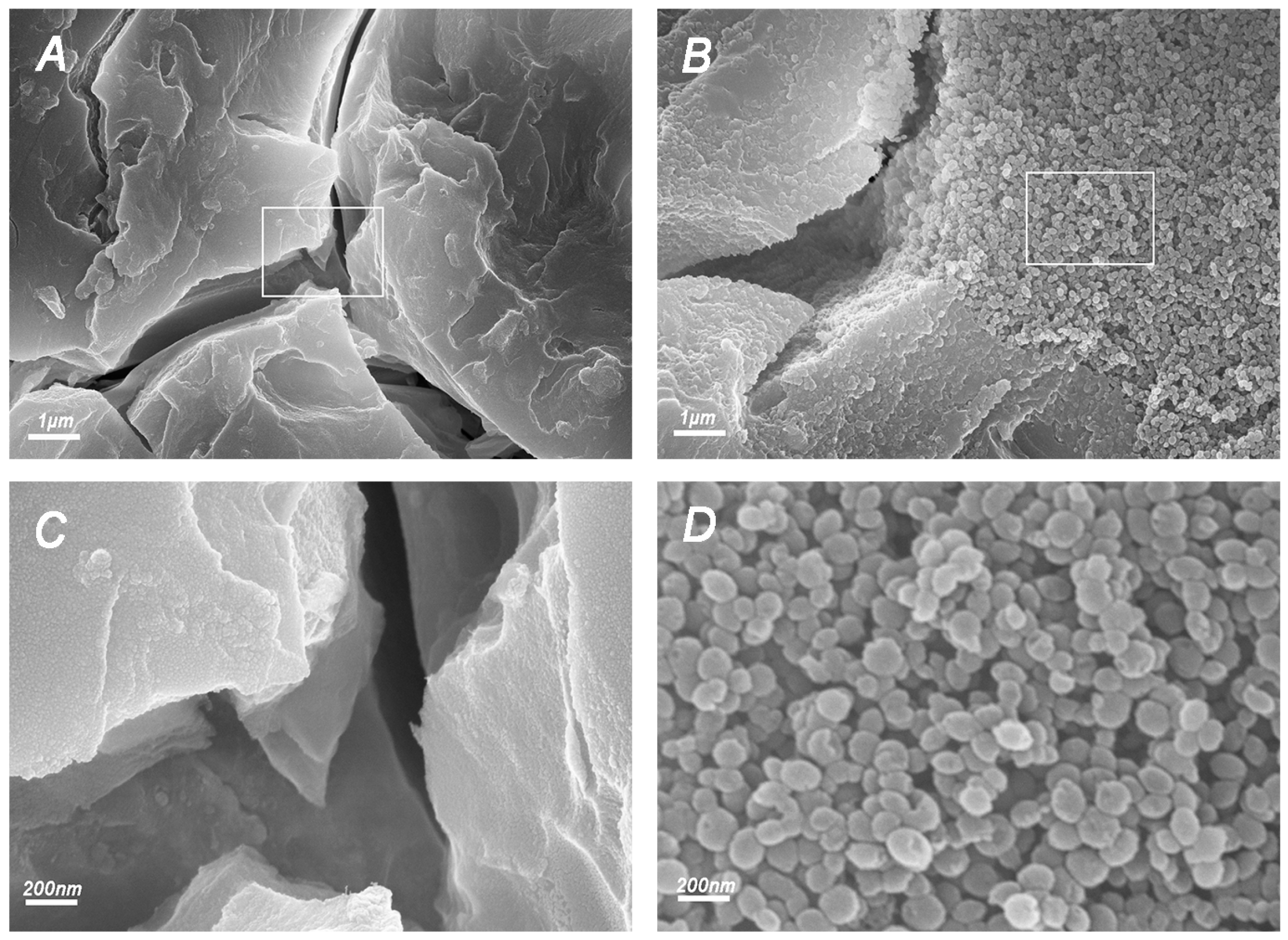
2.3.2. FESEM Observation of Fracture Surfaces
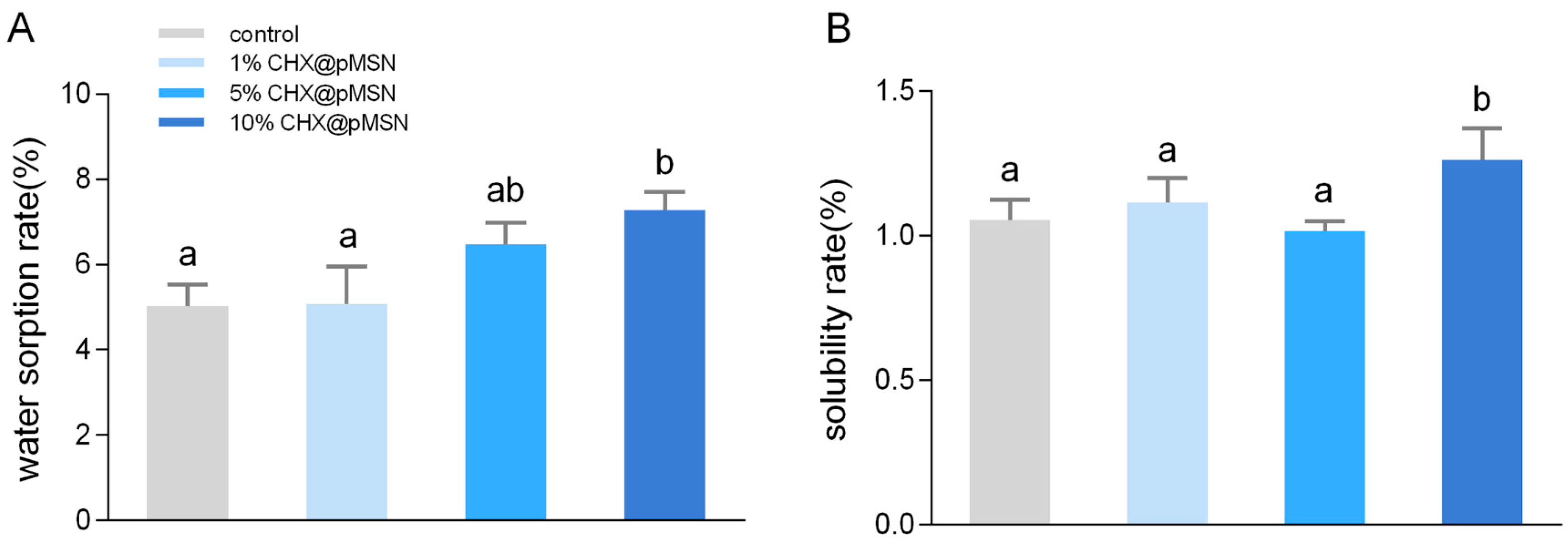
2.3.3. Water Sorption and Solubility
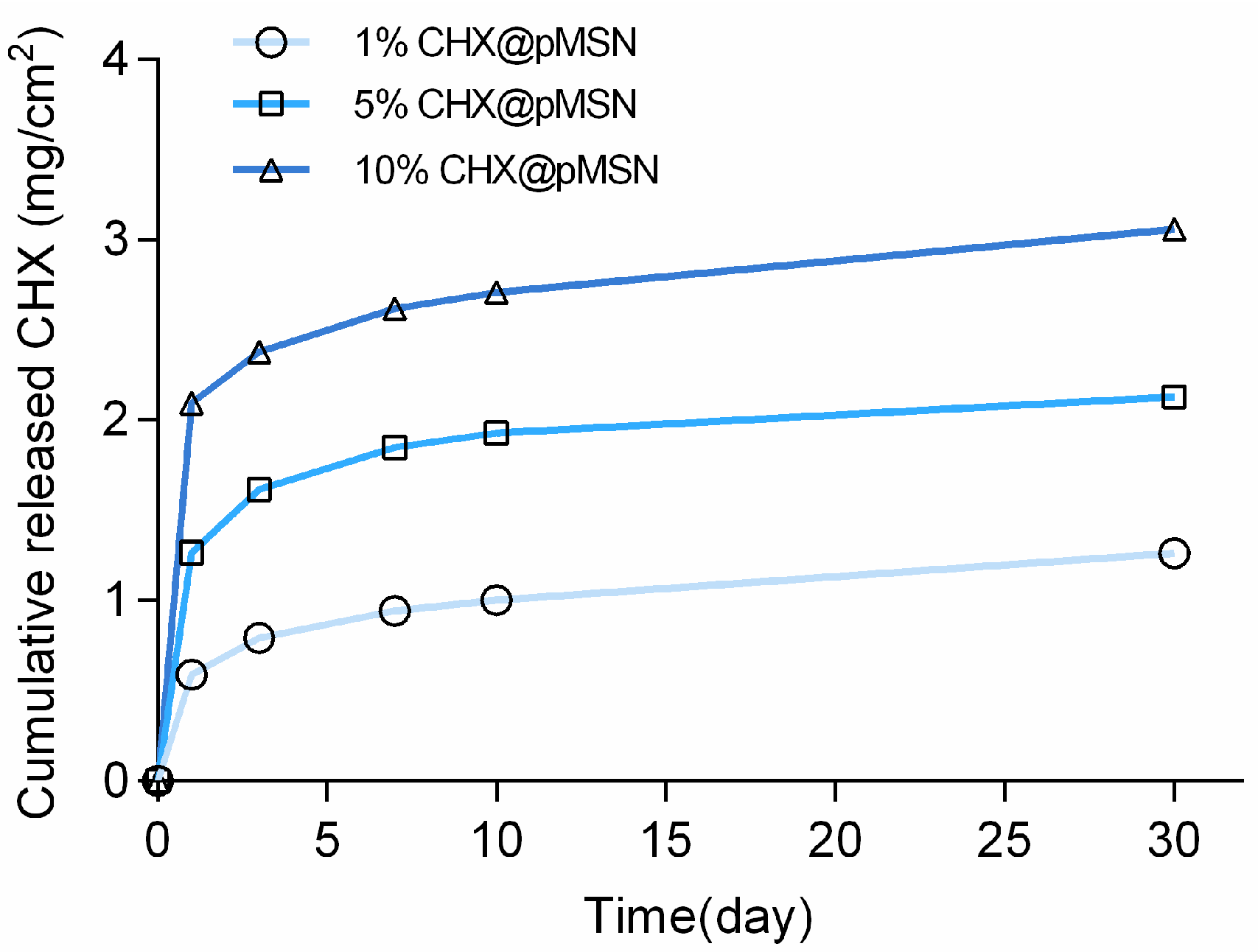
2.3.4. Release Profile of CHX@pMSN-Modified GIC
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of CHX@pMSN
3.3. Preparation of Experimental GIC
3.4. Specimen Preparation for Antibiofilm Test
3.4.1. MTT Assay of CHX@pMSN-Modified GIC at 1 Day and 30 Days
3.4.2. Confocal Laser Scanning Microscopy Analysis
3.4.3. FESEM Analysis
3.5. Surface Microhardness and Nanoindentation
3.6. Compressive Strength
3.7. Water Sorption and Solubility Rate
3.8. CHX Release
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Wilson, A.D.; Kent, B.E. A new translucent cement for dentistry. The glass ionomer cement. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C. Development of glass-ionomer cement systems. Biomaterials 1998, 19, 467–478. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, X.; Wang, L.; Qiu, W.; Wang, S.; Zhou, Y.; Li, M.; Li, Y.; Cheng, L.; Li, J.; et al. Combinatorial effects of arginine and fluoride on oral bacteria. J. Dent. Res. 2015, 94, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Imazato, S.; Kaneshiro, A.V.; Ebisu, S.; Frencken, J.E.; Tay, F.R. Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent. Mater. 2006, 22, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.; Khan, S.N.; Khan, A.U. Dental caries: From infection to prevention. Med. Sci. Monit. 2007, 13, RA196–RA203. [Google Scholar] [PubMed]
- Klai, S.; Altenburger, M.; Spitzmuller, B.; Anderson, A.; Hellwig, E.; Al-Ahmad, A. Antimicrobial effects of dental luting glass ionomer cements on Streptococcus mutans. Sci. World J. 2014, 2014, 807086. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.C.; Wilson, N.H. Glass-ionomer restoratives: A systematic review of a secondary caries treatment effect. J. Dent. Res. 1999, 78, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Yengopal, V.; Mickenautsch, S.; Bezerra, A.C.; Leal, S.C. Caries-preventive effect of glass ionomer and resin-based fissure sealants on permanent teeth: A meta analysis. J. Oral Sci. 2009, 51, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.L.; Simionato, M.R.; Imparato, J.C.; Oda, M. Antibacterial activity of glass-ionomer cement containing antibiotics on caries lesion microorganisms. Am. J. Dent. 2005, 18, 261–266. [Google Scholar] [PubMed]
- Feng, J.; Cheng, L.; Zhou, X.; Xu, H.H.; Weir, M.D.; Meyer, M.; Maurer, H.; Li, Q.; Hannig, M.; Rupf, S. In situ antibiofilm effect of glass-ionomer cement containing dimethylaminododecyl methacrylate. Dent. Mater. 2015, 31, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Hatunoglu, E.; Ozturk, F.; Bilenler, T.; Aksakalli, S.; Simsek, N. Antibacterial and mechanical properties of propolis added to glass ionomer cement. Angle Orthod. 2014, 84, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Du, X.; Huang, C.; Fu, D.; Ouyang, X.; Wang, Y. Antibacterial and physical properties of EGCG-containing glass ionomer cements. J. Dent. 2013, 41, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Hoszek, A.; Pierreville, F.; Schittek, M.; Ericson, D. Fissure penetration and antibacterial effect in vitro of a glass ionomer cement containing chlorhexidine gluconate. Swed. Dent. J. 1998, 22, 133–141. [Google Scholar] [PubMed]
- De Castilho, A.R.; Duque, C.; Negrini, T.C.; Sacono, N.T.; de Paula, A.B.; de Souza, C.C.; Spolidorio, D.M.; Puppin-Rontani, R.M. In vitro and in vivo investigation of the biological and mechanical behaviour of resin-modified glass-ionomer cement containing chlorhexidine. J. Dent. 2013, 41, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Addy, M.; Wade, W. The mechanism of action of chlorhexidine—A study of plaque growth on enamel inserts in vivo. J. Clin. Periodontol 1988, 15, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.; Geszke-Moritz, M. Mesoporous silica materials with different structures as the carriers for antimicrobial agent. Modeling of chlorhexidine adsorption and release. Appl. Surf. Sci. 2015, 356, 1327–1340. [Google Scholar] [CrossRef]
- Hook, E.R.; Owen, O.J.; Bellis, C.A.; Holder, J.A.; O’Sullivan, D.J.; Barbour, M.E. Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. J. Nanobiotechnol. 2014, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Marti, L.M.; Mata, M.; Ferraz-Santos, B.; Azevedo, E.R.; Giro, E.M.; Zuanon, A.C. Addition of chlorhexidine gluconate to a glass ionomer cement: A study on mechanical, physical and antibacterial properties. Braz. Dent. J. 2014, 25, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Imazato, S.; Toi, C.; Mulder, J.; Mickenautsch, S.; Takahashi, Y.; Ebisu, S. Antibacterial effect of chlorhexidine-containing glass ionomer cement in vivo: A pilot study. Caries Res 2007, 41, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.; Jones, F.H.; Billington, R.W.; Pearson, G.J. Chlorhexidine release from an experimental glass ionomer cement. Biomaterials 2004, 25, 5423–5431. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Vallet-Regi, M.; Kupferschmidt, N.; Terasaki, O.; Schmidtchen, A.; Malmsten, M. Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials 2009, 30, 5729–5736. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Koneru, B.; Shi, Y.; Wang, Y.C.; Chavala, S.H.; Miller, M.L.; Holbert, B.; Conson, M.; Ni, A.; Di Pasqua, A.J. Tetracycline-Containing MCM-41 Mesoporous Silica Nanoparticles for the Treatment of Escherichia coli. Molecules 2015, 20, 19690–19698. [Google Scholar] [CrossRef] [PubMed]
- Malfanti, A.; Miletto, I.; Bottinelli, E.; Zonari, D.; Blandino, G.; Berlier, G.; Arpicco, S. Delivery of Gemcitabine Prodrugs Employing Mesoporous Silica Nanoparticles. Molecules 2016, 21, 522. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, H.; Li, K.; Lei, J.; Zhou, L.; Huang, C. A novel application of nanohydroxyapatite/mesoporous silica biocomposite on treating dentin hypersensitivity: An in vitro study. J. Dent. 2016, 50, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; El-Fiqi, A.; Jo, J.K.; Kim, D.A.; Kim, S.C.; Jun, S.K.; Kim, H.W.; Lee, H.H. Development of long-term antimicrobial poly(methyl methacrylate) by incorporating mesoporous silica nanocarriers. Dent. Mater. 2016, 32, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Leung, K.C.; Wong, C.H.; Lee, S.F.; Li, X.; Leung, P.C.; Lau, C.B.; Wat, E.; Jin, L. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS ONE 2014, 9, e103234. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.; Kammler, H.K.; Karsten Wegner, A.; Pratsinis, S.E. OH Surface Density of SiO2 and TiO2 by Thermogravimetric Analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Barandehfard, F.; Rad, M.K.; Hosseinnia, A.; Khoshroo, K.; Tahriri, M.; Jazayeri, H.E.; Moharamzadeh, K.; Tayebi, L. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram. Int. 2016, 42, 17866–17875. [Google Scholar] [CrossRef]
- Shiekh, R.A.; Rahman, I.A.; Masudi, S.M.; Luddin, N. Modification of glass ionomer cement by incorporating hydroxyapatite-silica nano-powder composite: Sol-gel synthesis and characterization. Ceram. Int. 2014, 40, 3165–3170. [Google Scholar] [CrossRef]
- Tabaii, E.S.; Sari, M.N. Evaluation of Shear Bond Strength of Resin Reinforced Glass Ionomer Cement Modified by Nano-hydroxyapatite on Ceramic Bracket Debonding Using Full-dimension Wire. Annu. Rev. Res. Biol. 2014, 15, 379–385. [Google Scholar] [CrossRef]
- Arita, K.; Lucas, M.E.; Nishino, M. The effect of adding hydroxyapatite on the flexural strength of glass ionomer cement. Dent. Mater. J. 2003, 22, 126. [Google Scholar] [CrossRef] [PubMed]
- Slane, J.; Vivanco, J.; Meyer, J.; Ploeg, H.L.; Squire, M. Modification of acrylic bone cement with mesoporous silica nanoparticles: Effects on mechanical, fatigue and absorption properties. J. Mech. Behav. Biomed. Mater. 2014, 29, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Horszczaruk, E.; Mijowska, E.; Cendrowski, K.; Mijowska, S.; Sikora, P. Effect of incorporation route on dispersion of mesoporous silica nanospheres in cement mortar. Constr. Build. Mater. 2014, 66, 418–421. [Google Scholar] [CrossRef]
- De Caluwe, T.; Vercruysse, C.W.; Ladik, I.; Convents, R.; Declercq, H.; Martens, L.C.; Verbeeck, R.M. Addition of bioactive glass to glass ionomer cements: Effect on the physico-chemical properties and biocompatibility. Dent. Mater. 2017, 33, e186–e203. [Google Scholar] [CrossRef] [PubMed]
- Ana, I.D.; Matsuya, S.; Ohta, M.; Ishikawa, K. Effects of added bioactive glass on the setting and mechanical properties of resin-modified glass ionomer cement. Biomaterials 2003, 24, 3061–3067. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.M.; Thomas, A.M.; Koshy, G.; Dua, K. Evaluation of the Microleakage of Chlorhexidine-Modified Glass Ionomer Cement: An in vivo Study. Int. J. Clin. Pediatr. Dent. 2013, 6, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Osorio, R.; Osorio, E.; Fuentes, V.; Prati, C.; Garc A-Godoy, F. Sorption and solubility of resin-based restorative dental materials. J. Dent. 2003, 31, 43–50. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Lin, V.S. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J. Am. Chem. Soc. 2007, 129, 8845–8849. [Google Scholar] [CrossRef] [PubMed]
- Anstice, H.M.; Nicholson, J.W.; Mccabe, J.F. The effect of using layered specimens for determination of the compressive strength of glass-ionomer cements. J. Dent. Res. 1993, 71, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds reported herein are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Yang, H.; Li, K.; Yu, J.; Huang, C. Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement. Molecules 2017, 22, 1225. https://doi.org/10.3390/molecules22071225
Yan H, Yang H, Li K, Yu J, Huang C. Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement. Molecules. 2017; 22(7):1225. https://doi.org/10.3390/molecules22071225
Chicago/Turabian StyleYan, Huiyi, Hongye Yang, Kang Li, Jian Yu, and Cui Huang. 2017. "Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement" Molecules 22, no. 7: 1225. https://doi.org/10.3390/molecules22071225
APA StyleYan, H., Yang, H., Li, K., Yu, J., & Huang, C. (2017). Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement. Molecules, 22(7), 1225. https://doi.org/10.3390/molecules22071225




