Molecular Weights of Bovine and Porcine Heparin Samples: Comparison of Chromatographic Methods and Results of a Collaborative Survey
Abstract
:1. Introduction
Summary and Aims of the Study
2. Results and Discussion
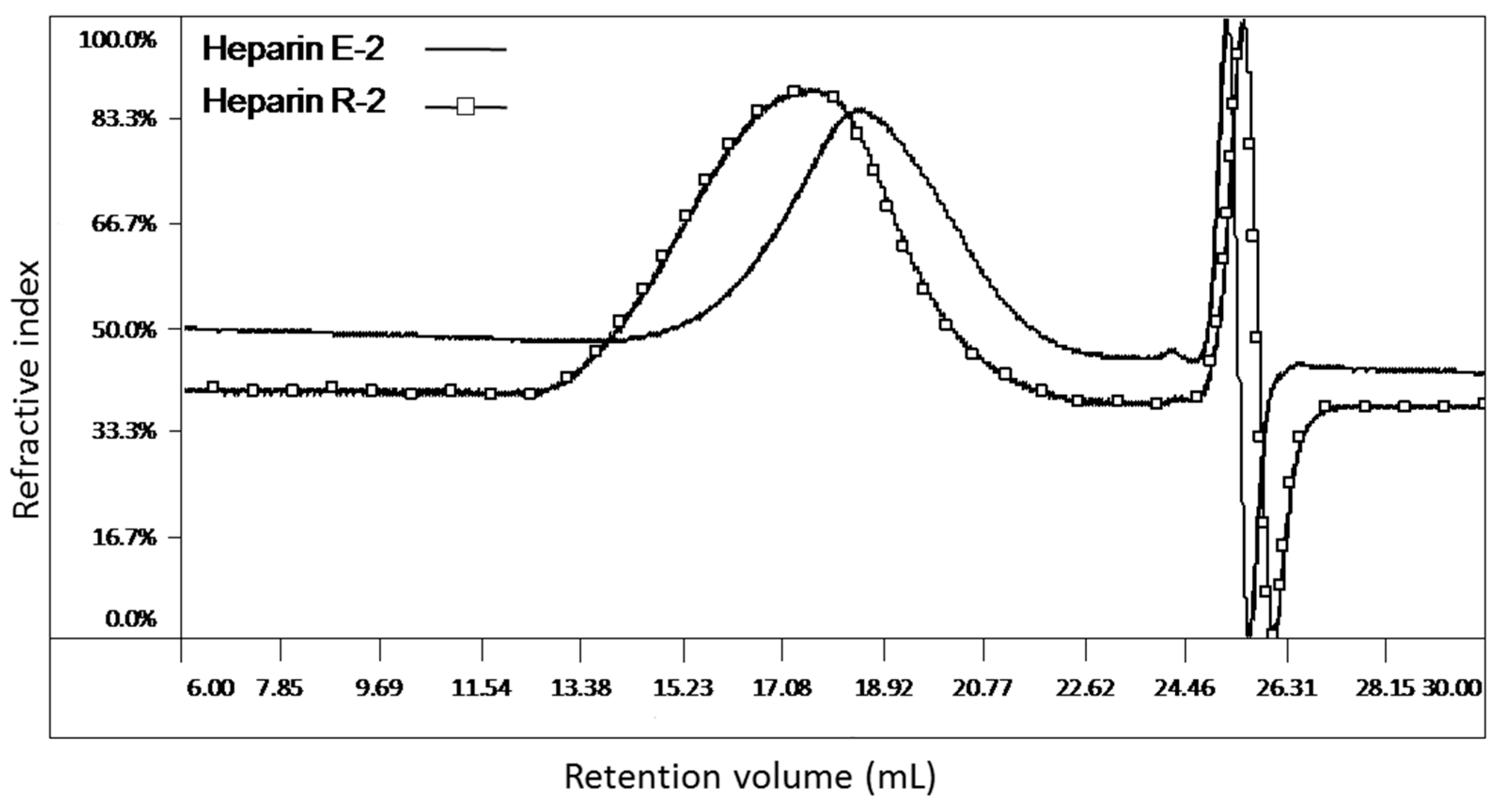
2.1. Phase 1: Collaborative Survey of Bovine and Porcine Heparin Samples
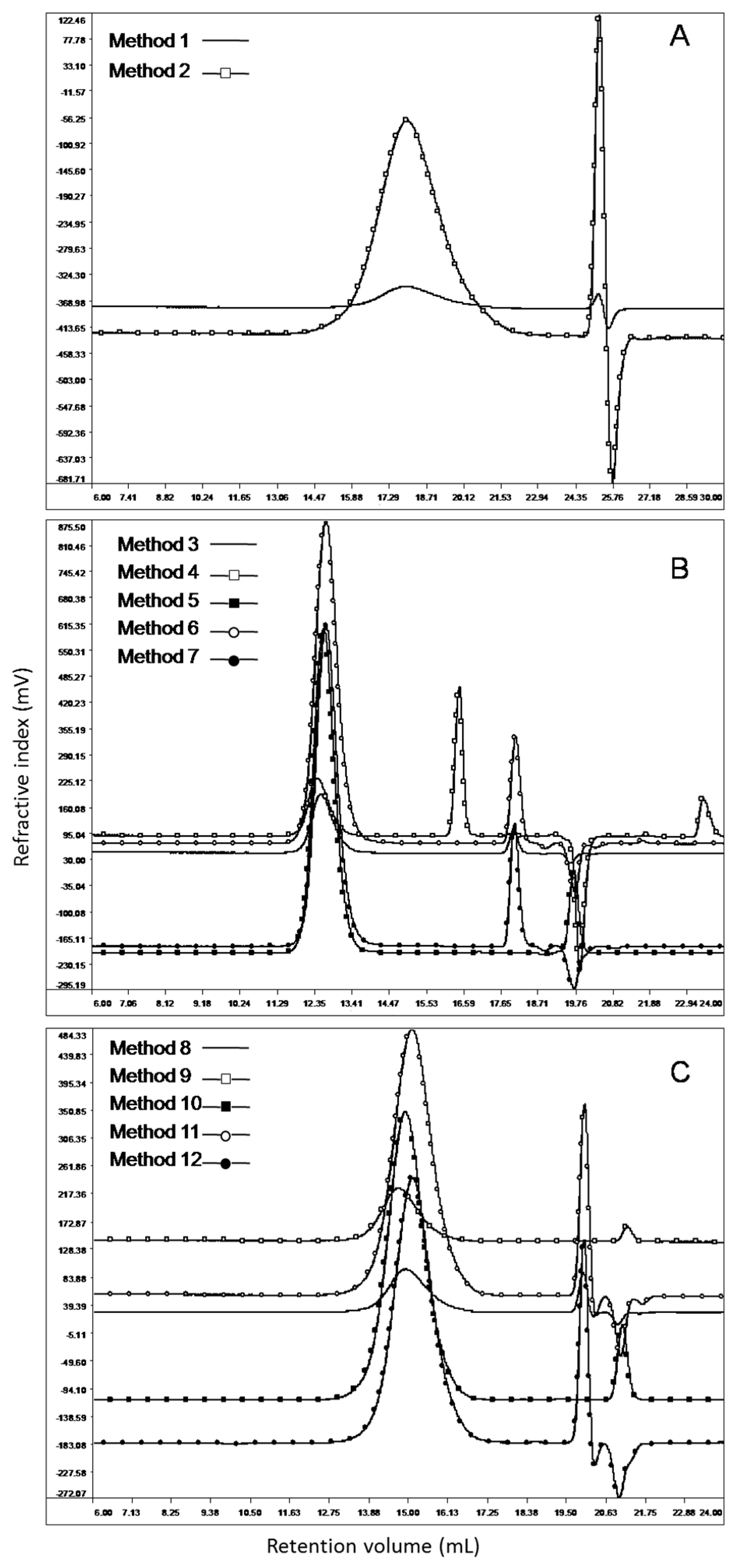
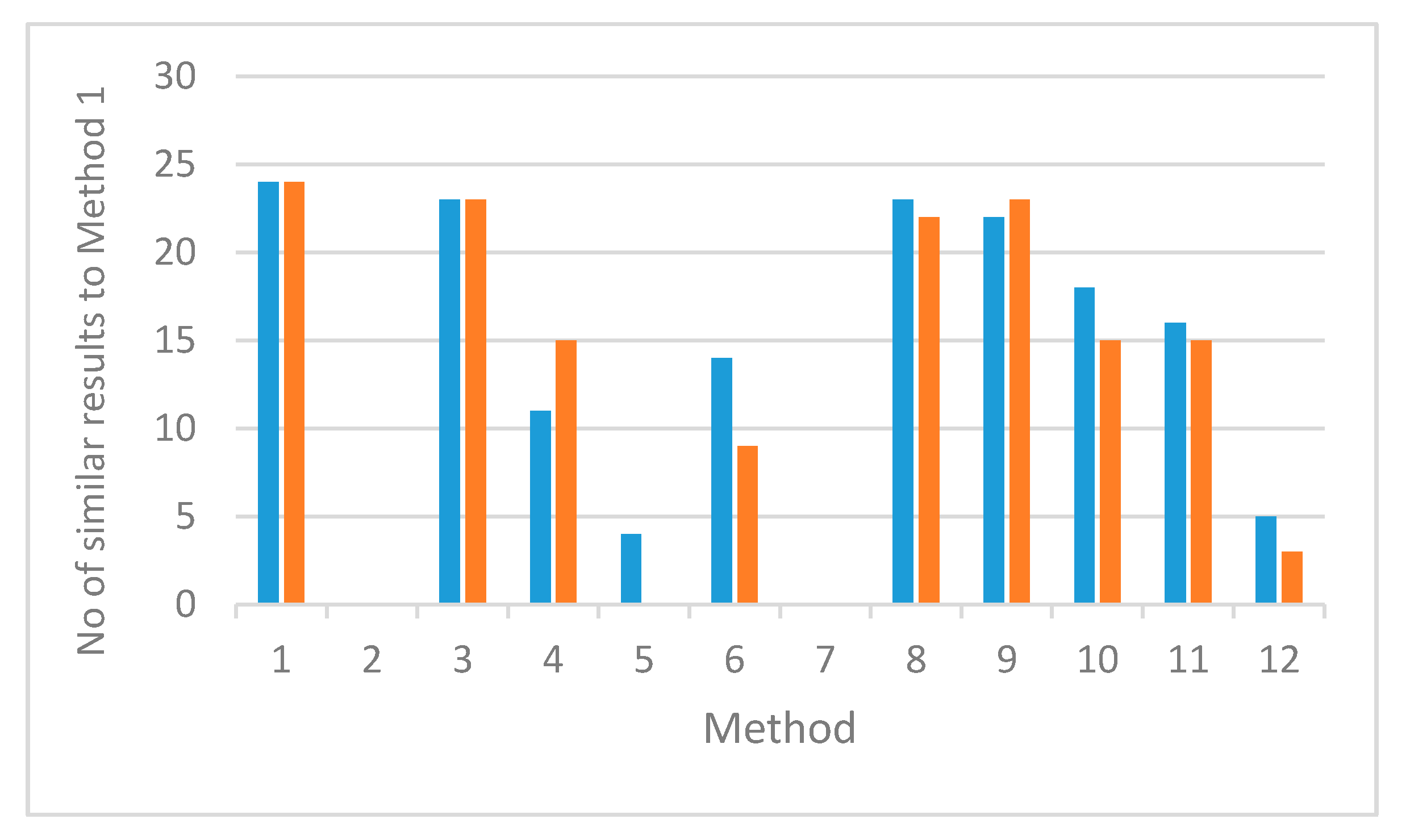
2.2. Phase 2: Comparison of Different Chromatographic Methods
3. Materials and Methods
3.1. Materials
3.2. Phase 1: The Collaborative Study
Analysis of the Chromatographic Data
3.3. Phase 2: Comparison of Different Chromatographic Methods
Analysis of the Chromatographic Data
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2016, 68, 76–141. [Google Scholar] [CrossRef] [PubMed]
- Bertini, S.; Bisio, A.; Torri, G.; Bensi, D.; Terbojevich, M. Molecular weight determination of heparin and dermatan sulfate by size exclusion chromatography with a triple detector array. Biomacromolecules 2005, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.D.; Ye, H.; Kolinski, R.E.; Nasr, M.; Buhse, L.F.; Al-Hakim, A.; Keire, D.A. Characterization of currently marketed heparin products: Analysis of molecular weight and heparinase-I digest patterns. Anal. Bioanal. Chem. 2011, 401, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. Heparin Sodium. In United States Pharmacopeia USP40-NF35; United States Pharmacopeial Convention: Rockville, MD, USA, 2017; pp. 4475–4480. [Google Scholar]
- Mulloy, B.; Heath, A.; Shriver, Z.; Jameison, F.; Al-Hakim, A.; Morris, T.S.; Szajek, A.Y. USP compendial methods for analysis of heparin: Chromatographic determination of molecular weight distributions for heparin sodium. Anal. Bioanal. Chem. 2014, 406, 4815–4823. [Google Scholar] [CrossRef] [PubMed]
- Keire, D.; Mulloy, B.; Chase, C.; Al-Hakim, A.; Cairatti, D.; Gray, E.; Hogwood, J.; Morris, T.; Mourão, P.; Soares, M.; et al. Diversifying the Global Heparin Supply Chain: Reintroduction of Bovine Heparin in the United States? Pharm. Technol. 2015, 39, 28–35. [Google Scholar]
- Santos, G.R.; Tovar, A.M.; Capille, N.V.; Pereira, M.S.; Pomin, V.H.; Mourao, P.A. Structural and functional analyses of bovine and porcine intestinal heparins confirm they are different drugs. Drug Discov. Today 2014, 19, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.M.; Santos, G.R.; Capille, N.V.; Piquet, A.A.; Glauser, B.F.; Pereira, M.S.; Vilanova, E.; Mourao, P.A. Structural and haemostatic features of pharmaceutical heparins from different animal sources: Challenges to define thresholds separating distinct drugs. Sci. Rep. 2016, 6, 35619. [Google Scholar] [CrossRef] [PubMed]
- St Ange, K.; Onishi, A.; Fu, L.; Sun, X.; Lin, L.; Mori, D.; Zhang, F.; Dordick, J.S.; Fareed, J.; Hoppensteadt, D.; et al. Analysis of Heparins Derived from Bovine Tissues and Comparison to Porcine Intestinal Heparins. Clin. Appl. Thromb. Hemost. 2016, 22, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, G.; Yang, B.; Onishi, A.; Li, L.; Sun, P.; Zhang, F.; Linhardt, R.J. Structural Characterization of Pharmaceutical Heparins Prepared from Different Animal Tissues. J. Pharm. Sci. 2013, 102, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Gray, E.; Barrowcliffe, T.W. Characterization of unfractionated heparin: Comparison of materials from the last 50 years. Thromb. Haemost. 2000, 84, 1052–1056. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Value | Chromatographic Methods (see Text and Table S5) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Mw (kDa) | 15.8 | 17.7 | 15.7 | 15.2 | 16.7 | 16.1 | 17.0 | 15.8 | 15.7 | 16.0 | 16.2 | 16.4 |
| M24,000 | 8.8 | 16.86 | 8.93 | 8.17 | 13.39 | 9.12 | 14.79 | 8.96 | 8.47 | 7.93 | 6.20 | 8.32 |
| Ratio | 1.89 | 0.99 | 1.82 | 1.97 | 1.49 | 1.64 | 1.48 | 1.72 | 1.82 | 1.37 | 1.24 | 1.15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertini, S.; Risi, G.; Guerrini, M.; Carrick, K.; Szajek, A.Y.; Mulloy, B. Molecular Weights of Bovine and Porcine Heparin Samples: Comparison of Chromatographic Methods and Results of a Collaborative Survey. Molecules 2017, 22, 1214. https://doi.org/10.3390/molecules22071214
Bertini S, Risi G, Guerrini M, Carrick K, Szajek AY, Mulloy B. Molecular Weights of Bovine and Porcine Heparin Samples: Comparison of Chromatographic Methods and Results of a Collaborative Survey. Molecules. 2017; 22(7):1214. https://doi.org/10.3390/molecules22071214
Chicago/Turabian StyleBertini, Sabrina, Giulia Risi, Marco Guerrini, Kevin Carrick, Anita Y. Szajek, and Barbara Mulloy. 2017. "Molecular Weights of Bovine and Porcine Heparin Samples: Comparison of Chromatographic Methods and Results of a Collaborative Survey" Molecules 22, no. 7: 1214. https://doi.org/10.3390/molecules22071214
APA StyleBertini, S., Risi, G., Guerrini, M., Carrick, K., Szajek, A. Y., & Mulloy, B. (2017). Molecular Weights of Bovine and Porcine Heparin Samples: Comparison of Chromatographic Methods and Results of a Collaborative Survey. Molecules, 22(7), 1214. https://doi.org/10.3390/molecules22071214





