Prediction of Antimicrobial and Antioxidant Activities of Mexican Propolis by 1H-NMR Spectroscopy and Chemometrics Data Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction, Antioxidant and Antibacterial Activities
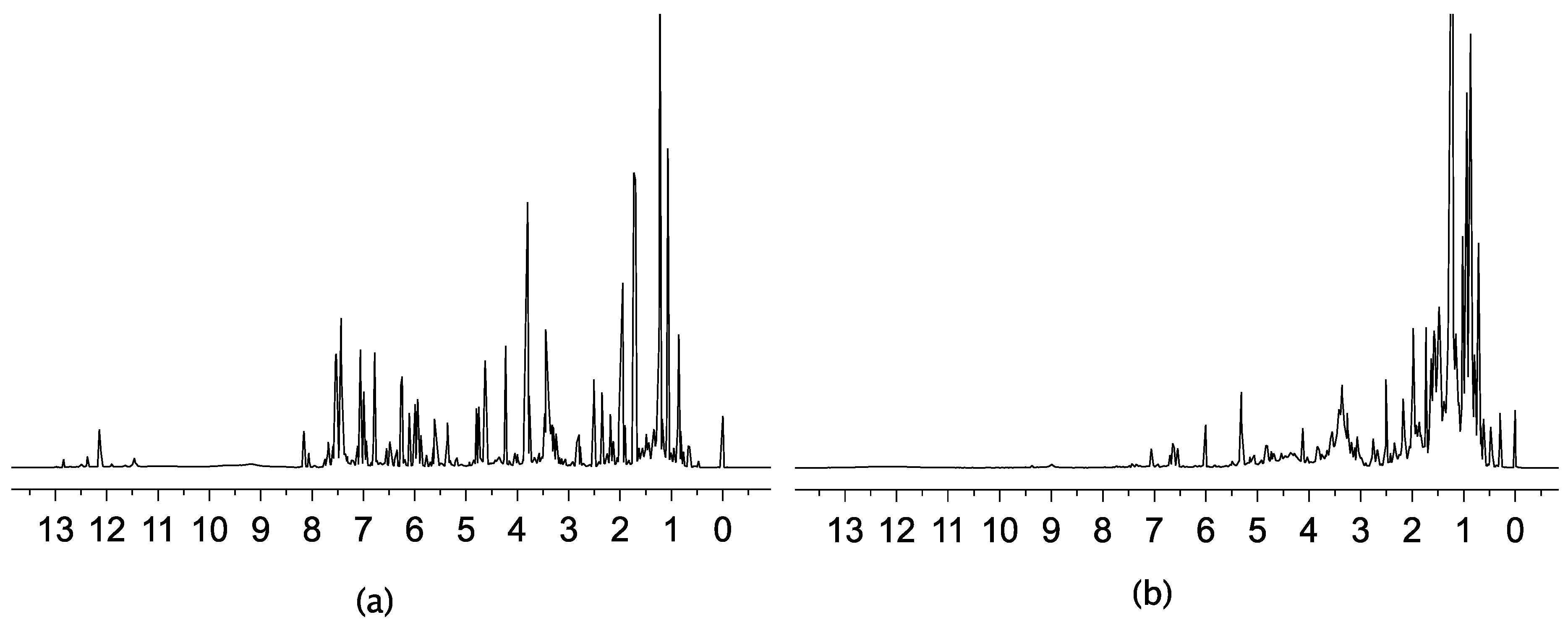
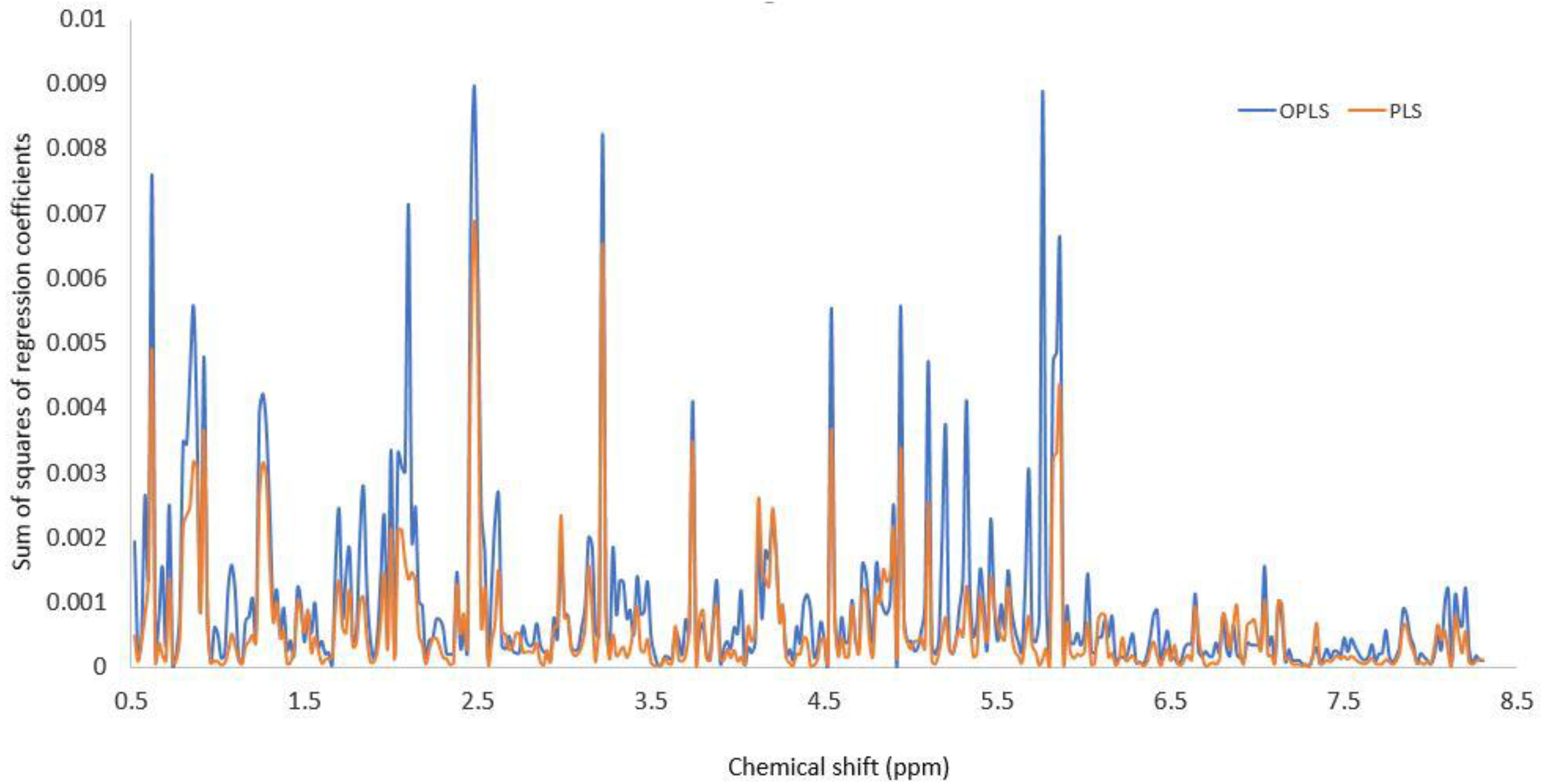
2.2. 1H-NMR
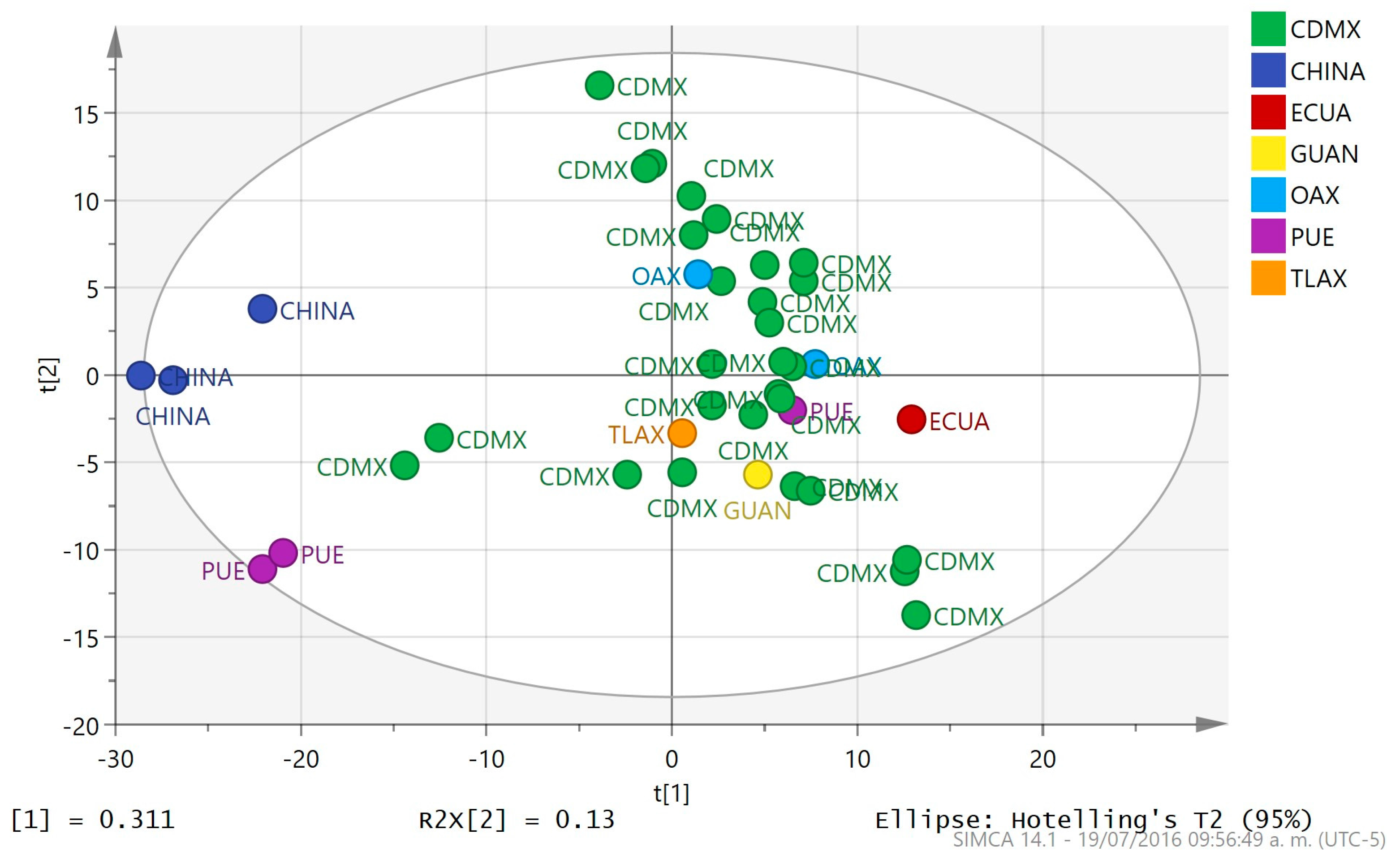
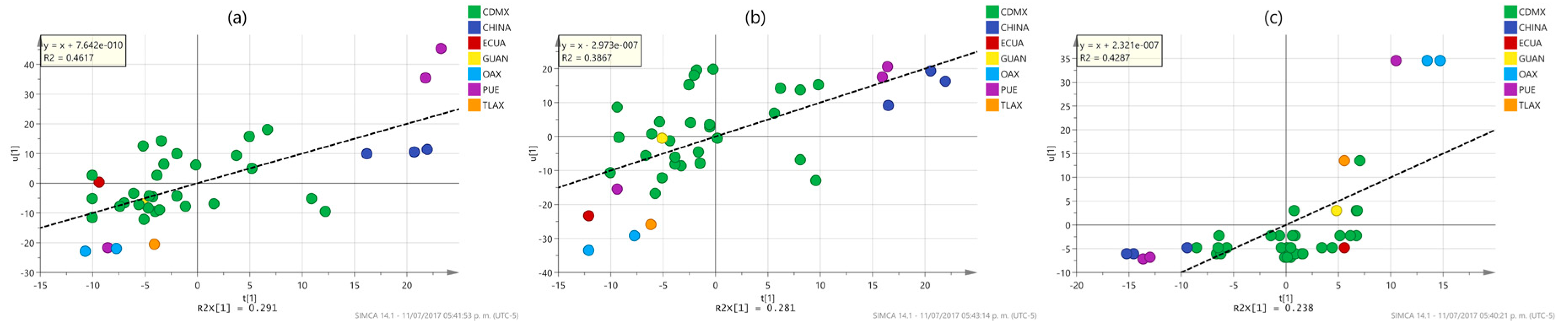
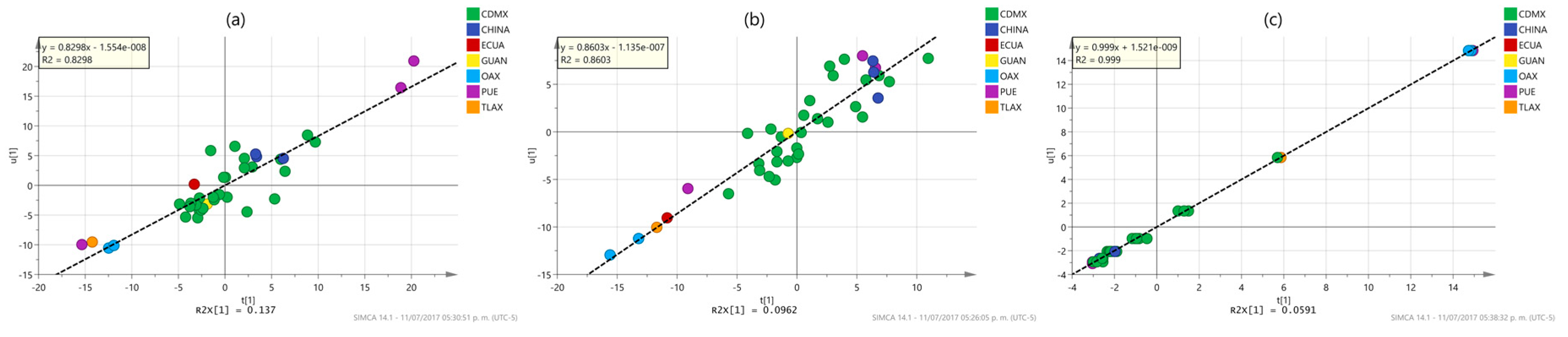
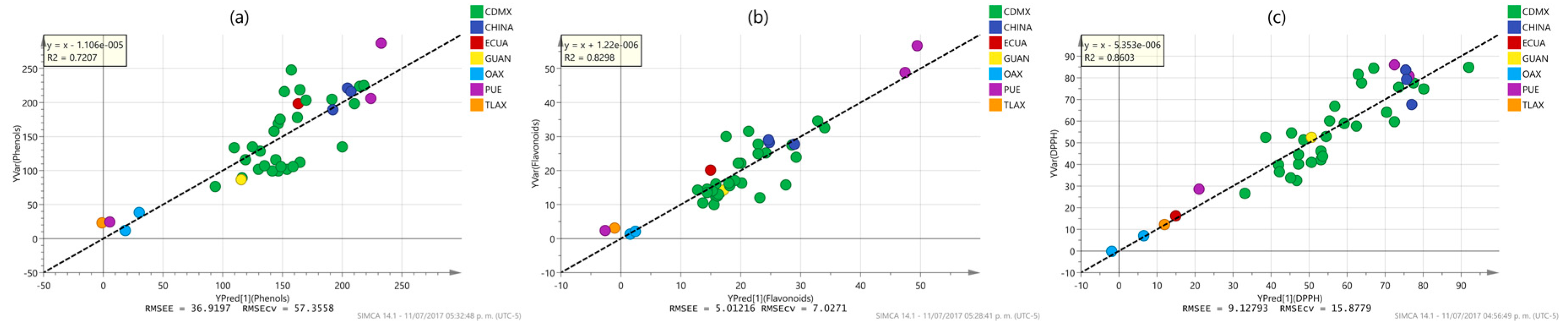
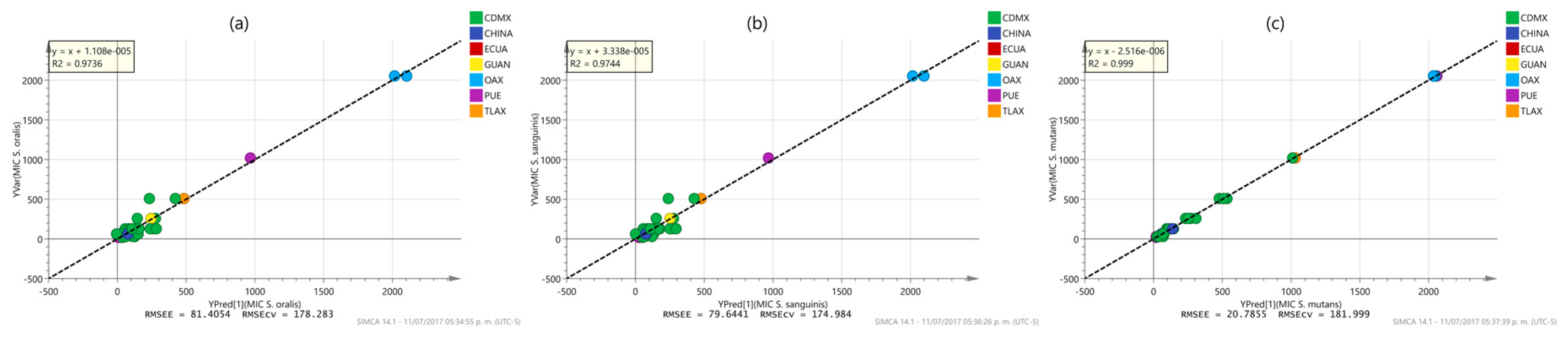
2.3. Multivariate Analysis
3. Materials and Methods
3.1. Samples
3.2. Chemicals and Reagents
3.3. Extract Preparation
3.4. DPPH Radical Scavenging Assay
3.5. Total Phenolic Content
3.6. Total Flavonoid Content
3.7. Determination of Minimum Inhibitory Concentration (MIC)
3.8. NMR Experiments
3.9. Data Processing for Multivariate Analysis
3.10. Multivariate Analysis
3.11. Figures of Merit
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sawaya, A.C.H.F.; Abdelnur, P.V.; Eberlin, M.N.; Kumazawa, S.; Ahn, M.-R.; Bang, K.-S.; Nagaraja, N.; Bankova, V.S.; Afrouzan, H. Fingerprinting of propolis by easy ambient sonic-spray ionization mass spectrometry. Talanta 2010, 81, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Omar, R.; Siheri, W.; Al Mutairi, S.; Clements, C.; Fearnley, J.; Edrada-Ebel, R.; Watson, D. Chromatographic analysis with different detectors in the chemical characterisation and dereplication of African propolis. Talanta 2014, 120, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Valencia, D.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Galvez-Ruiz, J.C.; Salas-Reyes, M.; Jiménez-Estrada, M.; Velazquez-Contreras, E.; Hernandez, J.; Velazquez, C. Seasonal effect on chemical composition and biological activities of Sonoran propolis. Food Chem. 2012, 131, 645–651. [Google Scholar] [CrossRef]
- Falcao, S.I.; Vilas-Boas, M.; Estevinho, L.M.; Barros, C.; Domingues, M.R.M.; Cardoso, S.M. Phenolic characterization of Northeast Portuguese propolis: Usual and unusual compounds. Anal. Bioanal. Chem. 2010, 396, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Fernandes-Silva, C.C.; Righi, A.A.; Salatino, M.L. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and functional characterization of Italian propolis obtained by different harvesting methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Papotti, G.; Bertelli, D.; Plessi, M.; Rossi, M.C. Use of HR-NMR to classify propolis obtained using different harvesting methods. Int. J. Food Sci. Technol. 2010, 45, 1610–1618. [Google Scholar] [CrossRef]
- Nina, N.; Quispe, C.; Jimenez-Aspee, F.; Theoduloz, C.; Feresin, G.E.; Lima, B.; Leiva, E.; Schmeda-Hirschmann, G. Antibacterial activity, antioxidant effect and chemical composition of propolis from the Región del Maule, Central Chile. Molecules 2015, 20, 18144–18167. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Barra, G.; Castro, C.; Figueroa, C.; Barriga, A.; Silva, X.; de Las Heras, B.; Hortelano, S.; Delporte, C. Anti-inflammatory activity and phenolic profile of propolis from two locations in Region Metropolitana de Santiago, Chile. J. Ethnopharmacol. 2015, 168, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, C.; Navarro, M.; Acosta, A.; Angulo, A.; Dominguez, Z.; Robles, R.; Robles-Zepeda, R.; Lugo, E.; Goycoolea, F.M.; Velazquez, E.F.; et al. Antibacterial and free-radical scavenging activities of Sonoran propolis. J. Appl. Microbiol. 2007, 103, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Navarro, M.; Ruiz-Bustos, P.; Valencia, D.; Robles-Zepeda, R.; Ruiz-Bustos, E.; Virues, C.; Hernandez, J.; Dominguez, Z.; Velazquez, C. Antibacterial activity of Sonoran propolis and some of its constituents against clinically significant Vibrio species. Foodborne Pathog. Dis. 2013, 10, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Buratti, S.; Benedetti, S.; Cosio, M.S. Evaluation of the antioxidant power of honey, propolis and royal jelly by amperometric flow injection analysis. Talanta 2007, 71, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dong, Y.; Du, H.; Shi, H.; Peng, Y.; Li, X. Antioxidant compounds from propolis collected in Anhui, China. Molecules 2011, 16, 3444–3455. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Scaglianti, M.; Pietta, P.; Simonetti, P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, D.; Papotti, G.; Bortolotti, L.; Marcazzan, G.L.; Plessi, M. 1H-NMR Simultaneous Identification of Health-Relevant Compounds in Propolis Extracts. Phytochem. Anal. 2012, 23, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Universal Quantitative NMR Analysis of Complex Natural Samples. Curr. Opin. Biotechnol. 2014, 25, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cuny, M.; Vigneau, E.; Le Gall, G.; Colquhoun, I.; Lees, M.; Rutledge, D.N. Fruit juice authentication by 1H NMR spectroscopy in combination with different chemometrics tools. Anal. Bioanal. Chem. 2008, 390, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Anđelković, B.; Vujisić, L.; Vučković, I.; Tešević, V.; Vajs, V.; Gođevac, D. Metabolomics study of Populus type propolis. J. Pharm. Biomed. Anal. 2017, 135, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.G.; Peyfoon, E.; Zheng, L.; Lu, D.; Seidel, V.; Johnston, B.; Parkinson, J.A.; Fearnley, J. Application of principal components analysis to 1H-NMR data obtained from propolis samples of different geographical origin. Phytochem. Anal. 2006, 17, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Maraschin, M.; Somensi-Zeggio, A.; Oliveira, S.K.; Kuhnen, S.; Tomazzoli, M.M.; Zeri, A.C.M.; Carreira, R.; Rocha, M. A machine learning and chemometrics assisted interpretation of spectroscopic data—A NMR-based metabolomics platform for the assessment of brazilian propolis. In Lecture Notes in Computer Science, Proceedings of the IAPR International Conference on Pattern Recognition in Bioinformatics, Tokyo, Japan, 8–10 November 2012; Springer: Berlin, Germany, 2012; Volume 7632, pp. 129–140. [Google Scholar]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Bowen, W.H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Moore, J.; Yu, L. High-Throughput Relative DPPH Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Marquele, F.D.; Di Mambro, V.M.; Georgetti, S.R.; Casagrande, R.; Valim, Y.M.L.; Fonseca, M.J.V. Assessment of the antioxidant activities of Brazilian extracts of propolis alone and in topical pharmaceutical formulations. J. Pharm. Biomed. Anal. 2005, 39, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests, 4th ed.; Approved Standards, M7-A4; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Cai, L. Compounds from Syzygium aromaticum Possessing Growth Inhibitory Activity Against Oral Pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Olivieri Alejandro, C.; Faber Nicolaas, M.; Ferré, J.; Boqué, R.; Kalivas John, H.; Mark, H. Uncertainty estimation and figures of merit for multivariate calibration (IUPAC Technical Report). Pure Appl. Chem. 2006, 78, 633. [Google Scholar]
- De Carvalho Rocha, W.F.; Nogueira, R.; Vaz, B.G. Validation of model of multivariate calibration: An application to the determination of biodiesel blend levels in diesel by near-infrared spectroscopy. J. Chemometrics. 2012, 26, 456–461. [Google Scholar] [CrossRef]
- Braga, J.W.B.; Trevizan, L.C.; Nunes, L.C.; Rufini, I.A.; Santos, D., Jr.; Krug, F.J. Comparison of univariate and multivariate calibration for the determination of micronutrients in pellets of plant materials by laser induced breakdown spectrometry. Spectrochim. Acta B 2010, 65, 66–74. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Source of Sample | Date of Harvesting | Harvesting Method | Total Phenols a | Total Flavonoids b | % DPPH c | MIC (µg/mL) | ||
|---|---|---|---|---|---|---|---|---|
| S. oralis | S. sanguinis | S. mutans | ||||||
| Mexico City (CDMX) | ||||||||
| Tlalpan | ||||||||
| Topilejo 1 | September 2011 | plastic nets | 112.7 | 15.7 | 42.3 | 128 | 128 | 256 |
| Topilejo 2 | October 2011 | plastic nets | 116 | 24.9 | 46.1 | 128 | 128 | 256 |
| Topilejo 3 | November 2011 | plastic nets | 106.7 | 12.6 | 40.2 | 32 | 32 | 64 |
| Topilejo 4 | October 2012 | plastic nets | 134.1 | 14.2 | 52.5 | 128 | 128 | 256 |
| Topilejo 5 | October 2013 | plastic nets | 101.3 | 13.6 | 40.8 | 128 | 128 | 256 |
| Topilejo 6 | October 2014 | plastic nets | 106.3 | 12.1 | 32.6 | 128 | 128 | 128 |
| Xochimilco | ||||||||
| San Luis Tlaxialtemalco 1 | October 2011 | scraping | 99.7 | 22.1 | 66.8 | 512 | 512 | 512 |
| San Luis Tlaxialtemalco 2 | November 2011 | scraping | 157.3 | 15.6 | 52.9 | 128 | 128 | 256 |
| San Luis Tlaxialtemalco 3 | October 2012 | scraping | 76.4 | 14.1 | 26.6 | 512 | 512 | 1024 |
| San Luis Tlaxialtemalco 4 | October 2013 | scraping | 175.9 | 17.1 | 60.2 | 64 | 128 | 128 |
| San Luis Tlaxialtemalco 5 | October 2013 | plastic nets | 116.4 | 14.5 | 54.6 | 128 | 128 | 256 |
| San Luis Tlaxialtemalco 6 | November 2013 | plastic nets | 134.6 | 13.5 | 44.6 | 128 | 128 | 256 |
| Milpa Alta | ||||||||
| San Pablo Oztotepec 1 | October 2011 | plastic nets | 101.3 | 25.2 | 59.6 | 128 | 128 | 128 |
| San Pablo Oztotepec 2 | October 2011 | scraping | 247.3 | 27.8 | 84.4 | 32 | 32 | 32 |
| San Pablo Oztotepec 3 | November 2012 | plastic nets | 128.9 | 12.2 | 39.6 | 128 | 128 | 256 |
| San Pablo Oztotepec 4 | October 2011 | scraping | 223.2 | 34.6 | 77.5 | 32 | 32 | 64 |
| San Pablo Oztotepec 5 | November 2012 | scraping | 225.4 | 32.6 | 75.6 | 32 | 32 | 64 |
| San Pablo Oztotepec 6 | October 2011 | plastic nets | 135.2 | 27.5 | 64 | 64 | 64 | 128 |
| San Pablo Oztotepec 7 | October 2012 | scraping | 203.8 | 31.5 | 81.7 | 16 | 16 | 32 |
| San Pablo Oztotepec 8 | October 2013 | plastic nets | 204.3 | 16.3 | 84.8 | 32 | 32 | 64 |
| San Pablo Oztotepec 9 | October 2012 | scraping | 218.9 | 22.1 | 77.5 | 32 | 32 | 32 |
| San Pablo Oztotepec 10 | November 2012 | scraping | 178.3 | 16.3 | 57.5 | 64 | 128 | 128 |
| San Pablo Oztotepec 11 | October 2013 | scraping | 168.6 | 16.1 | 58.8 | 64 | 64 | 128 |
| San Antonio Tecomitl 1 | November 2013 | scraping | 198.5 | 23.9 | 75 | 32 | 64 | 128 |
| San Antonio Tecomitl 2 | October 2011 | scraping | 215.7 | 30 | 51.2 | 32 | 64 | 64 |
| San Antonio Tecomitl 3 | October 2011 | plastic nets | 99.6 | 10.1 | 33.8 | 256 | 256 | 512 |
| San Antonio Tecomitl 4 | October 2013 | plastic nets | 105.3 | 13.1 | 43.6 | 64 | 64 | 128 |
| San Antonio Tecomitl 5 | October 2012 | plastic nets | 89.8 | 10.5 | 36.5 | 256 | 256 | 512 |
| Puebla (PUE) | ||||||||
| Valsequillo 1 | October 2011 | wooden wedges | 205.6 | 48.7 | 80.9 | 16 | 32 | 32 |
| Valsequillo 2 | October 2012 | scraping | 287.2 | 56.7 | 86 | 16 | 16 | 16 |
| Cuetzalan | October 2011 | scraping | 24.7 | 2.3 | 28.7 | 1024 | 1024 | 2048 |
| Oaxaca (OAX) | ||||||||
| Pinotepa Nacional 1 | October 2011 | scraping | 38.5 | 2.1 | 7.1 | 2048 | 2048 | 2048 |
| Pinotepa Nacional 2 | October 2012 | scraping | 12 | 1.3 | 0 | 2048 | 2048 | 2048 |
| Guanajuato (GUAN) | ||||||||
| Silao | October 2012 | plastic nets | 87.3 | 14.2 | 52.4 | 256 | 256 | 512 |
| Tlaxcala (TLAX) | ||||||||
| Tlaxcala | October 2011 | scraping | 23.5 | 3.1 | 12.1 | 512 | 512 | 1024 |
| South America (ECUA) | ||||||||
| Quito/Ecuador | 2011 | 198.3 | 20.1 | 16.3 | 64 | 128 | 128 | |
| China (CHINA) | ||||||||
| China 1 * | 2013 | 221.7 | 28.3 | 83.7 | 64 | 64 | 64 | |
| China 2 * | 2013 | 215.6 | 29.1 | 79.1 | 64 | 64 | 64 | |
| China 3 * | 2012 | 189.9 | 27.8 | 67.8 | 64 | 64 | 128 | |
| Positive control † | 0.12 | 0.12 | 0.24 | |||||
| Property | Number of Latent Variables | Regression Equation a | R2 | R2X (cum) | R2Y (cum) | Q2 (cum) | RMSEC | RMSECV |
|---|---|---|---|---|---|---|---|---|
| PLS | ||||||||
| Phenol content | 2 | x − 1.397 × 10−6 | 0.6003 | 0.400 | 0.600 | 0.263 | 43.548 | 56.086 |
| Flavonoid content | 2 | x + 1.212 × 10−7 | 0.7204 | 0.397 | 0.720 | 0.482 | 6.334 | 8.305 |
| DPPH | 2 | x + 1.61 × 10−7 | 0.7110 | 0.388 | 0.711 | 0.325 | 12.760 | 18.511 |
| MIC (S. oralis) | 3 | x + 1.165 × 10−5 | 0.9411 | 0.460 | 0.941 | 0.778 | 118.005 | 244.768 |
| MIC (S. sanguinis) | 3 | x + 7.404 × 10−6 | 0.9434 | 0.460 | 0.943 | 0.784 | 115.076 | 241.684 |
| MIC (S. mutants) | 3 | x + 2.033 × 10−6 | 0.9245 | 0.463 | 0.925 | 0.696 | 156.141 | 322.235 |
| OPLS | ||||||||
| Phenol content | 1 + 2+ 0 | x − 1.106 × 10−5 | 0.7207 | 0.472 | 0.721 | 0.249 | 36.920 | 57.356 |
| Flavonoid content | 1 + 2 + 0 | x + 1.22 × 10−6 | 0.8298 | 0.463 | 0.830 | 0.627 | 5.012 | 7.027 |
| DPPH | 1 + 3 + 0 | x − 5.353 × 10−6 | 0.8603 | 0.542 | 0.860 | 0.515 | 9.128 | 15.878 |
| MIC (S. oralis) | 1 + 4 + 0 | x + 1.108 × 10−5 | 0.9736 | 0.596 | 0.974 | 0.850 | 81.405 | 178.283 |
| MIC (S. sanguinis) | 1 + 4 + 0 | x + 3.338 × 10−5 | 0.9744 | 0.595 | 0.974 | 0.854 | 79.644 | 174.984 |
| MIC (S. mutants) | 1 + 12 + 0 | x − 2516 × 10−6 | 0.9990 | 0.861 | 0.999 | 0.886 | 20.785 | 181.999 |
| Figure of Merit | Phenol Content | Flavonoid Content | DPPH | MIC (S. oralis) | MIC (S. sanguinis) | MIC (S. mutants) |
|---|---|---|---|---|---|---|
| PLS | ||||||
| LD | 0.37 mg GAE g EEP−1 | 0.35 mg QE g EEP−1 | 0.40% | 0.51 µg mL−1 | 0.51 µg mL−1 | 0.53 µg mL−1 |
| LC | 1.12 mg GAE g EEP−1 | 1.08 mg QE g EEP−1 | 1.21% | 1.55 µg mL−1 | 1.55 µg mL−1 | 1.61 µg mL−1 |
| Evaluated linearity | 12–287.20 mg GAE g EEP−1 | 1.30–56.70 mg QE g EEP−1 | 0.40–86% | 16–2048 µg mL−1 | 16–2048 µg mL−1 | 16–2048 µg mL−1 |
| Sens | 39.93 g EEP mg GAE−1 | 41.39 g EEP mg QE−1 | 36.80%−1 | 25.23 mL µg−1 | 25.27 mL µg−1 | 25.89 mL µg−1 |
| Sel | 0.87 | 0.87 | 0.86 | 0.79 | 0.79 | 0.79 |
| γ | 8.94 g EEP mg GAE−1 | 9.28 g EEP mg QE−1 | 8.28%−1 | 6.45 mL µg−1 | 6.46 mL µg−1 | 6.19 mL µg−1 |
| γ−1 | 0.11 mg GAE g EEP−1 | 0.11 mg QE g EEP−1 | 0.12% | 0.15 µg mL−1 | 0.15 µg mL−1 | 0.16 µg mL−1 |
| OPLS | ||||||
| LD | 0.45 mg GAE g EEP−1 | 0.39 mg QE g EEP−1 | 0.49% | 0.67 µg mL−1 | 0.66 µg mL−1 | 0.66 µg mL−1 |
| LC | 1.38 mg GAE g EEP−1 | 1.20 mg QE g EEP−1 | 1.50% | 2.03 µg mL−1 | 1.86 µg mL−1 | 1.99 µg mL−1 |
| Evaluated linearity | 12–287.20 mg GAE g EEP−1 | 1.30–56.70 mg QE g EEP−1 | 0.40–86% | 16–2048 µg mL−1 | 16–2048 µg mL−1 | 16–2048 µg mL−1 |
| Sens | 40.34 g EEP mg GAE−1 | 45.12 g EEP mg QE−1 | 37.76%−1 | 28.92 mL µg−1 | 29.13 mL µg−1 | 29.59 mL µg−1 |
| Sel | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| γ | 7.24 g EEP mg GAE−1 | 8.35 g EEP mg QE−1 | 6.66%−1 | 4.92 mL µg−1 | 4.95 mL µg−1 | 5.03 mLµg−1 |
| γ−1 | 0.14 mg GAE g EEP−1 | 0.12 mg QE g EEP−1 | 0.15% | 0.20 µg mL−1 | 0.20 µg mL−1 | 0.20 µg mL−1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Cruz, J.F.; Rodríguez de San Miguel, E.; Robles-Obregón, S.; Hernández-Espino, C.C.; Rivero-Cruz, B.E.; Pedraza-Chaverri, J.; Esturau-Escofet, N. Prediction of Antimicrobial and Antioxidant Activities of Mexican Propolis by 1H-NMR Spectroscopy and Chemometrics Data Analysis. Molecules 2017, 22, 1184. https://doi.org/10.3390/molecules22071184
Rivero-Cruz JF, Rodríguez de San Miguel E, Robles-Obregón S, Hernández-Espino CC, Rivero-Cruz BE, Pedraza-Chaverri J, Esturau-Escofet N. Prediction of Antimicrobial and Antioxidant Activities of Mexican Propolis by 1H-NMR Spectroscopy and Chemometrics Data Analysis. Molecules. 2017; 22(7):1184. https://doi.org/10.3390/molecules22071184
Chicago/Turabian StyleRivero-Cruz, J. Fausto, Eduardo Rodríguez de San Miguel, Sergio Robles-Obregón, Circe C. Hernández-Espino, Blanca E. Rivero-Cruz, José Pedraza-Chaverri, and Nuria Esturau-Escofet. 2017. "Prediction of Antimicrobial and Antioxidant Activities of Mexican Propolis by 1H-NMR Spectroscopy and Chemometrics Data Analysis" Molecules 22, no. 7: 1184. https://doi.org/10.3390/molecules22071184
APA StyleRivero-Cruz, J. F., Rodríguez de San Miguel, E., Robles-Obregón, S., Hernández-Espino, C. C., Rivero-Cruz, B. E., Pedraza-Chaverri, J., & Esturau-Escofet, N. (2017). Prediction of Antimicrobial and Antioxidant Activities of Mexican Propolis by 1H-NMR Spectroscopy and Chemometrics Data Analysis. Molecules, 22(7), 1184. https://doi.org/10.3390/molecules22071184








