Characterization and Biological Evaluation of Propolis from Poland
Abstract
:1. Introduction
2. Results and Discussion
2.1. Propolis Composition
2.2. Antimicrobial Activity
2.3. Antiproliferative Activity
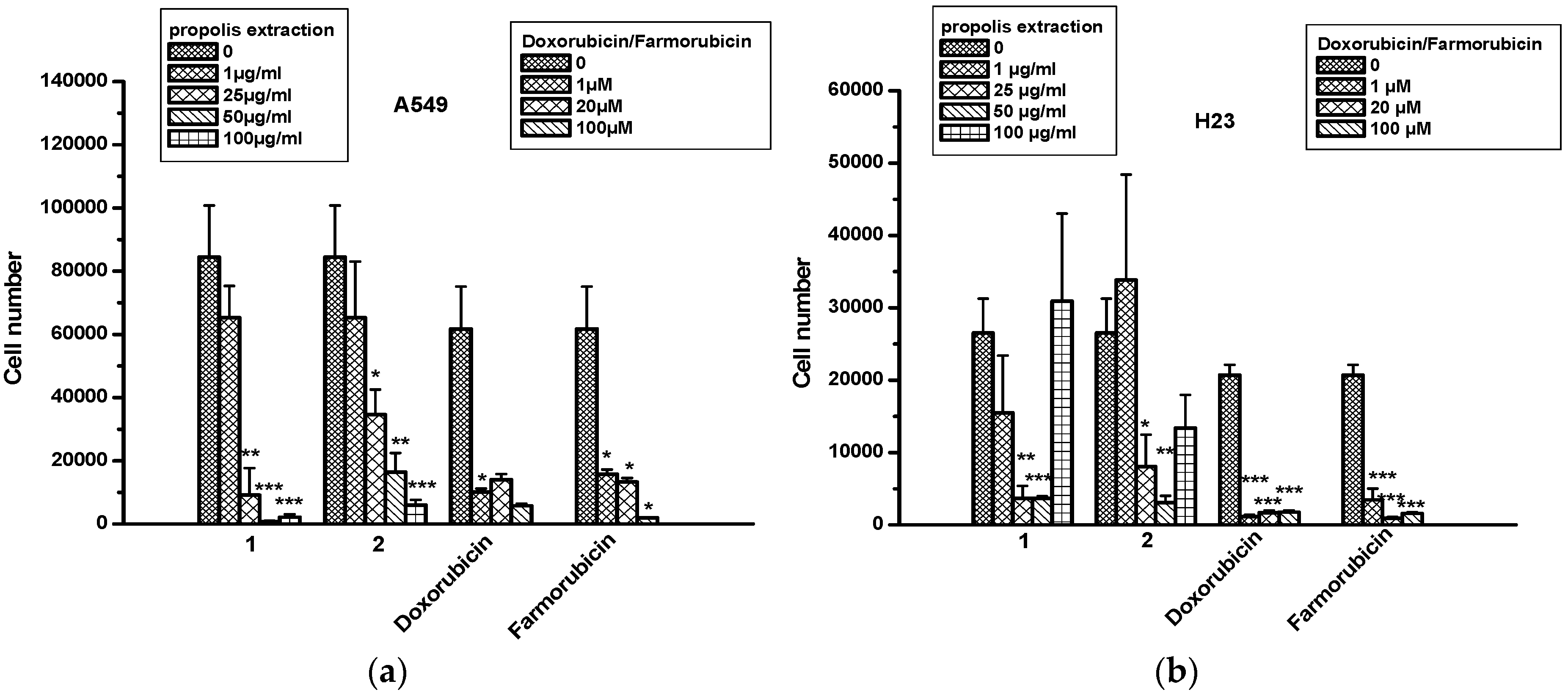
2.4. The Effect of Propolis Extracts in Lung Cancer Cell Proliferation and Toxicity
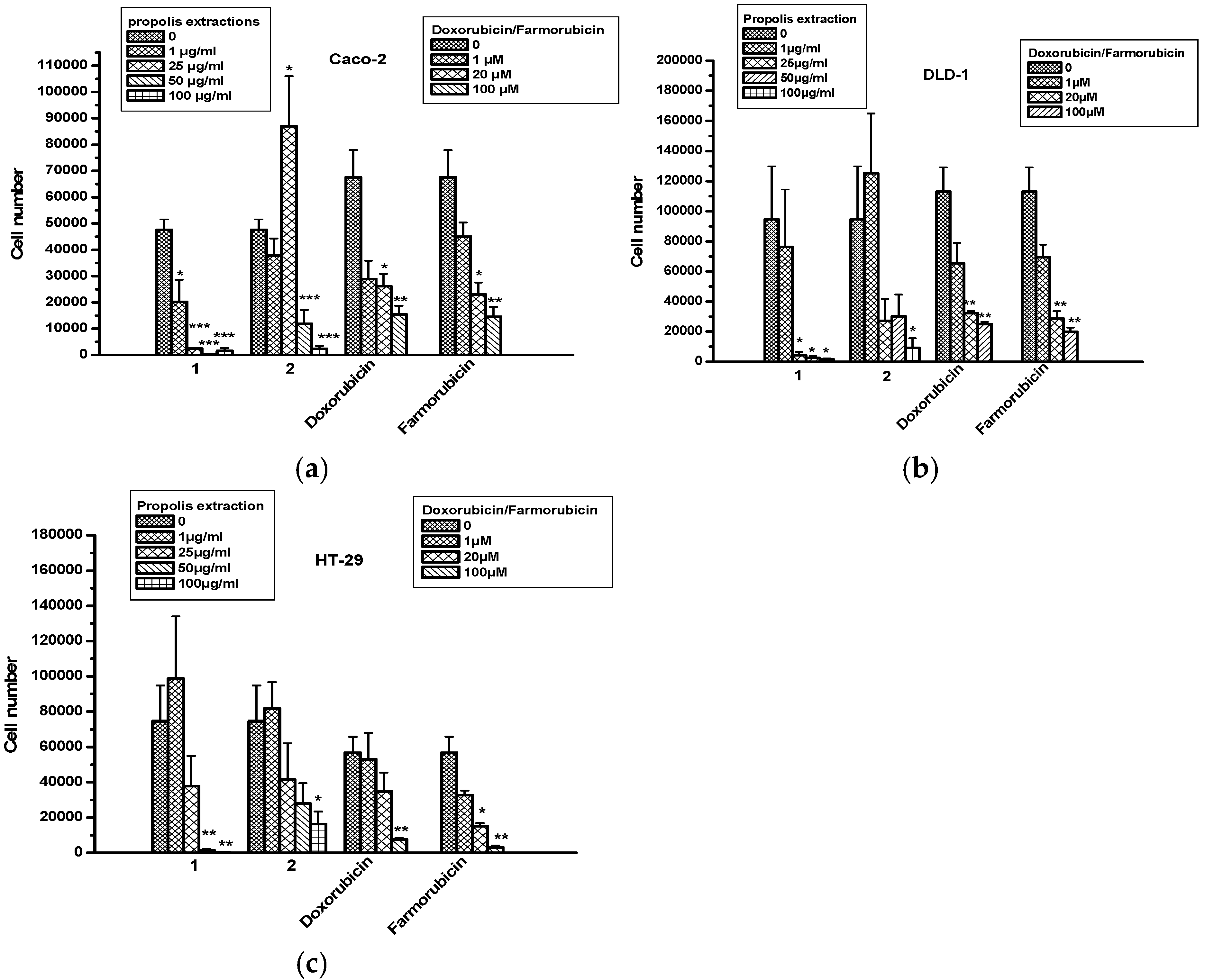
2.5. The Effect of Propolis Extracts in Colon Cancer Cell Proliferation and Toxicity
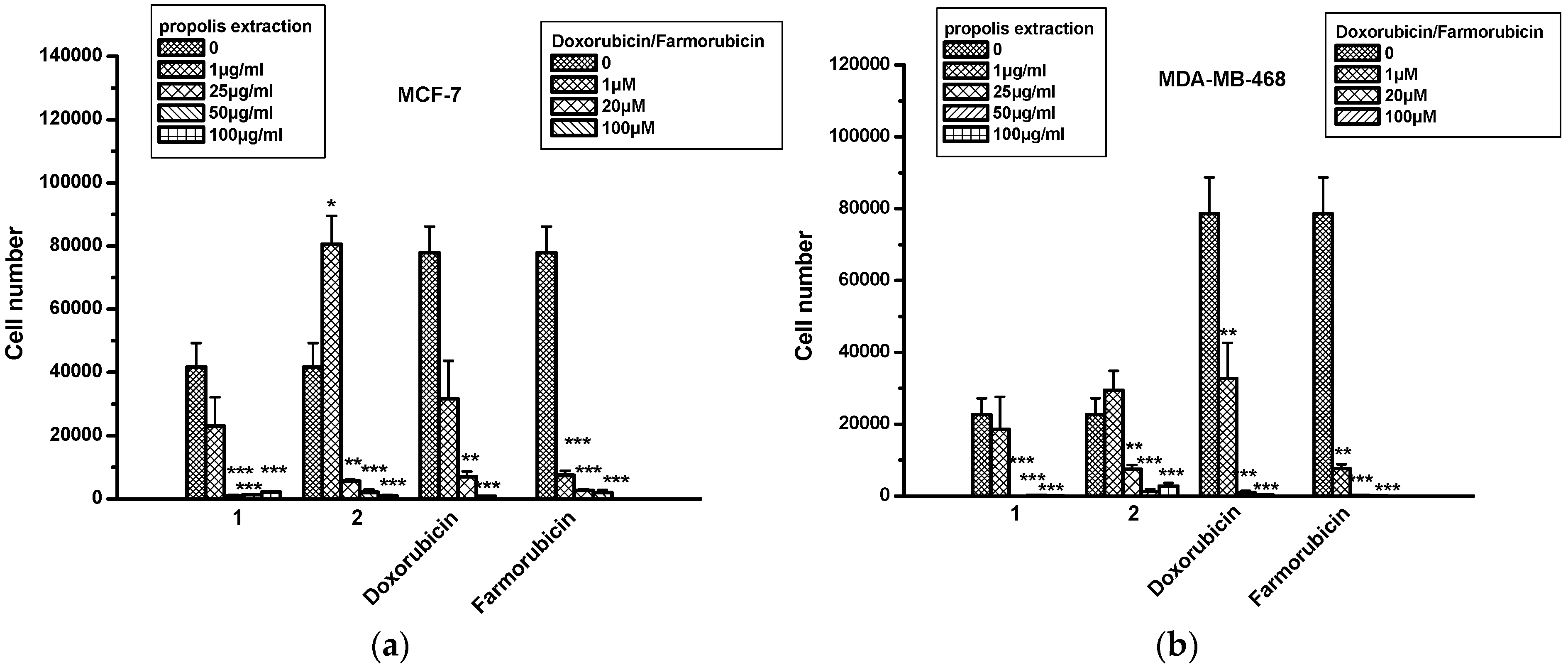
2.6. The Effect of Propolis Extracts in Breast Cancer Cell Proliferation and Toxicity
2.7. The Effect of Propolis Extracts in Glioblastoma Cell Proliferation and Toxicity
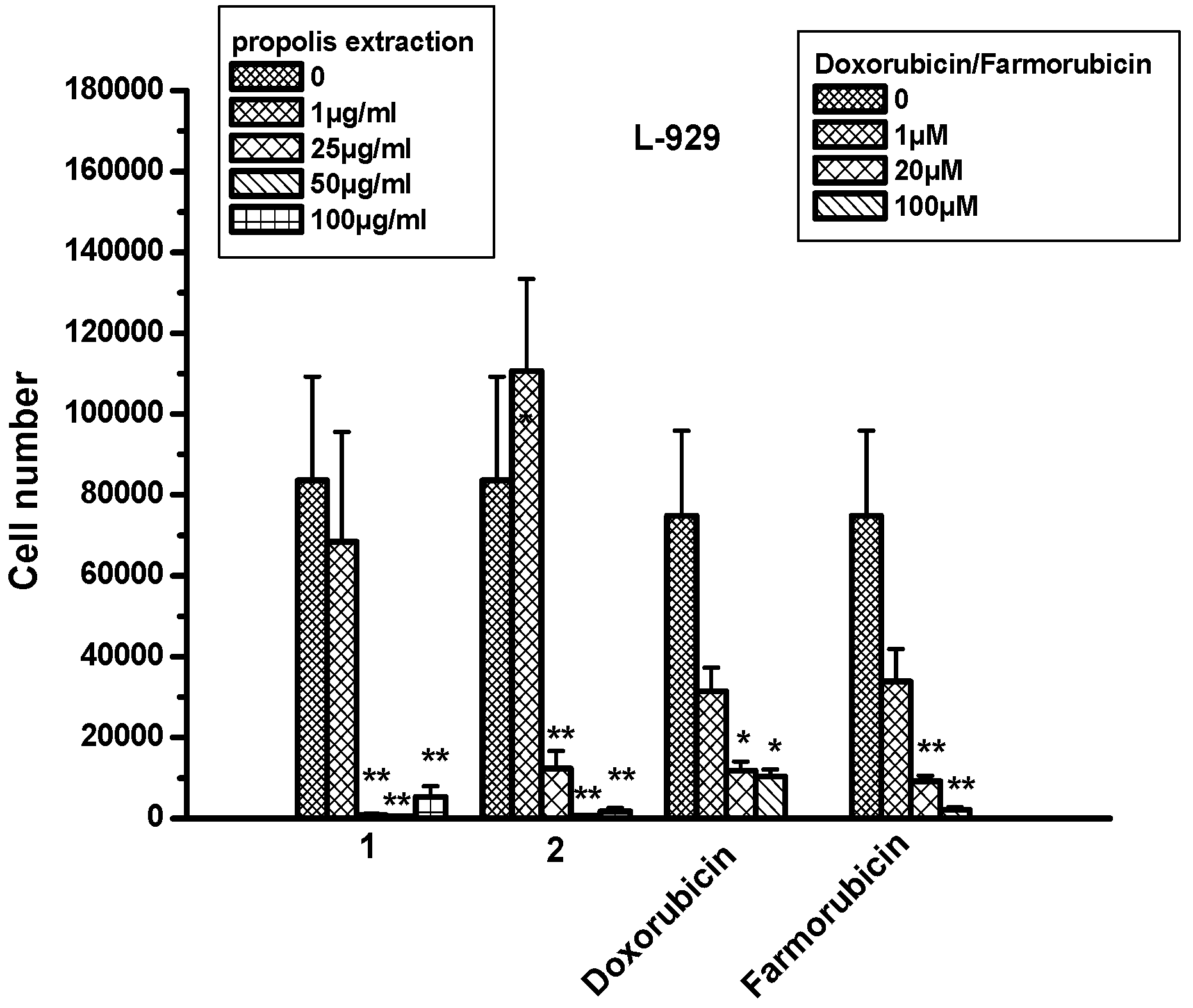
2.8. The Effect of Propolis Extracts in Fibroblast Cell Proliferation and Toxicity
3. Materials and Methods
3.1. Samples
3.2. Extraction and Sample Derivatization
3.3. GC–MS Analysis
3.4. Antimicrobial Bioassay
3.5. Antiproliferative Activity
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Resent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? LWT-Food Sci. Technol. 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Bułdak, R.J.; Wyszyńska, B.M.; Stawiarska-Pięta, B.; Szaflarska-Stojko, E. The Ethanol Extract of Polish Propolis Exhibits Anti-Proliferative and/or Pro-Apoptotic Effect on HCT 116 Colon Cancer and Me45 Malignant Melanoma Cells In Vitro Conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Krzek, J.; Kaleta, J.; Hubicka, U.; Niedzwiedz, A. Reversed-phase high-performance liquid chromatography determination of selected phenolic acids in propolis concentrates in terms of standardization for drug manufacturing purposes. J. AOAC Int. 2006, 89, 352–358. [Google Scholar] [PubMed]
- Maciejewicz, W. Isolation of flavonoid aglycones from propolis by a column chromatography method and their identification by GC-MS and TLC methods. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1171–1179. [Google Scholar] [CrossRef]
- Krol, W.; Scheller, S.; Shani, J.; Pietsz, G.; Czuba, Z. Synergistic effect of ethanolic extract of propolis and antibiotics on the growth of Staphylococcus aureus. Arznei-Forschung 1993, 43, 607–609. [Google Scholar]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective behaviour of honeybees in acquiring European propolis plant precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Scheller, S.; Krol, W.; Swiacik, J.; Owczarek, S.; Gabrys, J.; Shani, J. Antitumoral property of ethanolic extract of propolis in mice-bearing Ehrlich carcinoma, as compared to bleomycin. Z. Naturforsch. C 1989, 44, 1063–1065. [Google Scholar] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis- inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine 2005, 12, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.G. Chemical Composition of European Propolis: Expected and Unexpected Results. Z. Naturforsch. C 2002, 57, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Smoleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wąsik, T.J. Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Recent trends and important developments in propolis research. Evid. Based Complement. Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Lothstein, L.; Israel, M.; Sweatman, T.W. Anthracycline drug targeting: Cytoplasmic versus nuclear-a fork in the road. Drug Resist. Update 2001, 4, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, E.C.; Medema, J.P. Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat. Rev. 2008, 34, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Krol, W. Polyphenols Isolated from Propolis Augment TRAIL-Induced Apoptosis in Cancer Cells. Evid. Based Complement. Altern. Med. 2013, 2013, Article ID 731940. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, A-P.; Harquail, J.; Lassalle-Claux, G.; Belbraouet, M.; Jean-Francois, J.; Touaibia, M.; Robichaud, G.A. CAPE Analogs Induce Growth Arrest and Apoptosis in Breast Cancer Cells. Molecules 2015, 20, 12576–12589. [Google Scholar] [CrossRef]
- Kudugunti, S.K.; Vad, N.M.; Whiteside, A.J.; Naik, B.U.; Yusuf, M.A.; Srivenugopal, K.S.; Moridani, M.Y. Biochemical mechanism of caffeic acid phenylethyl ester (CAPE) selective toxicity towards melanoma cell lines. Chem. Biol. Interact. 2010, 188, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Kubina, R.; Kabała-Dzik, A.; Tanasiewicz, M. Induction of Cell Cycle Arrest and Apoptotic Response of Head and Neck Squamous Carcinoma Cells (Detroit 562) by Caffeic Acid and Caffeic Acid Phenethyl Ester Derivative. Evid. Based Complement. Altern. Med. 2017, 2017, Article ID 6793456. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Chinou, I. Chemistry and Bioactivity of Royal Jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, E.; Papadimitriou, E. Amifostine has antiangiogenic properties in vitro by changing the redox status of human endothelial cells. Free Radic. Res. 2003, 37, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Van Tienhoven, E.A.E.; Korbee, D.; Schipper, L.; Verharen, H.W.; de Jong, W.H. In vitro and in vivo cytotoxicity assays using PVC and LDPE as model materials. J. Biomed. Mater. Res. A 2006, 78, 175–182. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of studied propolis and previously isolated compounds, like pinocembrin, pinobanksin, chrysin, galangin, kaempferol (by KG and IC), which were used for co-chromatograph are available from the authors. |

| Chemical Category | Compounds | % Total Ion Current a |
|---|---|---|
| Aromatic Acids | Benzoic acid | 3.1 |
| Dihydrocinnamic acid | 0.1 | |
| Cinnamic acid | 0.1 | |
| p-Hydroxybenzoic acid | 0.1 | |
| Vanillic acid | 0.1 | |
| p-Coumaric acid (Z) | 0.3 | |
| p-Methoxycinnamic acid | 0.1 | |
| Ferulic acid (Z) | 0.1 | |
| p-Coumaric acid (E) | 5.0 | |
| Dimethoxycinnamic acid | 0.7 | |
| Isoferulic acid | 0.4 | |
| Ferulic acid (E) | 3.4 | |
| Caffeic acid | 0.1 | |
| Other Aromatics | Benzyl alcohol | 0.3 |
| Phenylethyl alcohol | 0.1 | |
| p-Hydroxybenzaldehyde | 0.1 | |
| Hydroquinone | 0.1 | |
| Vanilin | 1.0 | |
| Fatty Acids | Palmitic acid | 0.4 |
| Linoleic acid | 0.7 | |
| Oleic acid | 0.3 | |
| Esters | Benzyl benzoate | 0.2 |
| Propyl p-coumarate | 0.1 | |
| Pentyl p-coumarate | 0.2 | |
| Butyl p-coumarate | 0.8 | |
| Butenyl p-coumarate | 0.4 | |
| Benzyl cinnamate | 0.2 | |
| Pentyl p-coumarate | 2.6 | |
| Pentenyl p-coumarate | 3.4 | |
| Isobutyl caffeate | 0.1 | |
| Pentenyl ferulate | 0.1 | |
| Benzyl p-coumarate (Z) | 0.3 | |
| 2-Methylbutyl caffeate | 0.1 | |
| 3-Methyl-3-butenyl caffeate | 0.5 | |
| 3-Methyl-2-butenyl ferulate | 0.2 | |
| 2-Methyl-2-butenyl caffeate | 0.3 | |
| 3-Methyl-2-butenyl caffeate | 0.5 | |
| Phenylethyl p-coumarate | 0.1 | |
| Benzyl ferulate | 0.1 | |
| Benzyl p-coumarate (E) | 6.7 | |
| Coniferyl benzoate | 0.6 | |
| Benzyl ferulate | 1.9 | |
| Benzyl caffeate | 1.5 | |
| Phenylethyl caffeate | 0.8 | |
| Cinnamyl p-coumarate | 4.0 | |
| Cinnamyl ferulate | 0.2 | |
| Cinnamyl caffeate | 0.7 | |
| Glycerol ester of phenolic acids | 0.6 | |
| Coumaryl coumarate | 0.2 | |
| Flavonoids and Chalcones | 2′,6′-Dihydroxy-4′-methoxydihydrochalcone | 1.3 |
| Trihydroxymonomethoxy chalcone (m/z: 502) | 0.2 | |
| Pinostrobin chalcone | 1.2 | |
| Pinocembrin chalcone | 2.6 | |
| Pinocembrin * | 4.8 | |
| Pinobanksin chalcone | 0.3 | |
| Sakuranetin | 0.3 | |
| Pinobanksin * | 5.7 | |
| 3-Acetylpinobanksin chalcone | 0.2 | |
| Trihydroxyflavanone (m/z: 488) | 0.2 | |
| 2′,6′-Dihydroxy-4,4′-dimethoxydihydrochalcone | 0.3 | |
| 3-Acetylalpinon | 0.3 | |
| Dihydroxymethoxyflavanone (m/z: 430) | 0.5 | |
| 3-Acetylpinobanksin | 3.3 | |
| 2′,6′,4-Trihydroxy-4′-methoxydihydrochalcone | 0.5 | |
| Chrysin * | 3.0 | |
| Galangin * | 4.0 | |
| Dihydroxydimethoxychalcone (m/z: 444) | 0.7 | |
| Dimethoxyhydroxyflavanone (m/z: 372) | 0.5 | |
| Isosakuranetin chalcone | 1.9 | |
| Isosakuranetin | 2.0 | |
| Alpinon chalcone | 2.0 | |
| Alpinon | 1.9 | |
| Naringenin | 0.2 | |
| Kaempferol-dimethyl ether | 1.3 | |
| Kaempferol-methyl-ether | 2.4 | |
| Dihydroxymethoxyflavone (m/z: 428) | 0.6 | |
| Kaempferol * | 0.3 | |
| Quercetin-methyl-ether | 0.7 | |
| Others | Ethylamine | 0.4 |
| Glycerol | 0.1 |
| Tested Compounds | S. aureus | S. epidermidis | P. aeruginosa | E. cloacae | K. pneumoniae | E. coli | C. albicans | C. tropicalis | C. glabrata |
|---|---|---|---|---|---|---|---|---|---|
| Propolis extr1 | 18/0.95 | 19/0.90 | 16/1.10 | 16/1.15 | 17/1.03 | 14/1.34 | 12/1.40 | 13/1.28 | 14/1.25 |
| Propolis extr2 | 13 | 12 | 10 | 10 | 9 | 8 | NA | NA | NA |
| Netilmicin | 21/0.004 | 25/0.004 | 20/0.088 | 23/0.008 | 22/0.008 | 24/0.010 | NT | NT | NT |
| Itraconazole | NT | NT | NT | NT | NT | NT | 20/0.01 | 22/0.001 | 23/0.0001 |
| 5-flucytosine | NT | NT | NT | NT | NT | NT | 21/0.01 | 22/0.001 | 24/0.0001 |
| A549 | H23 | Caco-2 | DLD-1 | HT-29 | ||
|---|---|---|---|---|---|---|
| Propolis Extr1 | 1 μg/mL | 0 | 0 | 0 | 0 | 0 |
| 25 μg/mL | 59% ± 11 | 69% ± 1 | 96% ± 0.5 | 82% ± 2 | 35% ± 33 | |
| 50 μg/mL | 36% ± 34 | 78% ± 7 | 112% ± 60 | 49% ± 38 | 71% ± 14 | |
| 100 μg/mL | 23% ± 62 | 28% ± 7 | 67% ± 4 | 29% ± 55 | 60% ± 18 | |
| Propolis Extr2 | 1 μg/mL | 5% ± 18 | 0 | 0 | 0 | 0 |
| 25 μg/mL | 19% ± 9 | 27% ± 1 | 0 | 74% ± 35 | 39% ± 29 | |
| 50 μg/mL | 48% ± 50 | 82% ± 37 | 5% ± 93 | 72% ± 55 | 40% ± 48 | |
| 100 μg/mL | 84% ± 60 | 68% ± 54 | 23% ± 103 | 110% ± 72 | 60% ± 57 | |
| Doxorubicin | 20 μM | 0.3% ± 0.3 | 1.2% ± 1.2 | 0.3% ± 0.3 | 1.2% ± 1.2 | 0 |
| 100 μM | 22% ± 7 | 21% ± 6 | 22% ± 7 | 21% ± 6 | 17% ± 5 | |
| Farmorubicin | 20 μM | 0 | 9% ± 9 | 0 | 9% ± 9 | 0 |
| 100 μM | 33% ± 18 | 28% ± 6 | 33% ± 18 | 28% ± 6 | 30% ± 8 | |
| MCF-7 | MDA-MB-468 | U87 | LN18 | L-929 | ||
|---|---|---|---|---|---|---|
| Propolis extr1 | 1 μg/mL | 0 | 0 | 0 | 0 | 0 |
| 25 μg/mL | 196% ± 183 | 57% ± 30 | 9% ± 0.5 | 50% ± 38 | 112% ± 58 | |
| 50 μg/mL | 170% ± 148 | 42% ± 23 | 50% ± 43 | 25% ± 3 | 95% ± 58 | |
| 100 μg/mL | 178% ± 154 | 40% ± 24 | 52% ± 42 | 52% ± 16 | 172% ± 14 | |
| Propolis extr2 | 1 μg/mL | 6% ± 1 | 0 | 38% ± 39 | 15% ± 17 | 16% ± 33 |
| 25 μg/mL | 65% ± 57 | 0 | 7% ± 6 | 86% ± 85 | 202% ± 67 | |
| 50 μg/mL | 87% ± 72 | 13% ± 55 | 14% ± 3 | 64% ± 32 | 221% ± 7 | |
| 100 μg/mL | 146% ± 118 | 0 | 17% ± 0.5 | 92% ± 10 | 269% ± 62 | |
| Doxorubicin | 20 μM | 121% ± 2 | 60% ± 38 | 0 | 121% ± 2 | 60% ± 38 |
| 100 μM | 177% ± 16 | 21% ± 10 | 17% ± 5 | 177% ± 16 | 21% ± 10 | |
| Farmorubicin | 20 μM | 83% ± 33 | 16% ± 2 | 0 | 83% ± 33 | 16% ± 2 |
| 100 μM | 159% ± 9 | 38% ± 12 | 30% ± 8 | 159% ± 9 | 38% ± 12 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules 2017, 22, 1159. https://doi.org/10.3390/molecules22071159
Popova M, Giannopoulou E, Skalicka-Woźniak K, Graikou K, Widelski J, Bankova V, Kalofonos H, Sivolapenko G, Gaweł-Bęben K, Antosiewicz B, et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules. 2017; 22(7):1159. https://doi.org/10.3390/molecules22071159
Chicago/Turabian StylePopova, Milena, Efstathia Giannopoulou, Krystyna Skalicka-Woźniak, Konstantia Graikou, Jaroslaw Widelski, Vassya Bankova, Haralabos Kalofonos, Gregory Sivolapenko, Katarzyna Gaweł-Bęben, Beata Antosiewicz, and et al. 2017. "Characterization and Biological Evaluation of Propolis from Poland" Molecules 22, no. 7: 1159. https://doi.org/10.3390/molecules22071159
APA StylePopova, M., Giannopoulou, E., Skalicka-Woźniak, K., Graikou, K., Widelski, J., Bankova, V., Kalofonos, H., Sivolapenko, G., Gaweł-Bęben, K., Antosiewicz, B., & Chinou, I. (2017). Characterization and Biological Evaluation of Propolis from Poland. Molecules, 22(7), 1159. https://doi.org/10.3390/molecules22071159









