Antifungal and Ichthyotoxic Sesquiterpenoids from Santalum album Heartwood
Abstract
:1. Introduction
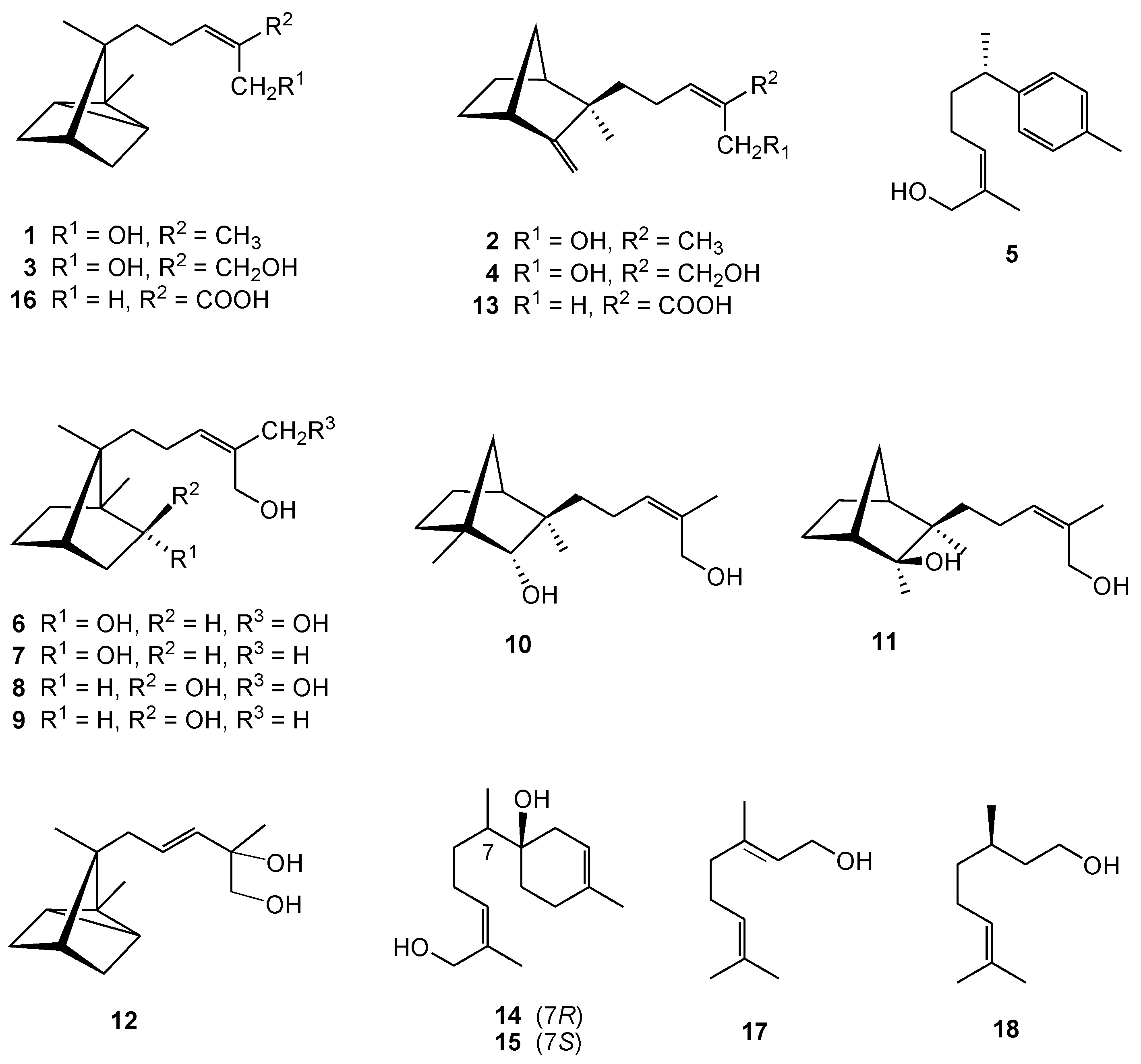
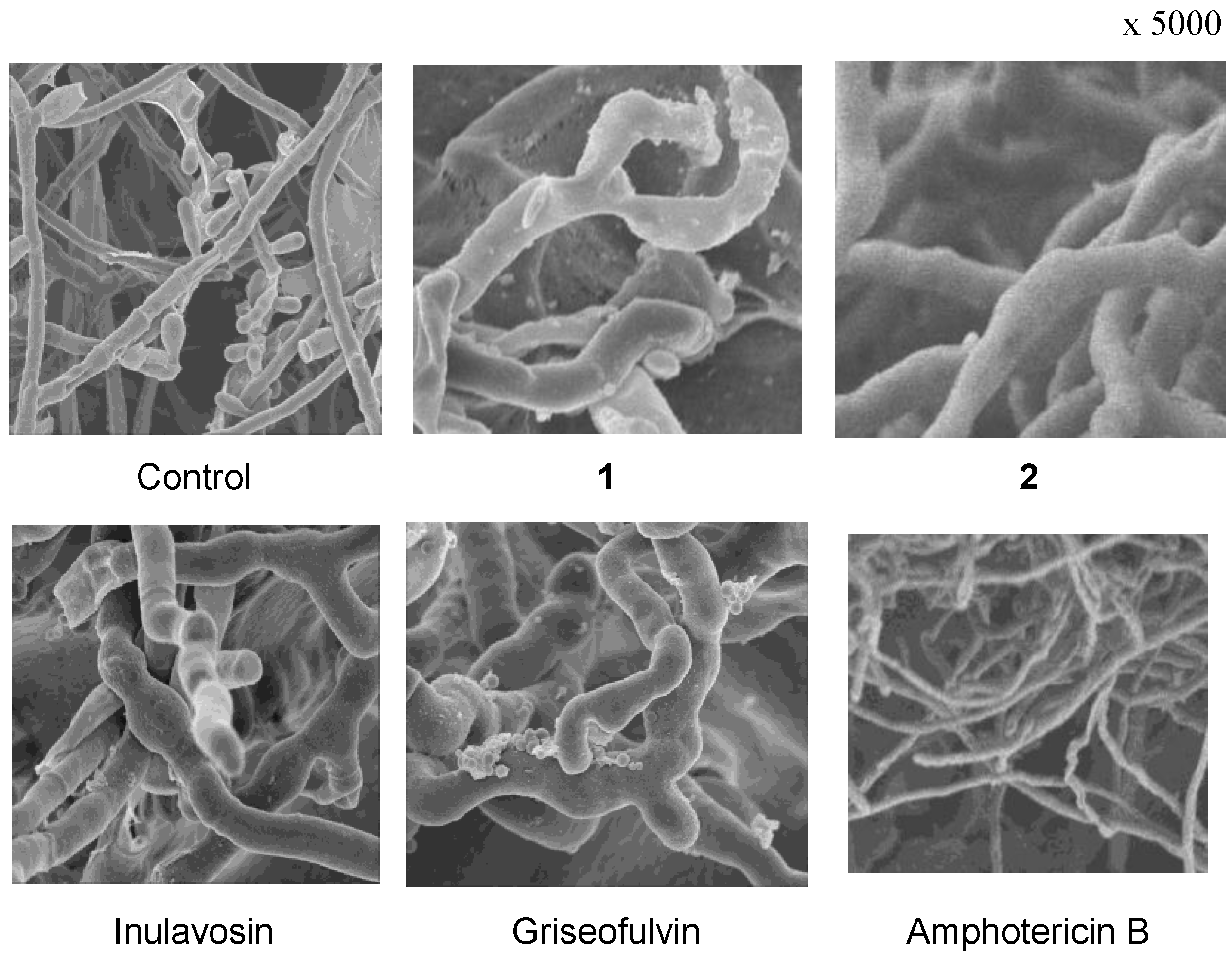
2. Results and Discussion
3. Materials and Methods
3.1. Isolation of the Test Compounds
3.2. Ichthyotoxic Assay
3.3. Assay for Antifungal Activity
3.4. Scanning Electron Microscopy (SEM)
3.5. Measurement of Tubulin Interactive Effects
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kapoor, L.D. Handbook of Ayurvedic Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Perry, L.M. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; The MIT Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Gibbard, S.; Schoental, R. Simple semi-quantitative estimation of sinapyl and certain related aldehydes in wood and in other materials. J. Chromatogr. 1969, 44, 396–398. [Google Scholar] [CrossRef]
- Shankaranarayana, K.H.; Ayyar, K.S.; Krishna Rao, G.S. Insect growth inhibitor from the bark of Santalum album. Phytochemistry 1980, 19, 1239–1240. [Google Scholar] [CrossRef]
- Shankaranarayana, K.H.; Ayyar, K.S.; Krishna Rao, G.S. Chemical constituents of the bark of Santalum album Linn. Curr. Sci. 1980, 49, 198–199. [Google Scholar]
- Adams, D.R.; Bhatnagar, S.P.; Cooksoon, R.C. Sesquiterpenes of Santalum album and Santalum spicatum. Phytochem. Rep. 1975, 14, 1459–1460. [Google Scholar] [CrossRef]
- Demole, E.; Demole, C.; Enggis, P. A chemical investigation of the volatile constituents of East Indian sandalwood oil (Santalum album L.). Helv. Chim. Acta 1976, 59, 737–747. [Google Scholar] [CrossRef]
- Christenson, P.A.; Secord, N.; Willis, B.J. Identification of trans-β-santalol and epi-cis-β-santalol in east Indian sandalwood oil. Phytochemistry 1981, 20, 1139–1141. [Google Scholar] [CrossRef]
- Ranibai, P.; Ghatge, B.B.; Patil, B.B.; Bhattacharyya, S.C. Ketosantalic acid, a new sesquiterpenic acid from Indian sandalwood oil. Indian J. Chem. 1986, 25B, 1006–1013. [Google Scholar]
- Okugawa, H.; Ueda, R.; Matsumoto, K.; Kawanishi, K.; Kato, A. Effect of α-santalol and β-santalol from sandalwood on the central nervous system in mice. Phytomedicine 1995, 2, 119–126. [Google Scholar] [CrossRef]
- Corey, E.J.; Kirst, H.A.; Katzenellenbogen, J.A. A sterospecific total synthesis of α-santalol. J. Am. Chem. Soc. 1970, 92, 6314–6319. [Google Scholar] [CrossRef]
- Christenson, P.A.; Willis, B.J. East Indian sandalwood oil. 1. Stereoselective synthesis of (±)-beta-santalene and (±)-beta-santalol. J. Org. Chem. 1979, 44, 2012–2018. [Google Scholar] [CrossRef]
- Benencia, F.; Courreges, M.C. Antiviral activity of sandalwood oil against herpes simplex viruses-1 and 2. Phytomedicine 1999, 6, 119–123. [Google Scholar] [CrossRef]
- Banerjee, S.; Ecavade, A.; Rao, A.R. Modulatory influence of sandalwood oil on mouse heptic glutathione S-transferase activity and acid soluble sulphydryl level. Cancer Lett. 1993, 68, 105–109. [Google Scholar] [CrossRef]
- Okugawa, H.; Ueda, R.; Matsumoto, K.; Kawanishi, K.; Kato, K. Effects of sesquitepenoids from “oriental incenses” on acetic acid-induced writhing and D and 5-HT receptors in rat brain. Phytomedicine 2000, 7, 417–422. [Google Scholar] [CrossRef]
- Hongratanaworakit, T.; Heuberger, E.; Buchbauer, G. Evaluation of the effects of East Indian sandalwood oil and alpha-santalol in humans after transdermal absortion. Planta Med. 2004, 70, 3–7. [Google Scholar] [PubMed]
- Dwivedi, C.; Abu-Ghazaleh, A. Chemopreventive effects of sandalwood oil on skin papillomas in mice. Eur. J. Cancer Prev. 1997, 6, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Zang, Y. Sandalwood oil prevents skin tumor development in CD1 mice. Eur. J. Cancer Prev. 1999, 8, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Ito, H.; Hayashi, K.; Hasegawa, T.; Machiguchi, T.; Yoshida, T. Aromatic constituents from the heartwood of Santalum album L. Chem. Pharm. Bull. 2005, 53, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Ito, H.; Hatano, T.; Akiba, A.; Machiguchi, T.; Yoshida, T. Bisabolane-and santalane-type sesquitepenoids from Santalum album of Indian origin. J. Nat. Prod. 2005, 68, 1805–1808. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Ito, H.; Hatano, T.; Takawasu, J.; Tokuda, H.; Nishino, H.; Machiguchi, T.; Yoshida, T. New antitumor sesquiterpenoids from Santalum album of Indian origin. Tetrahedron 2006, 62, 6981–6989. [Google Scholar] [CrossRef]
- Yoshida, T.; Ito, H. Naturally occurring ichthyotoxic substances and their biological activities. Curr. Top Phytochem. India 2000, 4, 135–145. [Google Scholar]
- Cunningham, M.L.; Soliman, M.S.; Badr, M.Z.; Matthews, H.B. Rotenone, an anticarcinogen, inhibits cellular proliferation but not peroxisome proliferation in mouse liver. Cancer Res. 1995, 95, 93–97. [Google Scholar] [CrossRef]
- Gomez, E.; de la Curz-Giron, O.; de la Cruz, A.A.; Joshi, B.S.; Chittawong, V.; Miles, D.H. Toxicants from mangrove plants, V. Isolation of the piscicide, 2-hydroxy-5methoxy-3-undecyl-1,4 benzoqhinone (5-O-methlembelin) from Aegiceras cormiculatum. J. Nat. Prod. 1989, 52, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Ito, H.; Yoshida, T. Identification of iridals as piscicidal components of Iridaceous plants and their conformations associated with CD spectra. Can. Chem. J. 1997, 75, 734–741. [Google Scholar] [CrossRef]
- Yoshida, T.; Mori, K.; He, G. Inulavosin, a new thymol dimer with piscicidal activity from Inula nervosa. Heterocycles 1995, 41, 1923–1926. [Google Scholar] [CrossRef]
- Yoshida, T.; Nobuhara, J.; Uchida, M.; Okuda, T. Studies on the constituents of Buddleja species. I. Structures of buddlendin A and B, two new toxic sesquiterpenes from Buddleja davidii FRANCH. Chem. Pharm. Bull. 1978, 26, 2535–2542. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, M.; Truck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
- Kanzaki, H.; Imura, D.; Nitoda, T.; Kwazu, K. Enzymatic conversion of cyclic dipeptides to dehydro derivatives that inhibit cell division. J. Biosci. Bioeng. 2000, 90, 86–89. [Google Scholar] [CrossRef]
- Alpha, T.; Raharivelomanana, P.; Bianchini, J.P.; Faure, R.; Cambon, A.; Joncheray, L. α-Santaldiol and β-santaldiol, two santalene sesquiterpenes from Santalum insulare. Phytochemistry 1996, 41, 829–831. [Google Scholar] [CrossRef]
- Yoneda, R.; Harusawa, S.; Kurihara, T. Synthesis of (Z)-predominant α,β-unsaturated nitriles from enone cyanohydrin diethyl phosphates: application to the synthesis of (±)-nuciferal, (±)-(E)- and -(Z)-nuciferol, and (±)-manicone. J. Chem. Soc. Perkin Trans. 1 1988, 12, 3163–3168. [Google Scholar]
- Fujita, H.; Motokawa, T.; Katagiri, T.; Yokita, S.; Yamamoto, A.; Himeno, M.; Tanaka, Y. Inulavosin, a melanogenesis inhibitor, leads to mitageting of tyrosinase to lysosomes and accelerates its degradation. J. Investig. Dermatol. 2009, 129, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.W. Griseofulvin: Biology and clinical usefulness. A review. Ann Allergy 1965, 23, 103–110. [Google Scholar] [PubMed]
- Roth, F.J., Jr.; Sallman, B.; Blanck, H. In vitro studies of the antifungal antibiotic griseofulvin. J. Investig. Dermatol. 1959, 33, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Gull, K.; Trinci, A.P. Griseofulvin inhibits fungal mitosis. Nature 1973, 244, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Ho, H.H.; Pettit, G.R.; Hamel, E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry 1989, 28, 6984–6991. [Google Scholar] [CrossRef] [PubMed]
- Beckers, T.; Mahboobi, S. Natural, semisynthetic and synthetic microtubule inhibitors for cancer therapy. Drugs Future 2003, 28, 767–785. [Google Scholar] [CrossRef]
- Sato, H.; Kobayashi, A.; Itoh, T. Molecular basis of physical and chemical probes for spindle assembly. Cell Struct. Funct. 1989, 14, 1–34. [Google Scholar] [CrossRef]
- Kaur, M.; Agarwal, C.; Singh, R.P.; Guan, X.; Dwivedi, C.; Agarwal, R. Skin cancer chemopreventive agent, α-santalol, induces apoptotic death of human epidermoid carcinoma A431 cells via caspase activation together with dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis 2005, 26, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Guan, X.; Harmsen, W.L.; Goetz-Parten, D.E.; Koopman, E.M.; Johnson, K.M.; Valluri, H.B.; Matthees, D.P. Chemopreventive effects of alpha-santalol on skin tumor development in CD-1 and SENCAR mice. Cancer Epidemiol. Biomark Prev. 2003, 12, 151–156. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compounds | MIC (μg/disc) a | Compound | MIC (μg/disc) |
|---|---|---|---|
| 1 | 12.5 | 11 | 31.3 |
| 2 | 25.0 | 12 | 62.5 |
| 3 | 50.0 | 13 | 125.0 |
| 4 | 25.0 | 14 | 125.0 |
| 5 | 31.3 | 15 | 125.0 |
| 6 | 250.0 | 16 | 62.5 |
| 7 | 62.5 | 17 | 62.5 |
| 8 | 250.0 | 18 | 62.5 |
| 9 | 62.5 | Inulavosin | 10.0 |
| 10 | 62.5 | Griseofulvin | 0.5 |
| MIC (μg/mL) a | |||
|---|---|---|---|
| Compounds | Hemicentrotus pulcherrimus | Anthocidaris crassispina | Scaphechinus mirabilis |
| 1 | 25 | 12.5 | 12.5 |
| 2 | >50 | >50 | >50 |
| 3 | >50 | >50 | >50 |
| 4 | >50 | >50 | >50 |
| 5 | >50 | >50 | >50 |
| Inulavosin | 50 | 25 | 50 |
| Griseofulvin | 3.13 | 3.13 | 3.13 |
| Paclitaxel b | 10 c | nt | 25 |
| Colchicine b | nt d | nt | 50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.H.; Hatano, T.; Okamoto, K.; Yoshida, T.; Kanzaki, H.; Arita, M.; Ito, H. Antifungal and Ichthyotoxic Sesquiterpenoids from Santalum album Heartwood. Molecules 2017, 22, 1139. https://doi.org/10.3390/molecules22071139
Kim TH, Hatano T, Okamoto K, Yoshida T, Kanzaki H, Arita M, Ito H. Antifungal and Ichthyotoxic Sesquiterpenoids from Santalum album Heartwood. Molecules. 2017; 22(7):1139. https://doi.org/10.3390/molecules22071139
Chicago/Turabian StyleKim, Tae Hoon, Tsutomu Hatano, Keinosuke Okamoto, Takashi Yoshida, Hiroshi Kanzaki, Michiko Arita, and Hideyuki Ito. 2017. "Antifungal and Ichthyotoxic Sesquiterpenoids from Santalum album Heartwood" Molecules 22, no. 7: 1139. https://doi.org/10.3390/molecules22071139
APA StyleKim, T. H., Hatano, T., Okamoto, K., Yoshida, T., Kanzaki, H., Arita, M., & Ito, H. (2017). Antifungal and Ichthyotoxic Sesquiterpenoids from Santalum album Heartwood. Molecules, 22(7), 1139. https://doi.org/10.3390/molecules22071139






