Synthesis and Fungicidal Activity of 1-(Carbamoylmethyl)-2-aryl-3,1-benzoxazines
Abstract
:1. Introduction
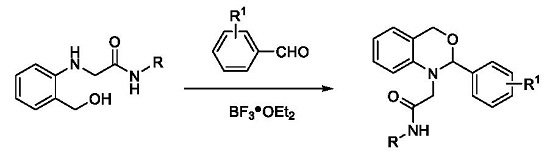
2. Results and Discussion
2.1. Chemistry
2.2. Fungicidal Activity Assay
3. Experimental Section
3.1. Materials and Reagents
3.2. Chemical Synthesis
3.2.1. Synthesis of N-Substituted 2-(2-(hydroxymethyl)phenylamino)acetamides 3a–f
3.2.2. Synthesis of 2,4-Dihydro-1H-3,1-benzoxazines 5a–r
3.3. Fungicidal Activity Testing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuch, H.; Schmitt, K.; Seudl, G.; Hoffmann, I. 2-Amino-4,4-di-substituted-4H-3,1-benzoxazines. U.S. Patent 3725404, 3 April 1973. [Google Scholar]
- Kobzina, J.W.; Creek, W. Herbicidal N-Haloacetyl-1,2-dihydro-4H-3,1-benzoxazine. U.S. Patent 4030906, 21 June 1977. [Google Scholar]
- Sugiyama, H.; Hosoda, K.; Kumagai, Y.; Takeuchi, M.; Okada, M. 4H-3,1-Benzoxazine Derivatives, Process for Producing the Same and Agricultural or Horticultural Fungicide Containing the Same. U.S. Patent 4596801, 24 June 1986. [Google Scholar]
- Tang, Z.; Wang, L.; Huang, T.; Gao, W.; Luo, X. The Fungicidal Activities and Application of 1-(Carbamoylmethyl)-2-aryl-2,4-dihydro-3,1-benzoxazines. CN104910094A, 16 September 2015. [Google Scholar]
- Charmantray, F.; Demeunynck, M.; Carrez, D.; Croisy, A.; Lansiaux, A.; Bailly, C.; Colson, P. 4-Hydroxymethyl-3-aminoacridine derivatives as a new family of anticancer agents. J. Med. Chem. 2003, 46, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Terefenko, E.A.; Fensome, A.; Zhang, Z.; Zhu, Y.; Cohen, J.; Winneker, R.; Wrobel, J.; Yardley, J. Potent nonsteroidal progesterone receptor agonists: Synthesis and SAR study of 6-aryl-benzoxazines. Bioorg. Med. Chem. Lett. 2002, 12, 787–790. [Google Scholar] [CrossRef]
- Dias, N.; Goossens, J.; Baldeyrou, B.; Lansiaux, A.; Colson, P.; Di Salvo, A.; Bernal, J.; Turnbull, A.; Mincher, D.J.; Bailly, C. Oxoazabenzo[de]anthracenes conjugated to amino acids: Synthesis and evaluation as DNA-binding antitumor agents. Bioconj. Chem. 2005, 16, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.D.; Caroon, J.M.; Kluge, A.F.; Repke, D.B.; Roszkowski, A.P.; Strosberg, A.M.; Baker, S.; Bitter, S.M.; Okada, M.D. Synthesis and antihypertensive activity of 4′-substituted spiro[4H-3,1-benzoxazine-4,4’-piperidin]-3(1H)-ones. J. Med. Chem. 1983, 26, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Badolato, M.; Carullo, G.; Armentano, B.; Panza, S.; Malivindi, R.; Aiello, F. Synthesis and anti-proliferative activity of a small library of 7-substituted 5H-pyrrole [1,2-a][3,1]benzoxazin-5-one derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 3092–3095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Terefenko, E.A.; Fensome, A.; Wrobel, J.; Winneker, R.; Lundeen, S.; Marschke, K.B.; Zhang, Z. 6-Aryl-1,4-dihydro-benzo[d][1,3]oxazin-2-ones: A novel class of potent, selective, and orally active nonsteroidal progesterone receptor antagonists. J. Med. Chem. 2002, 45, 4379–4382. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.C.; Terefenko, E.A.; Fensome, A.; Unwallla, R.; Wrobel, J.; Zhu, Y.; Cohen, J.; Winneker, R.; Zhang, Z.; Zhang, P. SAR studies of 6-(arylamino)-4,4-disubstituted-1-methyl-1,4-dihydro-benzo[d][1,3]-oxazin-2-ones as progesterone. Bioorg. Med. Chem. Lett. 2007, 17, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Krantz, A.; Spencer, R.W.; Tam, T.F.; Liak, T.J.; Copp, L.J.; Thomas, E.M.; Rafferty, S.P. Design and synthesis of 4H-3,1-benzoxazin-4-one as potent alternate substrate inhibitors of human leukocyte elastase. J. Med. Chem. 1990, 33, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.L.; Hays, S.J.; Caprathe, B.W.; Lee, C.; Emmerling, M.R.; Micheal, W.; Jean, J.C. Synthesis and valuation of 2-aryl-4H-3,1-benzoxazin-4-ones as C1r serine protease inhibitors. Bioorg. Med. Chem. Lett. 1996, 6, 679–682. [Google Scholar] [CrossRef]
- Hays, S.J.; Caprathe, B.W.; Gilmore, J.L.; Amin, N.; Emmerling, M.R.; Micheal, W.; Nadimpalli, R.; Nath, R.; Raser, K.J.; Stafford, D.; et al. 2-Amino-4H-3,1-benzoxazi-4-ones as C1r serine protease. J. Med. Chem. 1998, 41, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Gutschow, M.; Neumann, U.; Sieler, J.; Eger, K. Studies on 2-benzyloxy-4H-3,1-bnezoxazin-4-ones as serine protease inhibitors. Pharm. Acta Helv. 1998, 73, 95–103. [Google Scholar] [CrossRef]
- Mizutani, T.; Ishikawa, S.; Nagase, T.; Takahashi, H.; Fujimura, T.; Sasaki, T.; Nagumo, A.; Shimamura, K.; Miyamoto, Y.; Kitazawa, H.; et al. Discovery of novel benzoxazinones as potent and orally active long chain fatty acid elongase 6 inhibitors. J. Med. Chem. 1990, 52, 7289–7300. [Google Scholar] [CrossRef] [PubMed]
- Hanessian, S.; Jennequin, T.; Boyer, N.; Babonneau, V.; Soma, U.; La Cour, C.M.; Millan, M.J.; De Nanteuil, G. Design, synthesis, and optimization of balanced dual NK1/NK3 receptor antagonists. ACS Med. Chem. Lett. 2014, 5, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Marasini, B.P.; Rahim, F.; Perveen, S.; Karim, A.; Khan, K.M.; Rahman, A.; Choudhary, M.L. Synthesis, structure-activity relationships studies of benzoxazine derivatives as α-chymoitrypsin inhibitor. Bioorg. Chem. 2017, 70, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Martin-Martinez, M.; Perez-Gordillo, F.; Alvarez de la Rosa, D.; Rodriguez, Y.; Gerona-Navarro, G.; Gonzalez-Muniz, R.; Zhou, M.-M. Modulating mineralocorticoid receptor with non-steroidal antagonists. New opportunities for the development of potent and selective ligands without off-target side effects. J. Med. Chem. 2017, 60, 2629–2650. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Ko, S.S.; McHugh, R.J.; Markwalder, J.A.; Srivasta, A.S.; Cordova, B.C.; Klabe, R.M.; Erickson-Viitanen, S.; Trainor, G.L.; Seitz, S.P. Synthesis and evaluation of analogs of Efavirenz (SustivaTM) as HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 2805–2810. [Google Scholar] [CrossRef]
- Cocuzza, A.J.; Chidester, D.R.; Cordova, B.C.; Jeffrey, S.; Parsons, R.L.; Bacheler, L.T.; Erickson-Viitanen, S. Synthesis and evaluation of Efavirenz (SustivaTM) analogues as HIV-1 reverse transcriptase inhibitors: Replacement of the cyclopropylacetylene side chain. Bioorg. Med. Chem. Lett. 2001, 11, 1177–1179. [Google Scholar] [CrossRef]
- Holly, F.W.; Cope, A.C. Condensation products of aldehydes and ketones with o-aminobenzyl alcohol and o-hydroxybenzylamine. J. Am. Chem. Soc. 1944, 66, 1875–1879. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Amey, R.L.; Martin, J.C. Tridentate ligand useful in stabilizing coordination states of nonmetallic elements. An aryldialkoxydifluoroperiodinane. J. Org. Chem. 1982, 47, 1024–1207. [Google Scholar] [CrossRef]
- Saito, T.; Ogawa, S.; Takei, N.; Kutsumura, N.; Otani, T. Palladium-catalyzed highly regio- and stereoselective synthesis of 4-alkylidene-4H-3,1-benzoxazines from N-acyl-o-alkynylanilines. Org. Lett. 2011, 13, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cheng, R.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Hypervalent iodine- mediated oxygenation of N,N-diaryl tertiary amines: Intramolecular functionalization of sp3 C-H bonds adjacent to nitrogen. J. Org. Chem. 2014, 79, 10581–10587. [Google Scholar] [CrossRef] [PubMed]
- Lagu, B.; Pio, B.; Lebedev, R.; Yang, M.; Pelton, P.D. RXR-LXR heterodimer modulators for the potential treatment of dyslipidemia. Bioorg. Med. Chem. Lett. 2007, 17, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kikuchi, S.; Tsubo, T.; Yamada, T. Silver-catalyzedincorporation of carbon dioxide into o-alkynylanilides derivatives. Org. Lett. 2013, 15, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Chen, W.; Zhu, Z.; Liu, H. Synthesis of 2,3-diaryl-3,4-dihydro-2H-1,3-benzoxazines and their fungicidal activities. J. Heterocycl. Chem. 2011, 48, 255–260. [Google Scholar] [CrossRef]
- Tang, Z.; Zhu, Z.; Xia, Z.; Liu, H.; Chen, J.; Xiao, W.; Ou, X. Synthesis and fungicidal activity of novel 2,3-disubstituted-1,3-benzoxazines. Molecules 2012, 17, 8174–8185. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 5a–5r are available from the authors. |
| No. | R | R1 | Conditions | Product | Yield/% b |
|---|---|---|---|---|---|
| 1c | 2-CH3C6H4 | 3-NO2 | BF3·OEt2(10%), 65 °C, 6 hn(3):n(4) = 1:1.3 | 5a | 35 |
| 2c | 2-CH3C6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 6 hn(3):n(4) = 1:1.3 | 5a | 45 |
| 3 | 2-CH3C6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 6 hn(3):n(4) = 1:1.3 | 5a | 47 |
| 4 | 2-CH3C6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 8 hn(3):n(4) = 1:1.3 | 5a | 50 |
| 5 | 2-CH3C6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 10 hn(3):n(4) = 1:1.3 | 5a | 53 |
| 6 | 2-CH3C6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 14 hn(3):n(4) = 1:1.3 | 5a | 44 |
| 7 | 2-CH3C6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 10 hn(3):n(4) = 1:1.5 | 5a | 55 |
| 8 | 2-CH3C6H4 | 2-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5b | 45 |
| 9 | 2-CH3C6H4 | 4-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5c | 49 |
| 10 | 4-CH3C6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5d | 56 |
| 11 | 4-CH3C6H4 | 2-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5e | 40 |
| 12 | 4-CH3C6H4 | 4-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5f | 48 |
| 13 | 4-CH3OC6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5g | 85 |
| 14 | 4-CH3OC6H4 | 2-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5h | 66 |
| 15 | 4-CH3OC6H4 | 4-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5i | 80 |
| 16 | 3-CH3OC6H4 | 3-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5j | 74 |
| 17 | 3-CH3OC6H4 | 2-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5k | 41 |
| 18 | 3-CH3OC6H4 | 4-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5l | 60 |
| 19 | C6H5 | 3-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5m | 52 |
| 20 | C6H5 | 2-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5n | 44 |
| 21 | C6H5 | 4-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5o | 46 |
| 22 | C6H5CH2 | 3-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5p | 56 |
| 23 | C6H5CH2 | 2-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5q | 58 |
| 24 | C6H5CH2 | 4-NO2 | BF3·OEt2(20%), 65 °C, 10 h | 5r | 65 |
| Compd. | S. sclerotiorum/% | B. cinerea/% | R. solani/% | G. zeae/% | P. capsici/% | M. oryzae/% a |
|---|---|---|---|---|---|---|
| 5a | 33.3 | 43.8 | 21.1 | 12.5 | 7.7 | 18.2 |
| 5b | 33.3 | 37.5 | 18.4 | 37.5 | 30.8 | 27.3 |
| 5c | 33.3 | 31.3 | 5.3 | 12.5 | 15.4 | 13.6 |
| 5d | 14.3 | 43.8 | 26.3 | 31.3 | 15.4 | 60.1 |
| 5e | 14.3 | 31.3 | 21.1 | 6.3 | 38.5 | 36.4 |
| 5f | 47.6 | 56.3 | 28.9 | 31.3 | 15.4 | 22.7 |
| 5g | 57.1 | 12.5 | 21.1 | 25.0 | 15.4 | 54.5 |
| 5h | 23.8 | 18.8 | 31.6 | 31.3 | 38.5 | 22.7 |
| 5i | 71.9 | 25.0 | 31.6 | 12.5 | 15.4 | 31.8 |
| 5j | 14.3 | 12.5 | 18.4 | 37.5 | 30.8 | 22.7 |
| 5k | 42.9 | 25.0 | 21.1 | 12.5 | 7.7 | 22.7 |
| 5l | 23.8 | 43.8 | 31.6 | 12.5 | 15.4 | 27.3 |
| 5m | 14.3 | 50.0 | 26.3 | 37.5 | 15.4 | 27.3 |
| 5n | 28.6 | 37.5 | 18.4 | 18.8 | 38.5 | 22.7 |
| 5o | 27.3 | 23.8 | 51.7 | 53.8 | 33.3 | 51.7 |
| 5p | 11.6 | 11.5 | 19.6 | 17.1 | 11.1 | 41.2 |
| 5q | 46.5 | 38.5 | 25.5 | 17.1 | 29.6 | 41.2 |
| 5r | 16.3 | 30.8 | 15.7 | 12.2 | 11.1 | 11.8 |
| Chlorothalonil b | 84.9 | 92.9 | 85.2 | 67.6 | 78.6 | 53.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.-L.; Wang, L.; Tan, J.-Z.; Wan, Y.-C.; Jiao, Y.-C. Synthesis and Fungicidal Activity of 1-(Carbamoylmethyl)-2-aryl-3,1-benzoxazines. Molecules 2017, 22, 1103. https://doi.org/10.3390/molecules22071103
Tang Z-L, Wang L, Tan J-Z, Wan Y-C, Jiao Y-C. Synthesis and Fungicidal Activity of 1-(Carbamoylmethyl)-2-aryl-3,1-benzoxazines. Molecules. 2017; 22(7):1103. https://doi.org/10.3390/molecules22071103
Chicago/Turabian StyleTang, Zi-Long, Lian Wang, Jing-Zhao Tan, Yi-Chao Wan, and Yin-Chun Jiao. 2017. "Synthesis and Fungicidal Activity of 1-(Carbamoylmethyl)-2-aryl-3,1-benzoxazines" Molecules 22, no. 7: 1103. https://doi.org/10.3390/molecules22071103
APA StyleTang, Z.-L., Wang, L., Tan, J.-Z., Wan, Y.-C., & Jiao, Y.-C. (2017). Synthesis and Fungicidal Activity of 1-(Carbamoylmethyl)-2-aryl-3,1-benzoxazines. Molecules, 22(7), 1103. https://doi.org/10.3390/molecules22071103