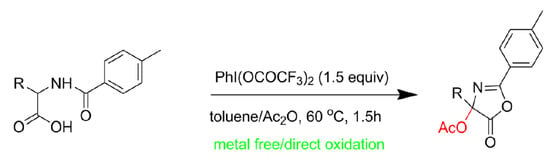
A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine
Abstract
:1. Introduction
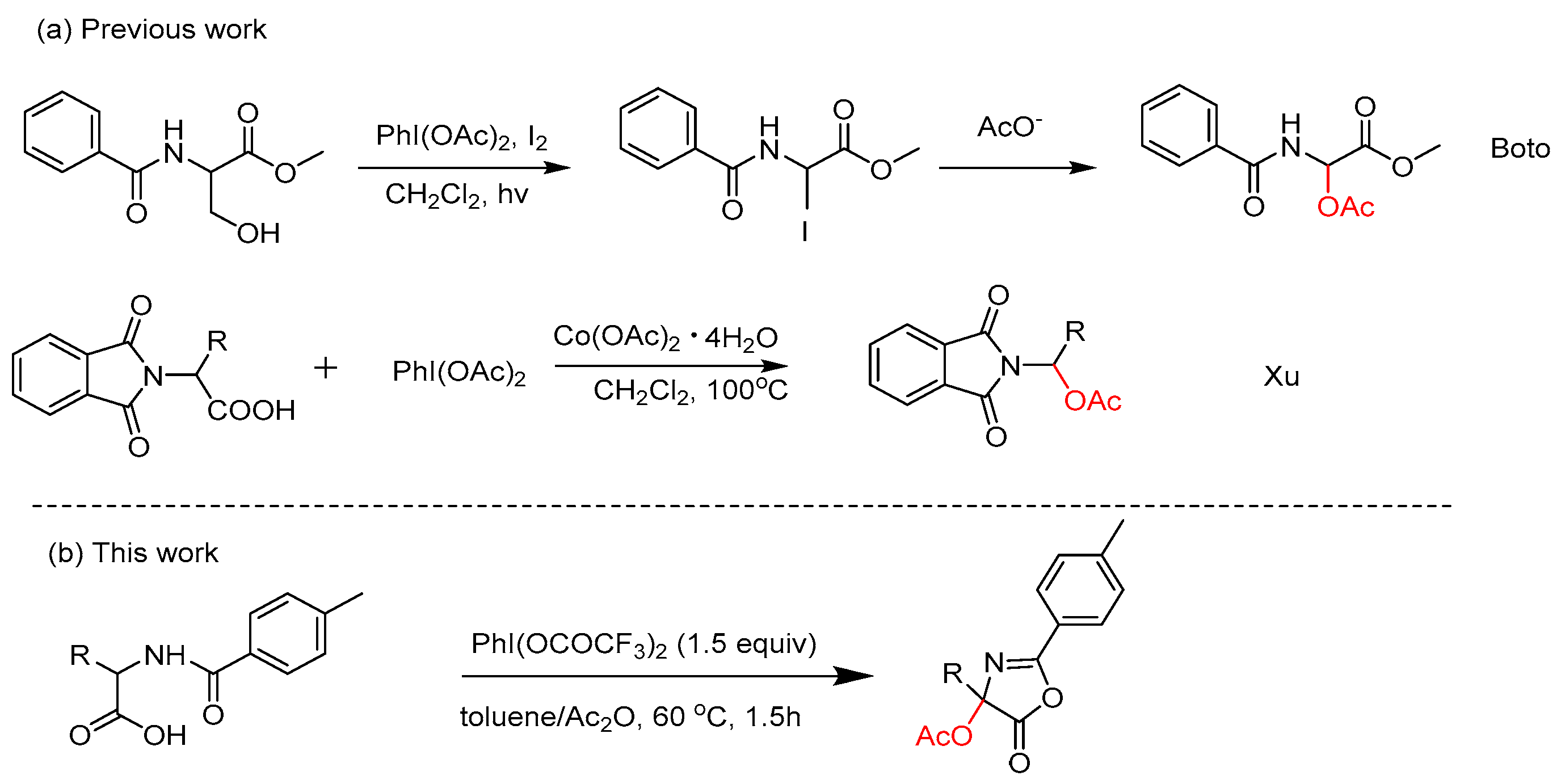
2. Results
3. Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iwasaki, T.; Horikawa, H.; Matsumoto, K.; Miyoshi, M. An electrochemical synthesis of 2-acetoxy-2-amino acid and 3-acetoxy-3-amino acid derivatives. J. Org. Chem. 1977, 42, 2419–2423. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.K.; Kolar, A.J. N-acylimines as intermediates in reactions of α-substituted α-amino acids and dehyroamino acids. Tetrahedron Lett. 1975, 16, 3579–3582. [Google Scholar] [CrossRef]
- Williams, R.M. Synthesis of Optically Active α-Amino Acids; Pergamon Press: Oxford, UK, 1993. [Google Scholar]
- Ben-Ishai, D.; Moshenberg, R.; Altman, J. A new synthesis of amino acids-II: Amidoalkylation of olefins with glyoxylic acid derivatives. Tetrahedron 1977, 33, 1533–1542. [Google Scholar] [CrossRef]
- Apitz, G.; Jäger, M.; Jaroch, S.; Kratzel, M.; Schäffeler, L.; Steglich, W. Generation of α-acetoxyglycine residues within peptide chains: a new strategy for the modification of oligopeptides. Tetrahedron 1993, 49, 8223–8232. [Google Scholar] [CrossRef]
- Weinstein, B.; Lande, S. Peptides: Chemistry and Biochemistry; Marcel Dekker, Inc.: New York, NY, USA, 1970. [Google Scholar]
- Apitz, G.; Steglich, W. Conversion of serine and threonine residues into α-acyloxy-, a-alkylthio-, and α-halogenoglycine moieties: A new strategy for the modification of peptides. Tetrahedron Lett. 1991, 32, 3163–3166. [Google Scholar] [CrossRef]
- Ishikawa, N.; Mustafa, E.S.; Takaoka, A. Trifluoropyruvic Acid Hydrate in Heterocyclic Cynthesis Part III: A Novel Synthesis of 4-(Trifluoromethyl)-oxazolones and other Related Compounds. Heterocycles 1986, 24, 1541–1547. [Google Scholar] [CrossRef]
- Morin, R.B.; Gordon, E.M. Formation of dehydropeptides by oxidation of peptide oxazolones. Tetrahedron Lett. 1973, 14, 2163–2166. [Google Scholar] [CrossRef]
- Murahash, S.I.; Saito, T.; Naota, T.; Kumobayashi, H.; Akutagawa, S. Ruthenium-catalyzed oxidation of β-lactams with molecular oxygen and aldehydes. Tetrahedron Lett. 1991, 32, 5991–5994. [Google Scholar] [CrossRef]
- Horikawa, H.; Iwasaki, T.; Matsumoto, K.; Miyoshi, M. A new synthesis of 2-alkoxy-and 2-acetoxy-2-amino acids by anodic oxidation. Tetrahedron Lett. 1976, 17, 191–194. [Google Scholar] [CrossRef]
- Pouységu, L.; Deffieux, D.; Quideau, S. Hypervalent iodine-mediated phenol dearomatization in natural product synthesis. Tetrahedron 2010, 66, 2235–2261. [Google Scholar] [CrossRef]
- Tian, T.; Zhong, W.-H.; Meng, S.; Meng, X.-B.; Li, Z.-J. Hypervalent Iodine Mediated para-Selective Fluorination of Anilides. J. Org. Chem. 2013, 78, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Samanta, R.; Matcha, K.; Antonchick, A.P. Metal-Free Oxidative Carbon-Heteroatom Bond Formation Through C–H Bond Functionalization. Eur. J. Org. Chem. 2013, 26, 5769–5804. [Google Scholar] [CrossRef]
- Moreno, I.; Tellitu, I.; Herrero, M.; SanMartin, R.; Dominguez, E. New perspectives for iodine (III) reagents in (hetero) biaryl coupling reactions. Curr. Org. Chem. 2002, 6, 1433–1452. [Google Scholar] [CrossRef]
- Kitamura, T.; Kuriki, S.; Morshed, M.H.; Hori, Y. A practical and convenient fluorination of 1, 3-dicarbonyl compounds using aqueous hf in the presence of iodosylbenzene. Org. Lett. 2011, 13, 2392–2394. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Miyamoto, K.; Ochiai, M. Hypervalent phenyl-λ 3-iodane-mediated para-selective aromatic fluorination of 3-phenylpropyl ethers. Chem. Commun. 2011, 47, 3410–3412. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ranjan, P.; Prakash, O. Facile Iodine (III)-Mediated Approach for the Regioselective Chlorination of 2-Aryl-2,3-dihydro-4(1H)-quinolones. Synth. Commun. 2009, 39, 596–603. [Google Scholar] [CrossRef]
- Wardrop, D.J.; Bowen, E.G.; Forslund, R.E.; Sussman, A.D.; Weerasekera, S.L. Intramolecular oxamidation of unsaturated O-alkyl hydroxamates: A remarkably versatile entry to hydroxy lactams. J. Am. Chem. Soc. 2009, 132, 1188–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-F.; Zhang, C. Iodobenzene dichloride as a stoichiometric oxidant for the conversion of alcohols into carbonyl compounds; two facile methods for its preparation. Synthesis 2007, 4, 551–557. [Google Scholar] [CrossRef]
- Zhong, W.-H.; Yang, J.; Meng, X.-B.; Li, Z.-J. BF3·OEt2-Promoted Diastereoselective Diacetoxylation of Alkenes by PhI(OAc)2. J. Org. Chem. 2011, 76, 9997–10004. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhao, Y.; Zhang, S.; Lu, C.; Chen, G. Highly efficient syntheses of azetidines, pyrrolidines, and indolines via palladium catalyzed intramolecular amination of C(sp3)-H and C(sp2)-H bonds at γ and δ positions. J. Am. Chem. Soc. 2011, 134, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Boto, A.; Gallardo, J.A.; Hernández, D.; Hernández, R. Synthesis of unnatural amino acids from serine derivatives by β-fragmentation of primary alkoxyl radicals. J. Org. Chem. 2007, 72, 7260–7269. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.J.; Horgan, S.W.; Shah, R.S.; Farr, R.A.; Schnettler, R.A.; Nevill, C.R.; Weiberth, F.J.; Huber, E.W.; Baron, B.M.; Webster, M.E. ChemInform abstract: Chemical development of MDL 103371: An N-methyl-d-aspartate-Type glycine receptor antagonist for the treatment of stroke. Org. Process. Res. Dev. 2000, 4, 477–487. [Google Scholar] [CrossRef]
- Stead, D.; Carbone, G.; O’Brien, P.; Campos, K.R.; Coldham, I.; Sanderson, A. Asymmetric deprotonation of N-boc piperidine: React IR monitoring and mechanistic aspects. J. Am. Chem. Soc. 2010, 132, 7260–7261. [Google Scholar] [CrossRef] [PubMed]
- Eisenbeis, S.A.; Chen, R.; Kang, M.; Barrila, M.; Buzon, R. Utilization of ReactIR in fit for purpose process enablement. Org. Process. Res. Dev. 2014, 19, 244–248. [Google Scholar] [CrossRef]
- Mizukami, F.; Ando, M.; Tanaka, T.; Imamura, J. The acetoxylation of p-substituted acetophenones and β-diketones with (diacetoxyiodo) benzene. Bull. Chem. Soc. Jpn. 1978, 51, 335–336. [Google Scholar] [CrossRef]
- Padwa, A.; Akiba, M.; Cohen, L.A.; Macdonald, J.G. Aza cope rearrangements in the cyclopropenyl- and allyl-substituted. DELTA. 2-oxazolinone systems. J. Org. Chem. 1983, 48, 695–703. [Google Scholar] [CrossRef]
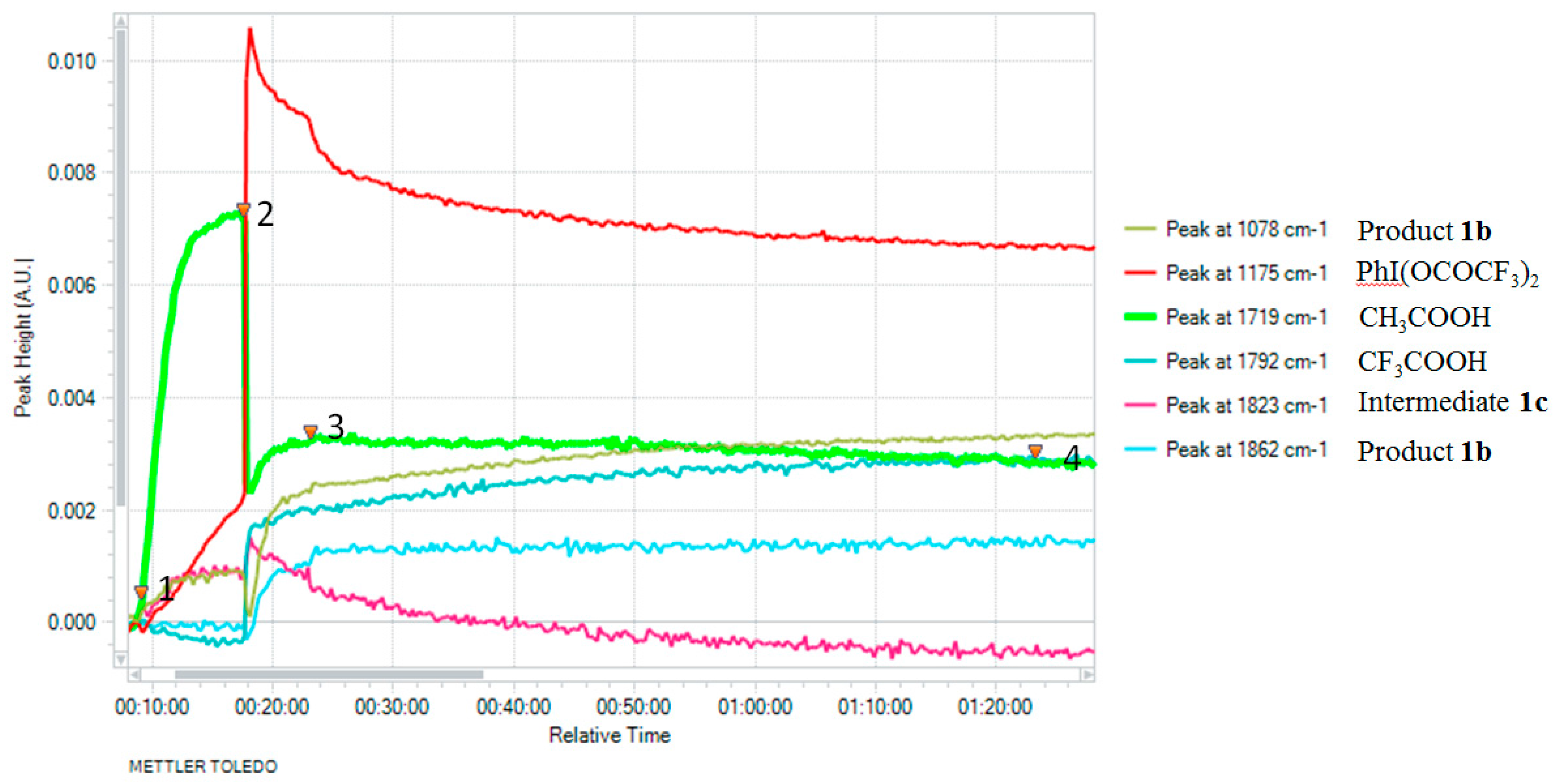
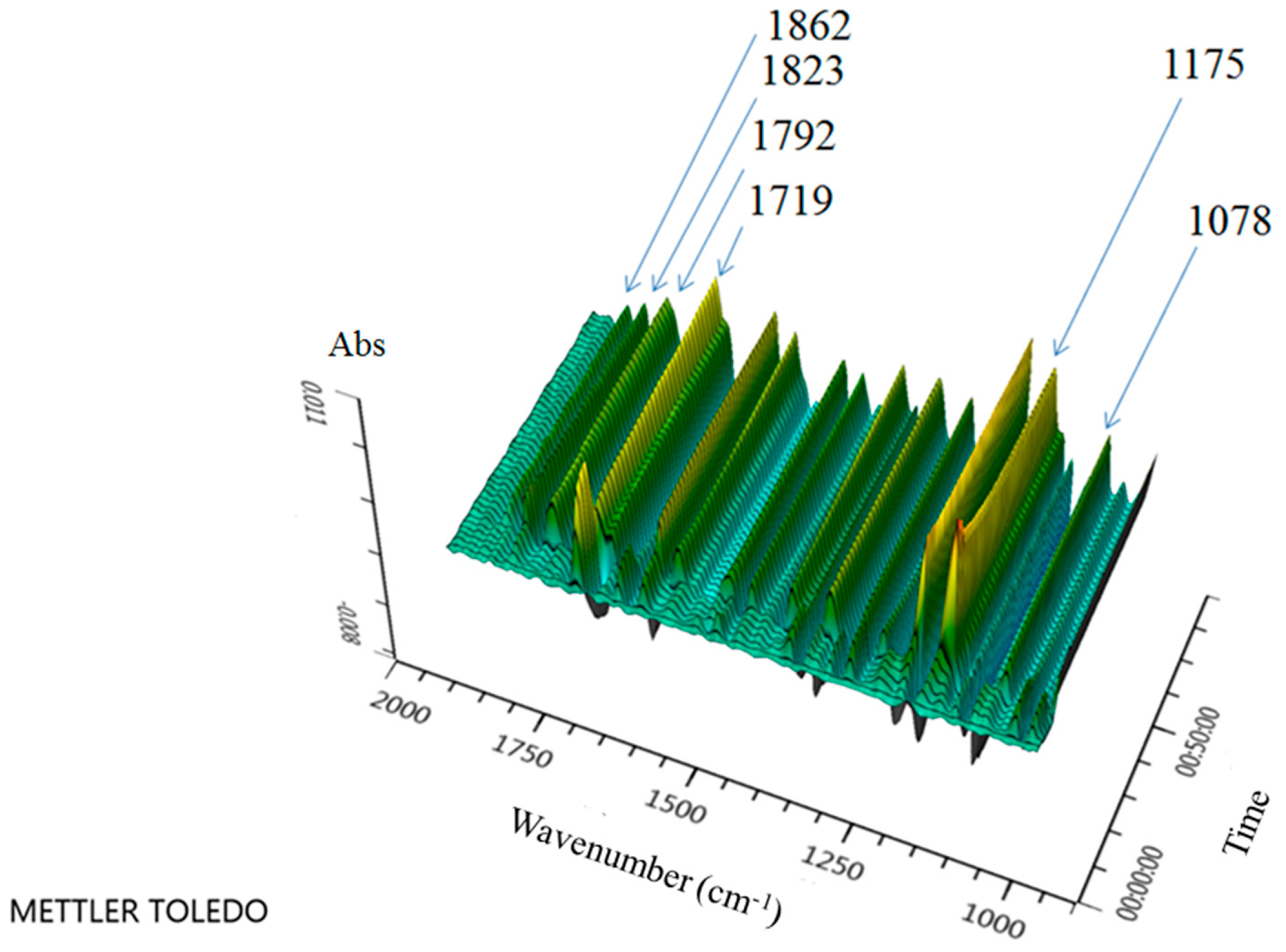
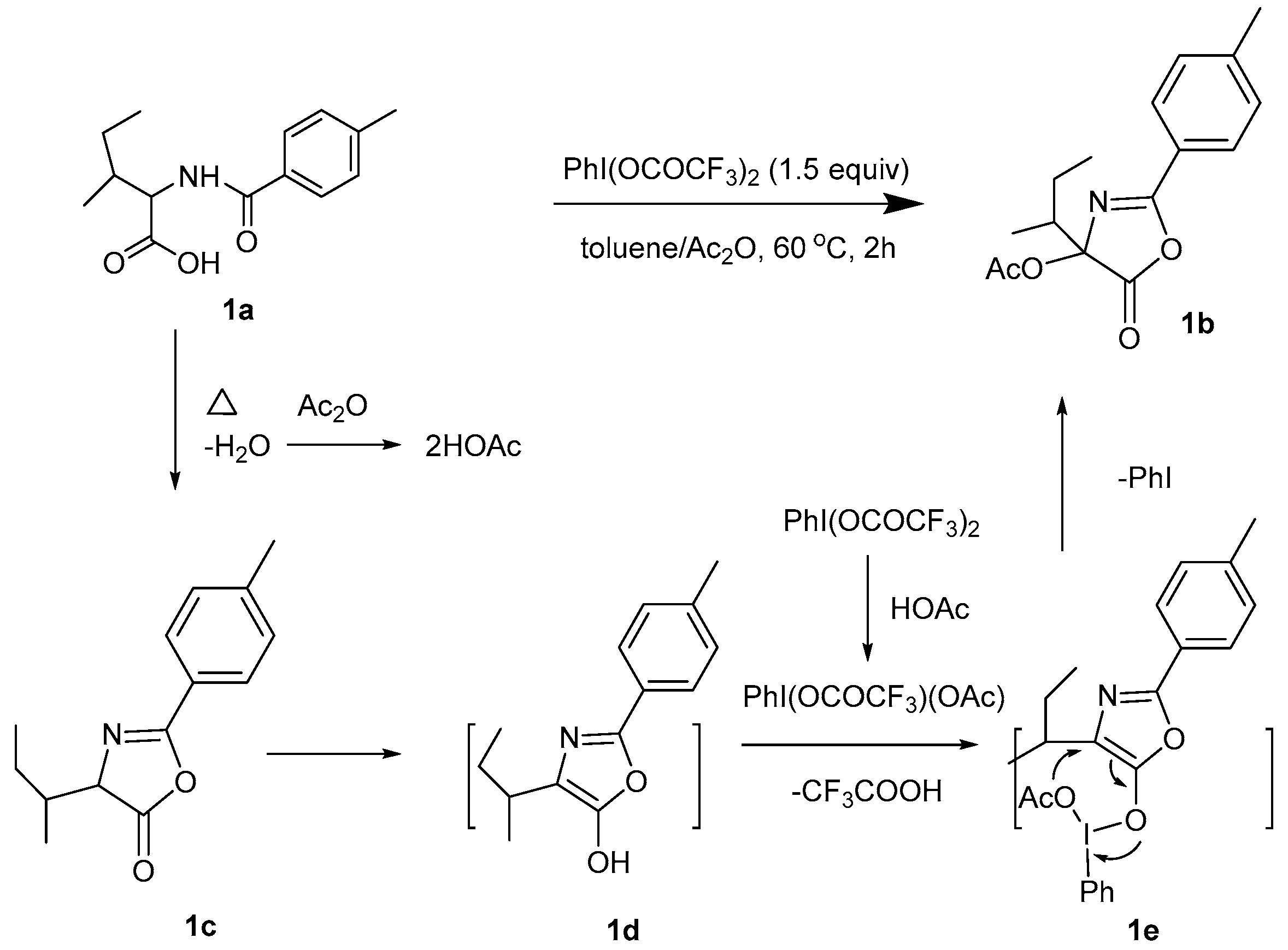
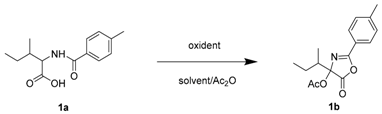
| Entry | Oxidant (equiv) | Temp (°C) | Solvent | Yield (%) a |
|---|---|---|---|---|
| 1 | PhI(OCOCF3)2 (1) | 60 | toluene | 40 |
| 2 | PhI(OCOCF3)2 (1.5) | 60 | toluene | 68 (63 b) |
| 3 | PhI(OCOCF3)2 (3) | 60 | toluene | 50 |
| 4 | PhI(OCOCF3)2 (0) | 60 | toluene | no |
| 5 | PhI(OAc)2 (1.5) | 60 | toluene | 42 |
| 6 | PhI(OPiv)2 (1.5) | 60 | toluene | 19 |
| 7 | AgF2 (1.5) | 60 | toluene | no |
| 8 | PhI(OCOCF3)2 (1.5) | 30 | toluene | 38 |
| 9 | PhI(OCOCF3)2 (1.5) | 90 | toluene | 43 |
| 10 | PhI(OCOCF3)2 (1.5) | 60 | ACN | 50 |
| 11 | PhI(OCOCF3)2 (1.5) | 60 | DCE | 43 |
| 12 | PhI(OCOCF3)2 (1.5) | 60 | Acetone | 8 |
| 13 | PhI(OCOCF3)2 (1.5) | 60 | THF | no |
| Entry | Substrates | Products (Isolated Yield) | Entry | Substrates | Products (Isolated Yield) |
|---|---|---|---|---|---|
| 1 | 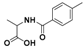 2a | 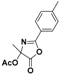 2b (53%) | 6 |  7a | 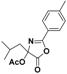 7b (47%) |
| 2 | 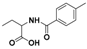 3a | 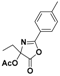 3b (47%) | 7 | 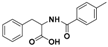 8a |  8b (45%) |
| 3 |  4a | 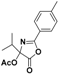 4b (53%) | 8 | 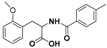 9a |  9b (51%) |
| 4 | 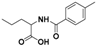 5a |  5b (47%) | 9 |  10a | 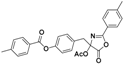 10b (45%) |
| 5 |  6a | 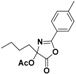 6b (60%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, G.; Zhang, W.-X.; Wu, S. A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine. Molecules 2017, 22, 1102. https://doi.org/10.3390/molecules22071102
Wen G, Zhang W-X, Wu S. A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine. Molecules. 2017; 22(7):1102. https://doi.org/10.3390/molecules22071102
Chicago/Turabian StyleWen, Gang, Wen-Xuan Zhang, and Song Wu. 2017. "A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine" Molecules 22, no. 7: 1102. https://doi.org/10.3390/molecules22071102
APA StyleWen, G., Zhang, W.-X., & Wu, S. (2017). A Novel Synthesis of 4-Acetoxyl 5(4H)-Oxazolones by Direct α-Oxidation of N-Benzoyl Amino-Acid Using Hypervalent Iodine. Molecules, 22(7), 1102. https://doi.org/10.3390/molecules22071102