An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs
Abstract
:1. Introduction
2. Chemical Classification of Marine Bioactive Compounds
2.1. Alkaloids
2.2. Polyketides
2.3. Terpenes
2.4. Peptides
2.5. Carbohydrates, Glycosides and Others
3. MNP-Based Drugs that Are Approved or in Ongoing Clinical Trials
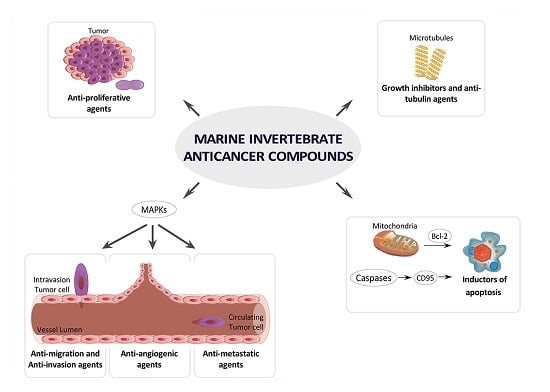
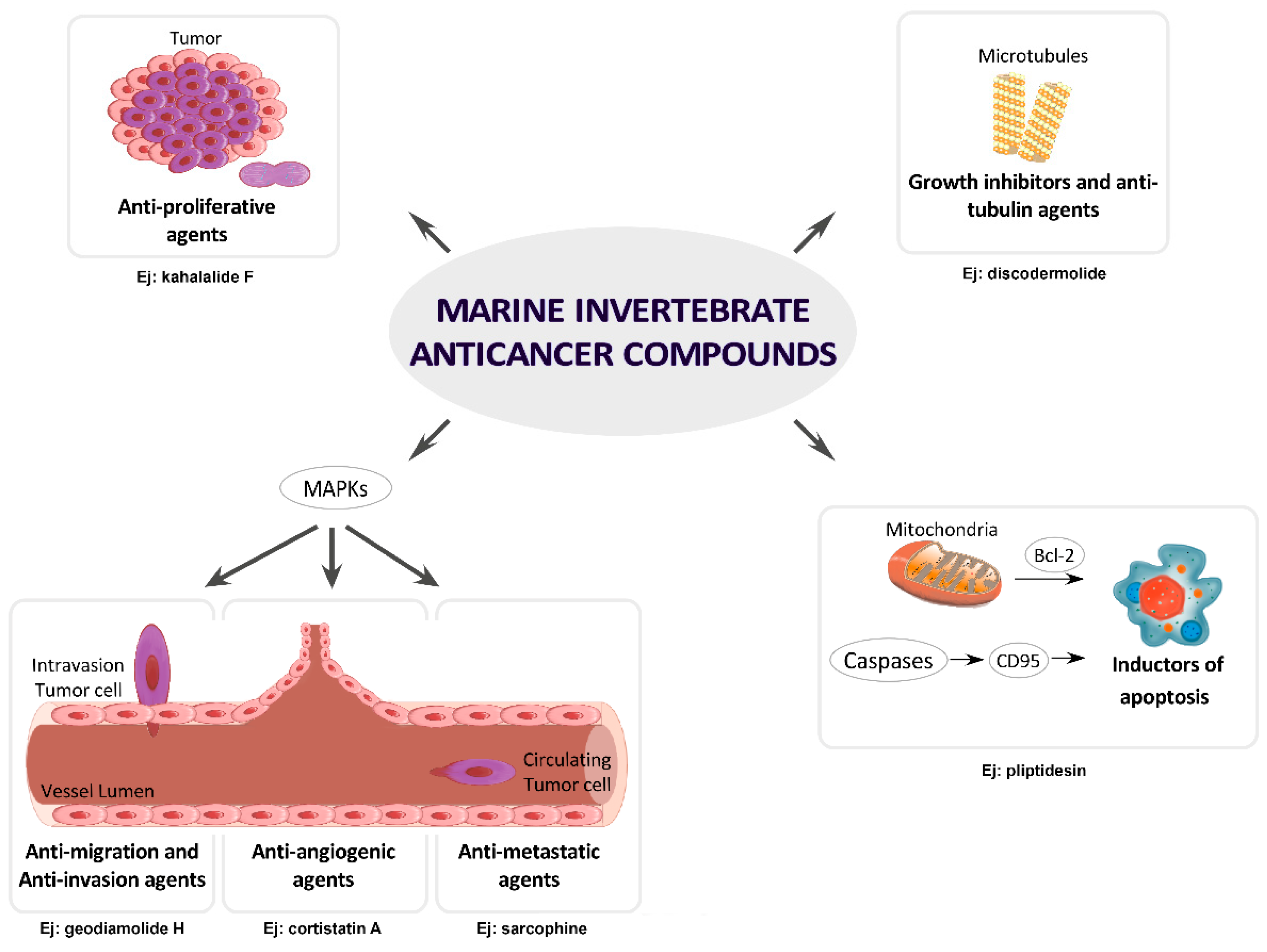
4. MNPs under Research or in Preclinical Stages Classified by Cancer Molecular Targets
4.1. Growth Inhibitors and Anti-Tubulin Agents
4.2. Inductors of Apoptosis and Autophagy
4.3. Inhibitors of Angiogenesis, Migration, Invasion or Metastasis
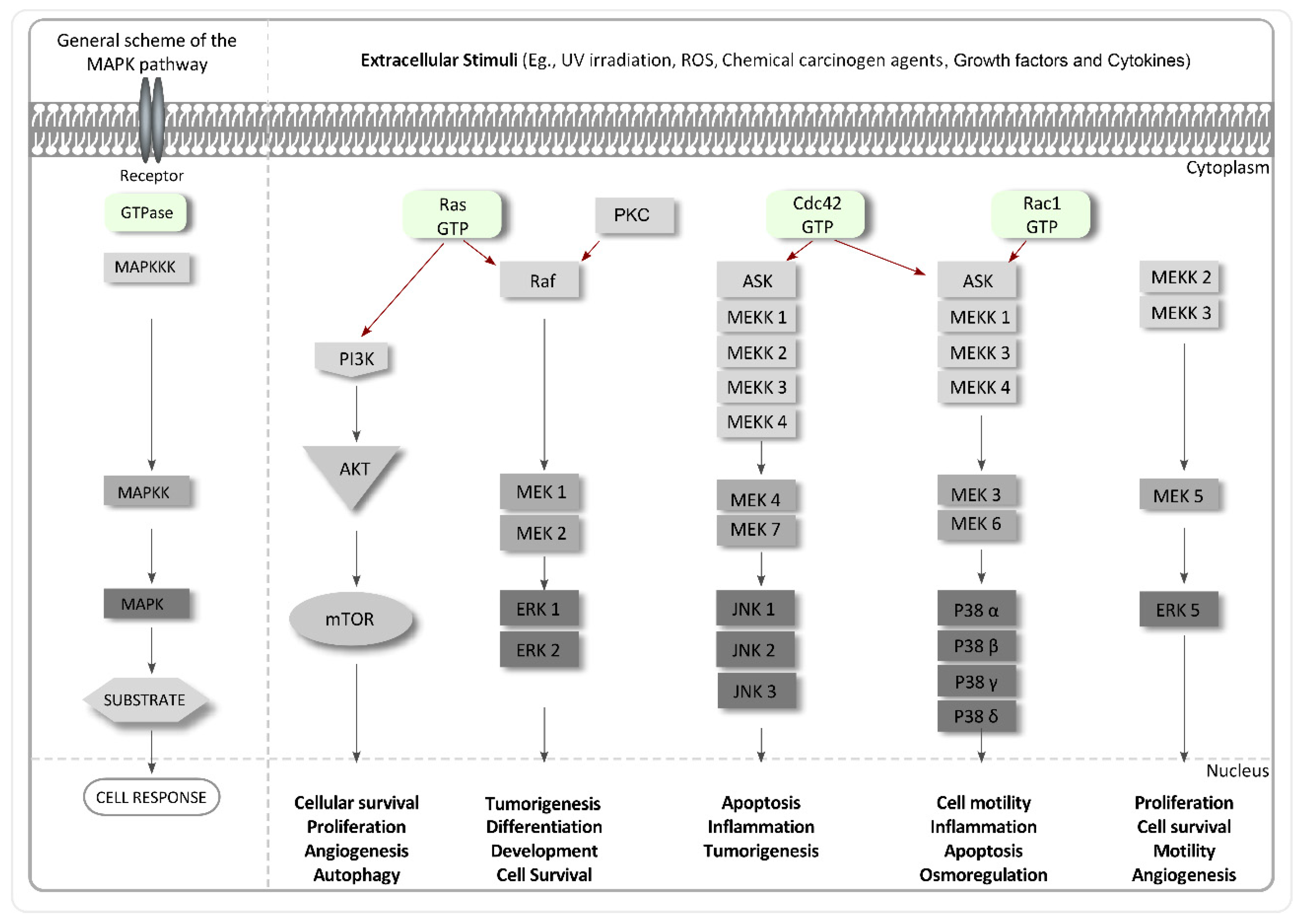
4.4. Inhibitors of MAPKs
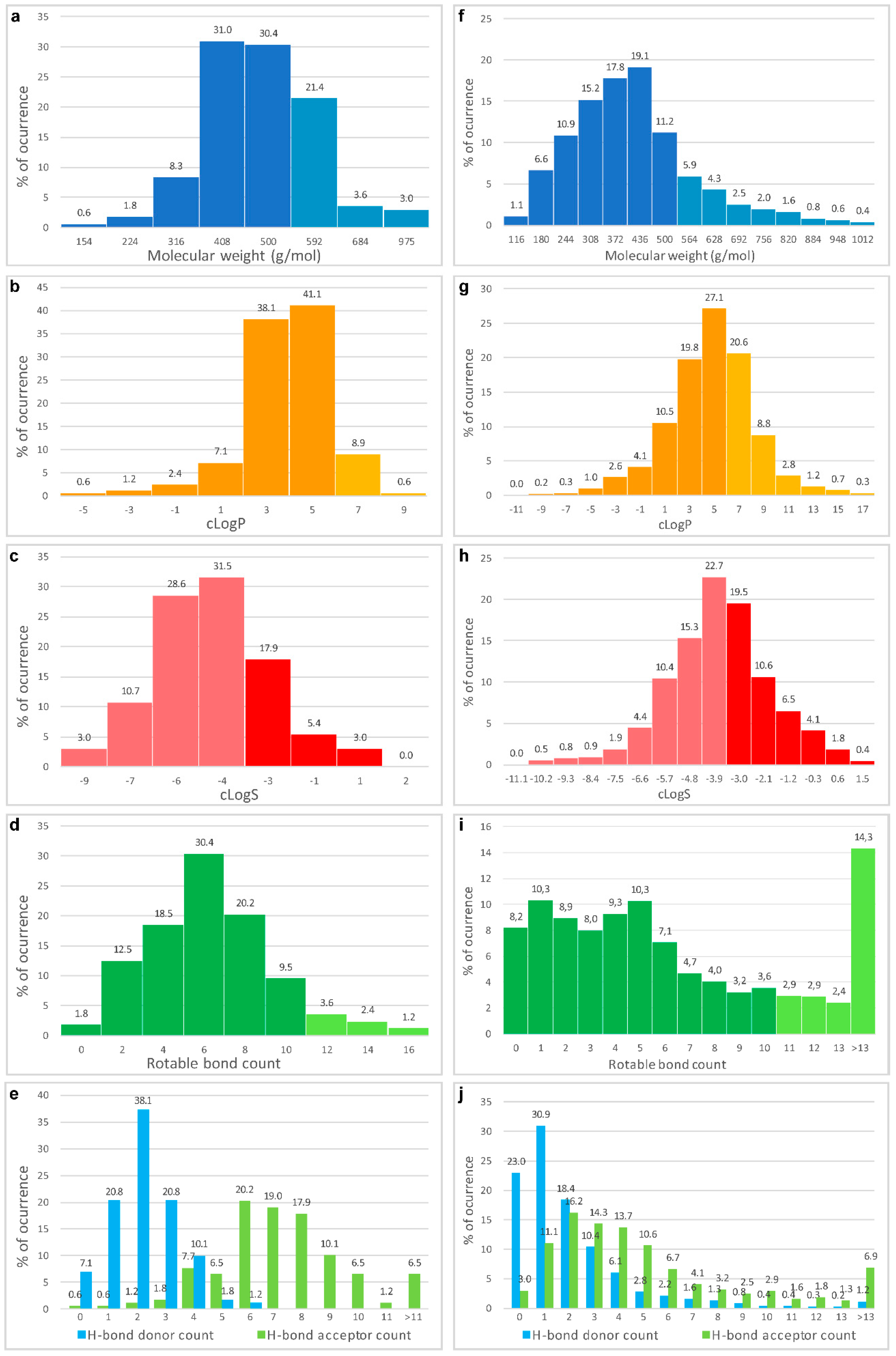
5. New Perspectives on the Virtual Screening of MNPs for the Discovery of Anticancer Compounds
6. Limitations of Marine Invertebrates as Source for Anticancer Agents
7. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | absorption, distribution, metabolism, excretion, and toxicity |
| AMPK | AMP-activated protein kinase |
| CAM | chorioallantoic membrane assay |
| MNPs | marine natural products |
| MAPK | mitogen-activated protein kinase |
| VEGF | vascular endothelial growth factor |
| ERS | endoplasmic reticulum stress |
| MMP | matrix metalloproteinase |
References
- American Cancer Society. Cancer Facts & Figures 2016; American Cancer Society: Atlanta, GA, USA, 2016. [Google Scholar]
- Mudit, M.; El Sayed, K.A. Cancer control potential of marine natural product scaffolds through inhibition of tumor cell migration and invasion. Drug Discov. Today 2016, 21, 1745–1760. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves first immunotherapy combo. Nat. Rev. Drug Discov. 2015, 14, 739. [Google Scholar] [CrossRef]
- Nicolini, A.; Carpi, A.; Ferrari, P.; Biava, P.M.; Rossi, G. Immunotherapy and hormone-therapy in metastatic breast cancer: A review and an update. Curr. Drug Targets 2016, 17, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.L.; Chaudhuri, P.K.; Ramalingam, N.; Tan, D.S.; Lim, C.T.; Warkiani, M.E. Single-cell profiling approaches to probing tumor heterogeneity. Int. J. Cancer 2016, 139, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Ohlsson, R.; Henikoff, S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 2006, 7, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Paterson, I.; Anderson, E.A. The renaissance of natural products as drug candidates. Science 2005, 310, 451. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Pietra, F. Secondary metabolites from marine microorganisms: Bacteria, protozoa, algae and fungi. Achievements and prospects. Nat. Prod. Rep. 1997, 14, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Microbial production of primary metabolites. Naturwissenschaften 1980, 67, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Role of secondary metabolites in chemical defence mechanisms in plants. Ciba Found. Symp. 1990, 154, 126–134. [Google Scholar] [PubMed]
- Bell, S.C.; Alford, R.A.; Garland, S.; Padilla, G.; Thomas, A.D. Screening bacterial metabolites for inhibitory effects against batrachochytrium dendrobatidis using a spectrophotometric assay. Dis. Aquat. Org. 2013, 103, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.O.; Badal, S. Chapter 1—Background to pharmacognosy. In Pharmacognosy; Academic Press: Boston, MA, USA, 2017; pp. 3–13. [Google Scholar]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.T.; Benkendorff, K.; Green, T.; Speck, P. Marine snails and slugs: A great place to look for antiviral drugs. J. Virol. 2015, 89, 8114–8118. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R.; Baker, H.L.; Bewley, C.A. Celebesides A-C and theopapuamides B-D, depsipeptides from an Indonesian sponge that inhibit HIV-1 entry. J. Org. Chem. 2009, 74, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Nieves, K.; Rodríguez, A.D. Neopetrosiamine A, biologically active bis-piperidine alkaloid from the caribbean sea sponge neopetrosia proxima. Bioorg. Med. Chem. Lett. 2010, 20, 5905–5908. [Google Scholar] [CrossRef] [PubMed]
- Nuijen, B.; Bouma, M.; Manada, C.; Jimeno, J.M.; Schellens, J.H.; Bult, A.; Beijnen, J.H. Pharmaceutical development of anticancer agents derived from marine sources. Anticancer Drugs 2000, 11, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Asolkar, R.N.; Freel, K.C.; Jensen, P.R.; Fenical, W.; Kondratyuk, T.P.; Park, E.J.; Pezzuto, J.M. Arenamides A-C, cytotoxic NFkappaB inhibitors from the marine actinomycete Salinispora arenicola. J. Nat. Prod. 2009, 72, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Lee, Y.-R.; Cho, K.-S.; Cho, Y.-N.; Lee, H.A.; Hwang, D.-Y.; Jung, Y.-J.; Son, H.-J. Stalked sea squirt (Styela clava) tunic waste as a valuable bioresource: Cosmetic and antioxidant activities. Process Biochem. 2015, 50, 1977–1984. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, T.A.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2017, 17, e30–e41. [Google Scholar] [CrossRef]
- Kong, D.-X.; Jiang, Y.-Y.; Zhang, H.-Y. Marine natural products as sources of novel scaffolds: Achievement and concern. Drug Discov. Today 2010, 15, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Pomponi, S.A. The bioprocess—Technological potential of the sea. J. Biotechnol. 1999, 70, 5–13. [Google Scholar] [CrossRef]
- Williams, P.G. Panning for chemical gold: Marine bacteria as a source of new therapeutics. Trends Biotechnol. 2009, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, J.; Faircloth, G.; Sousa-Faro, F.J.; Scheuer, P.; Rinehart, K. New marine derived anticancer therapeutics—A journey from the sea to clinical trials. Mar. Drugs 2004, 2, 14–29. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kim, S.-K. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid. Based Complement. Altern. Med. 2013, 2013, 572859. [Google Scholar] [CrossRef] [PubMed]
- Nikapitiya, C. Chapter 24—Bioactive secondary metabolites from marine microbes for drug discovery. In Advances in Food and Nutrition Research; Se-Kwon, K., Ed.; Academic Press: New York, NY, USA, 2012; Volume 65, pp. 363–387. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Pallela, R. Chapter 1—Medicinal foods from marine animals: Current status and prospects. In Advances in Food and Nutrition Research; Se-Kwon, K., Ed.; Academic Press: New York, NY, USA, 2012; Volume 65, pp. 1–9. [Google Scholar]
- Ramsey, U.P.; Bird, C.J.; Shacklock, P.F.; Laycock, M.V.; Wright, J.L. Kainic acid and 1′-hydroxykainic acid from palmariales. Nat. Toxins 1994, 2, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Landowne, R.A.; Bergmann, W. Contributions to the study of marine products. L. Phospholipids of sponges1,2. J. Org. Chem. 1961, 26, 1257–1261. [Google Scholar]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Cruz, L.J.; de Santos, V.; LeCheminant, G.W.; Griffin, D.; Zeikus, R.; McIntosh, J.M.; Galyean, R.; Varga, J.; Gray, W.R.; et al. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry 1987, 26, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- D’Incalci, M.; Badri, N.; Galmarini, C.M.; Allavena, P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br. J. Cancer 2014, 111, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin e7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Newland, A.M.; Li, J.X.; Wasco, L.E.; Aziz, M.T.; Lowe, D.K. Brentuximab vedotin: A CD30-directed antibody-cytotoxic drug conjugate. Pharmacotherapy 2013, 33, 93–104. [Google Scholar] [CrossRef] [PubMed]
- De Zoysa, M. Medicinal benefits of marine invertebrates: Sources for discovering natural drug candidates. Adv. Food Nutr. Res. 2012, 65, 153–169. [Google Scholar] [PubMed]
- Thorpe, J.P.; Solé-Cava, A.M.; Watts, P.C. Exploited marine invertebrates: Genetics and fisheries. Hydrobiologia 2000, 420, 165–184. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Kolita, B.; Dutta, P.P.; Dutta, D.J.; Neipihoi; Nath, S.; Bordoloi, M.; Quan, P.M.; Thuy, T.T.; Phuong, D.L.; et al. Marine steroids as potential anticancer drug candidates: In silico investigation in search of inhibitors of Bcl-2 and CDK-4/Cyclin D1. Steroids 2015, 102, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.-P.; Yuan, J.; Sun, L.; She, Z.-G.; Wu, J.-H.; Lan, X.-J.; Zhu, X.; Lin, Y.-C.; Chen, S.-P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Madeira, C.; Brandao, C.A.; Puga, J.; Calado, R. Bioprospecting of marine invertebrates for new natural products—A chemical and zoogeographical perspective. Molecules 2012, 17, 9842–9854. [Google Scholar] [CrossRef] [PubMed]
- Voultsiadou, E.; Vafidis, D. Marine invertebrate diversity in Aristotle’s zoology. Contrib. Zool. 2007, 76, 103–120. [Google Scholar]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [PubMed]
- Kukula-Koch, W.A.; Widelski, J. Chapter 9—Alkaloids A2—Badal, Simone. In Pharmacognosy; Delgoda, R., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 163–198. [Google Scholar]
- Zhang, Y.; Han, T.; Ming, Q.; Wu, L.; Rahman, K.; Qin, L. Alkaloids produced by endophytic fungi: A review. Nat. Prod. Commun. 2012, 7, 963–968. [Google Scholar] [PubMed]
- Rocha-Santos, T.; Duarte, A.C. Introduction to the analysis of bioactive compounds in marine samples. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 65, pp. 1–13. [Google Scholar]
- Molinski, T.F. Marine pyridoacridine alkaloids: Structure, synthesis, and biological chemistry. Chem. Rev. 1993, 93, 1825–1838. [Google Scholar] [CrossRef]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.M.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Müller, W.E.G.; Proksch, P. From anti-fouling to biofilm inhibition: New cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Hedner, E.; Charnock, C.; Samuelsen, Ø.; Larsson, R.; Gundersen, L.L.; Bohlin, L. (+)-Agelasine D: Improved synthesis and evaluation of antibacterial and cytotoxic activities. J. Nat. Prod. 2006, 69, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Seupel, R.; Feineis, D.; Zhang, G.; Xu, M.; Wu, J.; Kaiser, M.; Brun, R.; Seo, E.-J.; Efferth, T. Ancistectorine D, a naphthylisoquinoline alkaloid with antiprotozoal and antileukemic activities, and further 5,8′- and 7,1′-linked metabolites from the Chinese liana Ancistrocladus tectorius. Fitoterapia 2016, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, J.; Mihci, G.; Martin, M.T.; Gallard, J.F.; Menou, J.L.; Boury-Esnault, N.; Hooper, J.; Petek, S.; Chevalley, S.; Valentin, A.; et al. Agelasines J, K, and L from the Solomon Islands marine sponge Agelas cf. mauritiana. J. Nat. Prod. 2008, 71, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.-J.; Tang, X.-L.; Qin, G.-F.; de Voogd, N.J.; Li, P.-L.; Li, G.-Q. Three new non-brominated pyrrole alkaloids from the South China Sea sponge Agelas nakamurai. Chin. Chem. Lett. 2017, 28, 1210–1213. [Google Scholar] [CrossRef]
- Hopwood, D.A. Complex enzymes in microbial natural product biosynthesis, part B: Polyketides, aminocoumarins and carbohydrates. Preface. Methods Enzymol 2009, 459, 4624–4625. [Google Scholar]
- Hochmuth, T.; Piel, J. Polyketide synthases of bacterial symbionts in sponges—Evolution-based applications in natural products research. Phytochemistry 2009, 70, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Fukai, M.; Miki, K.; Shiraishi, T.; Suzuki, T.; Nishio, K.; Sugita, T.; Ishino, M.; Kinoshita, K.; Takahashi, K.; et al. Chemical constituents of a marine fungus, Arthrinium sacchari. J. Nat. Prod. 2011, 74, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Ebel, R. Terpenes from marine-derived fungi. Mar. Drugs 2010, 8, 2340–2368. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol. 2016, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Park, Y. The effects of marine carbohydrates and glycosylated compounds on human health. Int. J. Mol. Sci. 2015, 16, 6018–6056. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Safari, D.; Dekker, H.A.; Joosten, J.A.; Michalik, D.; de Souza, A.C.; Adamo, R.; Lahmann, M.; Sundgren, A.; Oscarson, S.; Kamerling, J.P.; et al. Identification of the smallest structure capable of evoking opsonophagocytic antibodies against Streptococcus pneumoniae type 14. Infect. Immun. 2008, 76, 4615–4623. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hwang, H.-M.; Aker, W.G.; Wang, P.; Lin, Y.; Jiang, X.; He, X. Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa. Microbiol. Res. 2014, 169, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Kren, V.; Martinkova, L. Glycosides in medicine: “The role of glycosidic residue in biological activity”. Curr. Med. Chem. 2001, 8, 1303–1328. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Coombe, D.R.; Kett, W.C. Heparan sulfate-protein interactions: Therapeutic potential through structure-function insights. Cell. Mol. Life Sci. 2005, 62, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Alonso, M.J.; Gonzalez-Santiago, L.; Martinez, T.; Losada, A.; Galmarini, C.M.; Munoz, A. The mechanism of action of plitidepsin. Curr. Opin. Investig. Drugs 2009, 10, 536–542. [Google Scholar] [PubMed]
- Krege, S.; Rexer, H.; vom Dorp, F.; de Geeter, P.; Klotz, T.; Retz, M.; Heidenreich, A.; Kuhn, M.; Kamradt, J.; Feyerabend, S.; et al. Prospective randomized double-blind multicentre phase II study comparing gemcitabine and cisplatin plus sorafenib chemotherapy with gemcitabine and cisplatin plus placebo in locally advanced and/or metastasized urothelial cancer: SUSE (AUO-AB 31/05). BJU Int. 2014, 113, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.; Saleh, M.; Rose, A.A.N.; Siegel, P.M.; Hart, L.; Sirpal, S.; Jones, S.; Green, J.; Crowley, E.; Simantov, R.; et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2014, 32, 3619–3625. [Google Scholar] [CrossRef] [PubMed]
- Molina-Guijarro, J.M.; García, C.; Macías, Á.; García-Fernández, L.F.; Moreno, C.; Reyes, F.; Martínez-Leal, J.F.; Fernández, R.; Martínez, V.; Valenzuela, C.; et al. Elisidepsin interacts directly with glycosylceramides in the plasma membrane of tumor cells to induce necrotic cell death. PLoS ONE 2015, 10, e0140782. [Google Scholar] [CrossRef] [PubMed]
- Mandin, P.; Gottesman, S. A genetic approach for finding small rnas regulators of genes of interest identifies RybC as regulating the DpiA/DpiB two-component system. Mol. Microbiol. 2009, 72, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Look, S.A.; Fenical, W.; Jacobs, R.S.; Clardy, J. The pseudopterosins: Anti-inflammatory and analgesic natural products from the sea whip Pseudopterogorgia elisabethae. Proc. Natl. Acad. Sci. USA 1986, 83, 6238–6240. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, R.; Zhu, H. An improved and efficient synthesis for IPL576,092 and its analogues. Monatshefte Chem. Chem. Mon. 2013, 144, 1081–1085. [Google Scholar] [CrossRef]
- Petek, B.J.; Jones, R.L. PM00104 (Zalypsis®): A marine derived alkylating agent. Molecules 2014, 19, 12328–12335. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Kim, S.-K. Marine antitumor drugs: Status, shortfalls and strategies. Mar. Drugs 2010, 8, 2702–2720. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Martinez-Bonet, M.; Sanchez, J.; Fernandez-Pineda, A.; Jimenez, J.L.; Munoz, E.; Moreno, S.; Alvarez, S.; Munoz-Fernandez, M.A. Bryostatin activates HIV-1 latent expression in human astrocytes through a PKC and NF-kB-dependent mechanism. Sci. Rep. 2015, 5, 12442. [Google Scholar] [CrossRef] [PubMed]
- Forero-Torres, A.; Kolibaba, K.S.; Lamy, T.; Jones, S.; Lee, C.; Sharman, J. Polatuzumab vedotin combined with obinutuzumab, cyclophosphamide, doxorubicin, and prednisone (G-CHP) for patients with previously untreated diffuse large B-cell lymphoma (DLBCL): Preliminary results of a phase Ib/II dose-escalation study. Blood 2016, 128, 1856. [Google Scholar]
- Kim, Y.H.; Duvic, M.; Obitz, E.; Gniadecki, R.; Iversen, L.; Osterborg, A.; Whittaker, S.; Illidge, T.M.; Schwarz, T.; Kaufmann, R.; et al. Clinical efficacy of zanolimumab (HuMax-CD4): Two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood 2007, 109, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Olson, D.E.; Bourassa, M.W.; Karuppagounder, S.S.; Zhang, Y.L.; Gale, J.; Wagner, F.F.; Basso, M.; Coppola, G.; Pinto, J.T.; et al. Hydroxamic acid-based histone deacetylase (HDAC) inhibitors can mediate neuroprotection independent of hdac inhibition. J. Neurosci. 2014, 34, 14328–14337. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, H.; Bozkurt, E.; Uzunoglu, S.; Uslu, R.; Karaca, B. A diverse induction of apoptosis by trabectedin in MCF-7 (HER2−/ER+) and MDA-MB-453 (HER2+/ER−) breast cancer cells. Toxicol. Lett. 2013, 221, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Cavaliere, P.; Wahidulla, S.; Naik, C.G.; Cimino, G. A new antitumor isoquinoline alkaloid from the marine nudibranch Jorunna funebris. Tetrahedron 2000, 56, 7305–7308. [Google Scholar] [CrossRef]
- Oku, N.; Matsunaga, S.; van Soest, R.W.M.; Fusetani, N. Renieramycin J, a highly cytotoxic tetrahydroisoquinoline alkaloid, from a marine sponge Neopetrosia sp. J. Nat. Prod. 2003, 66, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Scarpace, S.L. Eribulin mesylate (E7389): Review of efficacy and tolerability in breast, pancreatic, head and neck, and non–small cell lung cancer. Clin. Ther. 2012, 34, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.J. The structure activity relationship of discodermolide analogues. Mini Rev. Med. Chem. 2008, 8, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zonder, J.A.; Shields, A.F.; Zalupski, M.; Chaplen, R.; Heilbrun, L.K.; Arlauskas, P.; Philip, P.A. A phase II trial of bryostatin 1 in the treatment of metastatic colorectal cancer. Clin. Cancer Res. 2001, 7, 38–42. [Google Scholar] [PubMed]
- Caplan, S.L.; Zheng, B.; Dawson-Scully, K.; White, C.A.; West, L.M. Pseudopterosin A: Protection of synaptic function and potential as a neuromodulatory agent. Mar. Drugs 2016, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Jacobson, P.B.; Fenical, W.; Jacobs, R.S.; Glaser, K.B. Pharmacological characterization of the pseudopterosins: Novel anti-inflammatory natural products isolated from the caribbean soft coral, Pseudopterogorgia elisabethae. Life Sci. 1998, 62, L401–407. [Google Scholar] [CrossRef]
- Olivera, B.M. Conus Peptides: Biodiversity-based discovery and exogenomics. J. Biol. Chem. 2006, 281, 31173–31177. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.K.; Chen, R.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Connors, J.M.; Engert, A.; Larsen, E.K.; Chi, X.; et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory hodgkin lymphoma. Blood 2015, 125, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Riely, G.J.; Gadgeel, S.; Rothman, I.; Saidman, B.; Sabbath, K.; Feit, K.; Kris, M.G.; Rizvi, N.A. A phase 2 study of TZT-1027, administered weekly to patients with advanced non-small cell lung cancer following treatment with platinum-based chemotherapy. Lung Cancer 2007, 55, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Depenbrock, H.; Peter, R.; Faircloth, G.T.; Manzanares, I.; Jimeno, J.; Hanauske, A.R. In vitro activity of aplidine, a new marine-derived anti-cancer compound, on freshly explanted clonogenic human tumour cells and haematopoietic precursor cells. Br. J. Cancer 1998, 78, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Alonso, M.J.; Gonzalez-Santiago, L.; Zarich, N.; Martinez, T.; Alvarez, E.; Rojas, J.M.; Munoz, A. Plitidepsin has a dual effect inhibiting cell cycle and inducing apoptosis via Rac1/c-JUN NH2-terminal kinase activation in human melanoma cells. J. Pharmacol. Exp. Ther. 2008, 324, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Mitsiades, C.S.; Ocio, E.M.; Pandiella, A.; Maiso, P.; Gajate, C.; Garayoa, M.; Vilanova, D.; Montero, J.C.; Mitsiades, N.; McMullan, C.J.; et al. Aplidin, a marine organism-derived compound with potent antimyeloma activity in vitro and in vivo. Cancer Res. 2008, 68, 5216–5225. [Google Scholar] [CrossRef] [PubMed]
- Morande, P.E.; Zanetti, S.R.; Borge, M.; Nannini, P.; Jancic, C.; Bezares, R.F.; Bitsmans, A.; Gonzalez, M.; Rodriguez, A.L.; Galmarini, C.M.; et al. The cytotoxic activity of Aplidin in chronic lymphocytic leukemia (CLL) is mediated by a direct effect on leukemic cells and an indirect effect on monocyte-derived cells. Investig. New Drugs 2012, 30, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Natsume, T.; Watanabe, J.; Ogawa, K.; Yasumura, K.; Kobayashi, M. Tumor-specific antivascular effect of TZT-1027 (Soblidotin) elucidated by magnetic resonance imaging and confocal laser scanning microscopy. Cancer Sci. 2007, 98, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Mita, A.C.; Hammond, L.A.; Bonate, P.L.; Weiss, G.; McCreery, H.; Syed, S.; Garrison, M.; Chu, Q.S.C.; DeBono, J.S.; Jones, C.B.; et al. Phase I and pharmacokinetic study of tasidotin hydrochloride (ILX651), a third-generation dolastatin-15 analogue, administered weekly for 3 weeks every 28 days in patients with advanced solid tumors. Clin. Cancer Res. 2006, 12, 5207. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.T.; Otto, C.S.; Scheuer, P.J.; Dunbar, D.C. Kahalalides: Bioactive peptides from a marine mollusk Elysia rufescens and its algal diet Bryopsis sp. J. Org. Chem. 1996, 61, 6594–6600. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Pavlick, A.C.; Johnson, D.B.; Hart, L.L.; Infante, J.R.; Luke, J.J.; Lutzky, J.; Rothschild, N.; Spitler, L.; Cowey, C.L.; et al. A phase 2 study of glembatumumab vedotin (GV), an antibody-drug conjugate (ADC) targeting gpNMB, in advanced melanoma. Ann. Oncol. 2016, 27, 1147P. [Google Scholar] [CrossRef]
- Advani, R.H.; Lebovic, D.; Chen, A.; Brunvand, M.; Goy, A.; Chang, J.E.; Hochberg, E.; Yalamanchili, S.; Kahn, R.; Lu, D.; et al. Phase I study of the anti-CD22 antibody-drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma. Clin. Cancer Res. 2016, 6, 1078-0432. [Google Scholar] [CrossRef] [PubMed]
- De Goeij, B.E.; Lambert, J.M. New developments for antibody-drug conjugate-based therapeutic approaches. Curr. Opin. Immunol. 2016, 40, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Norton, E.B.; Kaplan, J.A.; Niu, C.; Loganzo, F.; Hernandez, R.; Beyer, C.F.; Annable, T.; Musto, S.; Discafani, C.; et al. Synthesis and activity of novel analogs of hemiasterlin as inhibitors of tubulin polymerization: Modification of the a segment. Bioorg. Med. Chem. Lett. 2004, 14, 5317–5322. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.J.; Coleman, J.E.; Andersen, R.J.; Roberge, M. Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother. Pharmacol. 1997, 39, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Pavan, B.; Simoni, D.; Baruchello, R.; Rondanin, R.; Mischiati, C.; Feriotto, G.; Ferraro, L.; Hsu, L.-C.; Lee, R.M.; et al. A novel hybrid drug between two potent anti-tubulin agents as a potential prolonged anticancer approach. Eur. J. Pharm. Sci. 2016, 91, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, D.; He, W.; Zhang, H.; Li, Z.; Luan, Y. Nanoassemblies from amphiphilic cytarabine prodrug for leukemia targeted therapy. J. Colloid Interface Sci. 2017, 487, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.; Oda, T.; Ohara, Y.; Miyamoto, R.; Kurokawa, T.; Hashimoto, S.; Enomoto, T.; Yamada, K.; Satake, M.; Ohkohchi, N. Anticancer effects of gemcitabine are enhanced by co-administered iRGD peptide in murine pancreatic cancer models that overexpressed neuropilin-1. Br. J. Cancer 2014, 110, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.-T.; Huang, J.-Y.; Chen, M.-H.; Chen, C.-Y.; Shyong, Y.-J.; Yen, K.-C.; Sun, Y.-J.; Ke, C.-J.; Cheng, Y.-H.; Lin, F.-H. Development of gelatin nanoparticles conjugated with phytohemagglutinin erythroagglutinating loaded with gemcitabine for inducing apoptosis in non-small cell lung cancer cells. J. Mater. Chem. B 2016, 4, 2444–2454. [Google Scholar] [CrossRef]
- De Bono, J.S.; Kristeleit, R.; Tolcher, A.; Fong, P.; Pacey, S.; Karavasilis, V.; Mita, M.; Shaw, H.; Workman, P.; Kaye, S.; et al. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 6663–6673. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.A.; Ducki, S.; Hirst, N.; McGown, A.T. Tubulin and microtubules as targets for anticancer drugs. Prog. Cell Cycle Res. 2003, 5, 309–325. [Google Scholar] [PubMed]
- Avendaño, C.; Menéndez, J.C. Chapter 9—Anticancer drugs targeting tubulin and microtubules. In Medicinal Chemistry of Anticancer Drugs, 2nd ed.; Elsevier: Boston, MA, USA, 2015; pp. 359–390. [Google Scholar]
- Wood, K.W.; Cornwell, W.D.; Jackson, J.R. Past and future of the mitotic spindle as an oncology target. Curr. Opin. Pharmacol. 2001, 1, 370–377. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, G.; Gill, R.K.; Soni, R.; Bariwal, J. Recent developments in tubulin polymerization inhibitors: An overview. Eur. J. Med. Chem. 2014, 87, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting microtubules by natural agents for cancer therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anticancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J. Nat. Prod. 2001, 64, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Petit, G.R.; Hamel, E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Biochem. Pharmacol. 1990, 39, 1941–1949. [Google Scholar] [CrossRef]
- Edler, M.C.; Fernandez, A.M.; Lassota, P.; Ireland, C.M.; Barrows, L.R. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the vinca alkaloids, and dolastatin 10. Biochem. Pharmacol. 2002, 63, 707–715. [Google Scholar] [CrossRef]
- Lachia, M.; Moody, C.J. The synthetic challenge of diazonamide a, a macrocyclic indole bis-oxazole marine natural product. Nat. Prod. Rep. 2008, 25, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Monserrate, Z.; Vervoort, H.C.; Bai, R.; Newman, D.J.; Howell, S.B.; Los, G.; Mullaney, J.T.; Williams, M.D.; Pettit, G.R.; Fenical, W.; et al. Diazonamide a and a synthetic structural analog: Disruptive effects on mitosis and cellular microtubules and analysis of their interactions with tubulin. Mol. Pharmacol. 2003, 63, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Guduru, R.; Sun, Y.P.; Banerji, B.; Chen, D.Y. Total synthesis of the originally proposed and revised structures of palmerolide A. Angew Chem. Int. Ed. Engl. 2007, 46, 5896–5900. [Google Scholar] [CrossRef] [PubMed]
- Llorca, O.; Martin-Benito, J.; Gomez-Puertas, P.; Ritco-Vonsovici, M.; Willison, K.R.; Carrascosa, J.L.; Valpuesta, J.M. Analysis of the interaction between the eukaryotic chaperonin CCT and its substrates actin and tubulin. J. Struct. Biol. 2001, 135, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M.; Mao, C.; Jan, S.T.; Huang, H.; Vassilev, A.O.; Navara, C.S.; Narla, R.K. Spongistatins as tubulin targeting agents. Curr. Pharm. Des. 2001, 7, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Zask, A.; Kaplan, J.; Musto, S.; Loganzo, F. Hybrids of the hemiasterlin analogue taltobulin and the dolastatins are potent antimicrotubule agents. J. Am. Chem. Soc. 2005, 127, 17667–17671. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Andreae, P.M.; Northcote, P.T.; Miller, J.H. Peloruside A inhibits microtubule dynamics in a breast cancer cell line MCF7. Investig. New Drugs 2011, 29, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.H.; Kingston, D.G. Zampanolide and dactylolide: Cytotoxic tubulin-assembly agents and promising anticancer leads. Nat. Prod. Rep. 2014, 31, 1202–1226. [Google Scholar] [CrossRef] [PubMed]
- Field, J.J.; Singh, A.J.; Kanakkanthara, A.; Halafihi, T.; Northcote, P.T.; Miller, J.H. Microtubule-stabilizing activity of zampanolide, a potent macrolide isolated from the tongan marine sponge Cacospongia mycofijiensis. J. Med. Chem. 2009, 52, 7328–7332. [Google Scholar] [CrossRef] [PubMed]
- Field, J.J.; Pera, B.; Calvo, E.; Canales, A.; Zurwerra, D.; Trigili, C.; Rodríguez-Salarichs, J.; Matesanz, R.; Kanakkanthara, A.; Wakefield, S.J.; et al. Zampanolide, a potent new microtubule stabilizing agent, covalently reacts with the taxane luminal site in both tubulin α,β-heterodimers and microtubules. Chem. Biol. 2012, 19, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R.A.; Cummins, J.; Pomponi, S.A.; Longley, R.E.; Wright, A.E. Tubulin polymerizing activity of dictyostatin-1, a polyketide of marine sponge origin. Biochem. Pharmacol. 2003, 66, 75–82. [Google Scholar] [CrossRef]
- Paterson, I.; Britton, R.; Delgado, O.; Wright, A.E. Stereochemical determination of dictyostatin, a novel microtubule-stabilising macrolide from the marine sponge Corallistidae sp. Chem. Commun. (Camb.) 2004, 632–633. [Google Scholar] [CrossRef] [PubMed]
- Madiraju, C.; Edler, M.C.; Hamel, E.; Raccor, B.S.; Balachandran, R.; Zhu, G.; Giuliano, K.A.; Vogt, A.; Shin, Y.; Fournier, J.H.; et al. Tubulin assembly, taxoid site binding, and cellular effects of the microtubule-stabilizing agent dictyostatin. Biochemistry 2005, 44, 15053–15063. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, L.L.; Jimenez, M.; Camarco, D.P.; Zhu, W.; Daghestani, H.N.; Balachandran, R.; Reese, C.E.; Lazo, J.S.; Hukriede, N.A.; Curran, D.P.; et al. A simplified synthesis of novel dictyostatin analogues with in vitro activity against epothilone b-resistant cells and antiangiogenic activity in zebrafish embryos. Mol. Cancer Ther. 2011, 10, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Brunden, K.R.; Gardner, N.M.; James, M.J.; Yao, Y.; Trojanowski, J.Q.; Lee, V.M.; Paterson, I.; Ballatore, C.; Smith, A.B., 3rd. Mt-stabilizer, dictyostatin, exhibits prolonged brain retention and activity: Potential therapeutic implications. ACS Med. Chem. Lett. 2013, 4, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Churchill, C.D.; Klobukowski, M.; Tuszynski, J.A. The unique binding mode of laulimalide to two tubulin protofilaments. Chem. Biol. Drug Des. 2015, 86, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, Z.L. Programmed cell death and cancer. Postgrad. Med. J. 2009, 85, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Baehrecke, E.H.; Kroemer, G. Does autophagy contribute to cell death? Autophagy 2005, 1, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Pervaiz, S. Apoptosis signaling in cancer stem cells. Int. J. Biochem. Cell Biol. 2010, 42, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G. Mitochondrial control of apoptosis: An introduction. Biochem. Biophys. Res. Commun. 2003, 304, 433–435. [Google Scholar] [CrossRef]
- Oliver, L.; Vallette, F.M. The role of caspases in cell death and differentiation. Drug Resist. Updat. 2005, 8, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of autophagy in cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Khan, N.; Mukhtar, H. Apoptosis and autophagy induction as mechanism of cancer prevention by naturally occurring dietary agents. Curr. Drug Targets 2012, 13, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Perez-Rojas, J.M.; Hernandez-Damian, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Odaka, C.; Sanders, M.L.; Crews, P. Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway. Clin. Diagn. Lab. Immunol. 2000, 7, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Wray, V.; de Voogd, N.J.; Deng, Z.; Lin, W.; Proksch, P. Two new jaspamide derivatives from the marine sponge Jaspis splendens. Mar. Drugs 2009, 7, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.J.; Morinaka, B.I.; Amagata, T.; Tenney, K.; Bray, W.M.; Gassner, N.C.; Lokey, R.S.; Crews, P. New structures and bioactivity properties of jasplakinolide (jaspamide) analogues from marine sponges. J. Med. Chem. 2010, 53, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Aherne, G.W.; Hardcastle, A.; Valenti, M.; Bryant, A.; Rogers, P.; Pettit, G.R.; Srirangam, J.K.; Kelland, L.R. Antitumour evaluation of dolastatins 10 and 15 and their measurement in plasma by radioimmunoassay. Cancer Chemother. Pharmacol. 1996, 38, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Maki, A.; Diwakaran, H.; Redman, B.; Al-Asfar, S.; Pettit, G.R.; Mohammad, R.M.; Al-Katib, A. The bcl-2 and p53 oncoproteins can be modulated by bryostatin 1 and dolastatins in human diffuse large cell lymphoma. Anticancer Drugs 1995, 6, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Liu, H.; Wang, F.; Zheng, L.; Zhao, J.; Chu, E.; Lin, X. A novel polypeptide extracted from ciona savignyi induces apoptosis through a mitochondrial-mediated pathway in human colorectal carcinoma cells. Clin. Colorectal Cancer 2012, 11, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Cheng, L.; Wei, J.; Wu, N.; Zheng, L.; Lin, X. A novel polypeptide from meretrix meretrix linnaeus inhibits the growth of human lung adenocarcinoma. Exp. Biol. Med. 2012, 237, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Potts, M.B.; McMillan, E.A.; Rosales, T.I.; Kim, H.S.; Ou, Y.-H.; Toombs, J.E.; Brekken, R.A.; Minden, M.D.; MacMillan, J.B.; White, M.A. Mode of action and pharmacogenomic biomarkers for exceptional responders to didemnin B. Nat. Chem. Biol. 2015, 11, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Guzii, A.G.; Makarieva, T.N.; Denisenko, V.A.; Dmitrenok, P.S.; Kuzmich, A.S.; Dyshlovoy, S.A.; Krasokhin, V.B.; Stonik, V.A. Monanchocidin: A new apoptosis-inducing polycyclic guanidine alkaloid from the marine sponge Monanchora pulchra. Org. Lett. 2010, 12, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Hauschild, J.; Amann, K.; Tabakmakher, K.M.; Venz, S.; Walther, R.; Guzii, A.G.; Makarieva, T.N.; Shubina, L.K.; Fedorov, S.N.; et al. Marine alkaloid monanchocidin a overcomes drug resistance by induction of autophagy and lysosomal membrane permeabilization. Oncotarget 2015, 6, 17328–17341. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; McIver, C.M.; Abbott, C.A. Bioactivity of the Murex Homeopathic Remedy and of Extracts from an Australian Muricid Mollusc against Human Cancer Cells. Evid. Based Complement. Altern. Med. 2011, 2011, 879585. [Google Scholar] [CrossRef] [PubMed]
- Vine, K.L.; Locke, J.M.; Ranson, M.; Pyne, S.G.; Bremner, J.B. In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorg. Med. Chem. 2007, 15, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Westley, C.B.; McIver, C.M.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. Enhanced acute apoptotic response to azoxymethane-induced DNA damage in the rodent colonic epithelium by tyrian purple precursors: A potential colorectal cancer chemopreventative. Cancer Biol. Ther. 2010, 9, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Edwards, V.; Benkendorff, K.; Young, F. Marine compounds selectively induce apoptosis in female reproductive cancer cells but not in primary-derived human reproductive granulosa cells. Mar. Drugs 2012, 10, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Casapullo, A.; Cutignano, A.; Bruno, I.; Bifulco, G.; Debitus, C.; Gomez-Paloma, L.; Riccio, R. Makaluvamine P, a new cytotoxic pyrroloiminoquinone from Zyzzya cf. fuliginosa. J. Nat. Prod. 2001, 64, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Shinkre, B.A.; Raisch, K.P.; Fan, L.; Velu, S.E. Analogs of the marine alkaloid makaluvamines: Synthesis, topoisomerase II inhibition, and anticancer activity. Bioorg. Med. Chem. Lett. 2007, 17, 2890–2893. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, Y.; Guo, H.; Liu, Y.; Hu, P.; Yang, X.; Li, X.; Ge, S.; Velu, S.E.; Nadkarni, D.H.; et al. Experimental therapy of ovarian cancer with synthetic makaluvamine analog: In vitro and in vivo anticancer activity and molecular mechanisms of action. PLoS ONE 2011, 6, e20729. [Google Scholar] [CrossRef] [PubMed]
- Tomasic, T.; Nabergoj, D.; Vrbek, S.; Zidar, N.; Jakopin, Z.; Zula, A.; Hodnik, Z.; Jukic, M.; Anderluh, M.; Ilas, J.; et al. Analogues of the marine alkaloids oroidin, clathrodin, and hymenidin induce apoptosis in human HepG2 and THP-1 cancer cells. MedChemComm 2015, 6, 105–110. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, Y.; Wang, J.F.; Li, H.; Long, T.T.; Li, Z.; Wang, Y.M.; Dong, P.; Xue, C.H. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearsonothuria graeffei. J. Sci. Food Agric. 2012, 92, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Q.; Peng, X.; Zhou, C.; Zhong, Y.; Chen, X.; Qiu, Y.; Jin, M.; Gong, M.; Kong, D. Stellettin B induces G1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci. Rep. 2016, 6, 27071. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Bélanger, J.; ApSimon, J.W.; Garneau, F.-X.; Harvey, C.; Brisson, J.-R. Frondoside A. A novel triterpene glycoside from the holothurian Cucumariafrondosa. Can. J. Chem. 1990, 68, 11–18. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Menchinskaya, E.S.; Venz, S.; Rast, S.; Amann, K.; Hauschild, J.; Otte, K.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; et al. The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Int. J. Cancer 2016, 138, 2450–2465. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Na, M.; Kim, H.G.; Khanal, T.; Choi, J.H.; Jin, S.W.; Oh, S.H.; Hwang, I.H.; Chung, Y.C.; Kim, H.S.; et al. Ilimaquinone induces death receptor expression and sensitizes human colon cancer cells to TRAIL-induced apoptosis through activation of ROS-ERK/p38 MAPK–CHOP signaling pathways. Food Chem. Toxicol. 2014, 71, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Okada, T.; Jahangeer, S.; Nakamura, S. Requirement of phospholipase D for ilimaquinone-induced Golgi membrane fragmentation. J. Biol. Chem. 2007, 282, 34085–34092. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhou, Y.D.; Nagle, D.G. Inducers of hypoxic response: Marine sesquiterpene quinones activate HIF-1. J. Nat. Prod. 2013, 76, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.C.; Huang, S.Y.; Chen, S.P.; Su, C.C.; Chiu, T.L.; Pang, C.Y. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 2013, 16, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Chung, J.K.; Hwang, H.I.; Gwak, J.; Park, S.; Ju, G.B.; Yun, E.; Kim, D.-E.; Chung, Y.-H.; Na, M.; et al. Activation of p53 with ilimaquinone and ethylsmenoquinone, marine sponge metabolites, induces apoptosis and autophagy in colon cancer cells. Mar. Drugs 2015, 13, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Sakemi, S.; Sun, H.H. Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991, 56, 4304–4307. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Parrino, B.; Lopergolo, A.; Pennati, M.; Zaffaroni, N.; Cirrincione, G. Synthesis and antitumor activity of 3-(2-phenyl-1,3-thiazol-4-yl)-1h-indoles and 3-(2-phenyl-1,3-thiazol-4-yl)-1h-7-azaindoles. ChemMedChem 2011, 6, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Barraja, P.; Montalbano, A.; Parrino, B.; Spano, V.; Lopergolo, A.; Sbarra, S.; Doldi, V.; Zaffaroni, N.; et al. Synthesis and antiproliferative activity of substituted 3[2-(1H-indol-3-yl)-1,3-thiazol-4-yl]-1H-pyrrolo[3,2-b]pyridines, marine alkaloid nortopsentin analogues. Curr. Med. Chem. 2014, 21, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spano, V.; Sbarra, S.; Doldi, V.; de Cesare, M.; et al. Novel 1h-pyrrolo[2,3-b]pyridine derivative nortopsentin analogues: Synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Parrino, B.; Di Vita, G.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; Diana, P.; et al. Synthesis and antiproliferative activity of thiazolyl-bis-pyrrolo[2,3-b]pyridines and indolyl-thiazolyl-pyrrolo[2,3-c]pyridines, nortopsentin analogues. Mar. Drugs 2015, 13, 460–492. [Google Scholar] [CrossRef] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The contribution of angiogenesis to the process of metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial cells and VEGF in vascular development. Nature 2005, 438, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Klagsbrun, M.; Moses, M.A. Molecular angiogenesis. Chem. Biol. 1999, 6, R217–R224. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, Z.; Tian, H.; Shen, G.; Ma, Y.; Xie, H.; Liu, Y.; Zhao, C.; Deng, S.; Yang, Y.; et al. SKLB1002, a novel potent inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and tumor growth in vivo. Clin. Cancer Res. 2011, 17, 4439–4450. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Bohman, S.; Dixelius, J.; Berge, T.; Dimberg, A.; Magnusson, P.; Wang, L.; Wikner, C.; Qi, J.H.; Wernstedt, C.; et al. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005, 24, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Abe, Y.; Tomii, Y.; Tsukamoto, H.; Kijima, H.; Yamazaki, H.; Ohnishi, Y.; Iwasaki, M.; Inoue, H.; Ueyama, Y.; et al. Cell binding isoforms of vascular endothelial growth factor-A (VEGF189) contribute to blood flow-distant metastasis of pulmonary adenocarcinoma. Int. J. Oncol. 2005, 26, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Zetter, B.R. Angiogenesis and tumor metastasis. Annu. Rev. Med. 1998, 49, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Noujaim, D.; van Golen, C.M.; van Golen, K.L.; Grauman, A.; Feldman, E.L. N-Myc and Bcl-2 coexpression induces MMP-2 secretion and activation in human neuroblastoma cells. Oncogene 2002, 21, 4549–4557. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Esposito, A.; Curigliano, G. Tumor-stroma crosstalk: Targeting stroma in breast cancer. Curr. Opin. Oncol. 2014, 26, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Cobleigh, M.A.; Langmuir, V.K.; Sledge, G.W.; Miller, K.D.; Haney, L.; Novotny, W.F.; Reimann, J.D.; Vassel, A. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin. Oncol. 2003, 30, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J. Med. 2012, 61, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yamano, Y.; Fujita, M.; Setiawan, A.; Kobayashi, M. Stylissamide X, a new proline-rich cyclic octapeptide as an inhibitor of cell migration, from an indonesian marine sponge of Stylissa sp. Bioorg. Med. Chem. Lett. 2012, 22, 1818–1821. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Qian, Z.J.; Ryu, B.; Kim, K.N.; Kim, D.; Kim, Y.M.; Jeon, Y.J.; Park, W.S.; Choi, I.W.; Kim, G.H.; et al. Matrix metalloproteinases (MMPs) inhibitory effects of an octameric oligopeptide isolated from abalone Haliotis discus hannai. Food Chem. 2013, 141, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Sulaiman, M.; Behery, F.A.; Foudah, A.I.; Sayed, K.A.E. Subereamolline A as a potent breast cancer migration, invasion and proliferation inhibitor and bioactive dibrominated alkaloids from the red sea sponge Pseudoceratina arabica. Mar. Drugs 2012, 10, 2492–2508. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Watanabe, Y.; Sanagawa, M.; Setiawan, A.; Kotoku, N.; Kobayashi, M. Cortistatins A, B, C, and D, anti-angiogenic steroidal alkaloids, from the marine sponge Corticium simplex. J. Am. Chem. Soc. 2006, 128, 3148–3149. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nieto, S.; Gonzalez-Iriarte, M.; Carmona, R.; Munoz-Chapuli, R.; Medina, M.A.; Quesada, A.R. Antiangiogenic activity of aeroplysinin-1, a brominated compound isolated from a marine sponge. FASEB J. 2002, 16, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Roskelley, C.D.; Williams, D.E.; McHardy, L.M.; Leong, K.G.; Troussard, A.; Karsan, A.; Andersen, R.J.; Dedhar, S.; Roberge, M. Inhibition of tumor cell invasion and angiogenesis by motuporamines. Cancer Res. 2001, 61, 6788–6794. [Google Scholar] [PubMed]
- Mathieu, V.; Wauthoz, N.; Lefranc, F.; Niemann, H.; Amighi, K.; Kiss, R.; Proksch, P. Cyclic versus hemi-bastadins. Pleiotropic anti-cancer effects: From apoptosis to anti-angiogenic and anti-migratory effects. Molecules 2013, 18, 3543–3561. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Thellung, S.; Wurth, R.; Gatto, F.; Corsaro, A.; Villa, V.; Nizzari, M.; Albertelli, M.; Ferone, D.; Florio, T. Emerging targets in pituitary adenomas: Role of the CXCL12/CXCR4-R7 system. Int. J. Endocrinol. 2014, 2014, 753524. [Google Scholar] [CrossRef] [PubMed]
- Cipres, A.; O’Malley, D.P.; Li, K.; Finlay, D.; Baran, P.S.; Vuori, K. Sceptrin, a marine natural compound, inhibits cell motility in a variety of cancer cell lines. ACS Chem. Biol. 2010, 5, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Spector, I.; Shochet, N.R.; Blasberger, D.; Kashman, Y. Latrunculins—Novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton 1989, 13, 127–144. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A.; Youssef, D.T.; Marchetti, D. Bioactive natural and semisynthetic latrunculins. J. Nat. Prod. 2006, 69, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Sayed, K.A.; Khanfar, M.A.; Shallal, H.M.; Muralidharan, A.; Awate, B.; Youssef, D.T.; Liu, Y.; Zhou, Y.D.; Nagle, D.G.; Shah, G. Latrunculin A and its C-17-O-carbamates inhibit prostate tumor cell invasion and HIF-1 activation in breast tumor cells. J. Nat. Prod. 2008, 71, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Murtagh, J.; Schwartz, E.L. The microtubule binding drug laulimalide inhibits vascular endothelial growth factor-induced human endothelial cell migration and is synergistic when combined with docetaxel (taxotere). Mol. Pharmacol. 2006, 69, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Z.D.; Xue, Y.; Wang, J.F.; Li, H.; Tang, Q.J.; Wang, Y.M.; Dong, P.; Xue, C.H. Ds-echinoside A, a new triterpene glycoside derived from sea cucumber, exhibits antimetastatic activity via the inhibition of NF-κB-dependent MMP-9 and VEGF expressions. J. Zhejiang Univ. Sci. B 2011, 12, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xue, Y.; Liu, Z.D.; Li, H.; Wang, J.F.; Li, Z.J.; Wang, Y.M.; Dong, P.; Xue, C.H. Differential effects of sulfated triterpene glycosides, holothurin A1, and 24-dehydroechinoside A, on antimetastasic activity via regulation of the MMP-9 signal pathway. J. Food Sci. 2010, 75, H280–H288. [Google Scholar] [CrossRef] [PubMed]
- Al Marzouqi, N.; Iratni, R.; Nemmar, A.; Arafat, K.; Ahmed Al Sultan, M.; Yasin, J.; Collin, P.; Mester, J.; Adrian, T.E.; Attoub, S. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur. J. Pharmacol. 2011, 668, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Jain, S.; Busnena, B.A.; El Sayed, K.A. Optimization of marine triterpene sipholenols as inhibitors of breast cancer migration and invasion. ChemMedChem 2013, 8, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, N.; Tamada, N.; Hayashi, A.; Kobayashi, M. Synthesis of BC-ring model of globostellatic acid X methyl ester, an anti-angiogenic substance from marine sponge. Bioorg. Med. Chem. Lett. 2008, 18, 3532–3535. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J. Heteronemin, a new scalarin type sesterterpene from the sponge Heteronema Erecta. Tetrahedron Lett. 1976, 17, 2631–2634. [Google Scholar] [CrossRef]
- Kopf, S.; Viola, K.; Atanasov, A.G.; Jarukamjorn, K.; Rarova, L.; Kretschy, N.; Teichmann, M.; Vonach, C.; Saiko, P.; Giessrigl, B.; et al. In vitro characterisation of the anti-intravasative properties of the marine product heteronemin. Arch. Toxicol. 2013, 87, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Shmeuli, U.; Zadock, E.; Kashman, Y.; Néeman, I. Sarcophine, a new epoxy cembranolide from marine origin. Tetrahedron 1974, 30, 2817–2824. [Google Scholar] [CrossRef]
- Sawant, S.S.; Youssef, D.T.; Reiland, J.; Ferniz, M.; Marchetti, D.; El Sayed, K.A. Biocatalytic and antimetastatic studies of the marine cembranoids sarcophine and 2-epi-16-deoxysarcophine. J. Nat. Prod. 2006, 69, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Sallam, A.A.; Mohammed, R.; Hifnawy, M.S.; Youssef, D.T.A.; El Sayed, K.A. Semisynthetic analogues of the marine cembranoid sarcophine as prostate and breast cancer migration inhibitors. Bioorg. Med. Chem. 2011, 19, 4928–4934. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Manly, S.P.; El Sayed, K.A.; Wali, V.B.; Sylvester, P.W.; Awate, B.; Shah, G.; Ross, S.A. Sinulodurins A and B, antiproliferative and anti-invasive diterpenes from the soft coral Sinularia dura. J. Nat. Prod. 2008, 71, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Warabi, K.; McHardy, L.M.; Matainaho, L.; van Soest, R.; Roskelley, C.D.; Roberge, M.; Andersen, R.J. Strongylophorine-26, a new meroditerpenoid isolated from the marine sponge Petrosia (Strongylophora) corticata that exhibits anti-invasion activity. J. Nat. Prod. 2004, 67, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yamori, T.; Kobayashi, M.; Duan, H. Antiproliferative and antiangiogenic activities of smenospongine, a marine sponge sesquiterpene aminoquinone. Mar. Drugs 2011, 9, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-H.; Chao, C.-H.; Wu, M.-H.; Sheu, J.-H. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur. J. Med. Chem. 2010, 45, 5998–6004. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Umeyama, A.; Shoji, N.; Hashimoto, T. Polyacetylenediols regulate the function of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2010, 10, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Gibson, T.B.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (map) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Sacks, D.B. Protein scaffolds in map kinase signalling. Cell Signal. 2009, 21, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Song, R.X.; McPherson, R.; Kumar, R.; Adam, L.; Jeng, M.-H.; Yue, W. The role of mitogen-activated protein (map) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 80, 239–256. [Google Scholar] [CrossRef]
- Avruch, J. Map kinase pathways: The first twenty years. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting ras signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Griner, E.M.; Kazanietz, M.G. Protein kinase c and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 2007, 7, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.M.; Coleman, R.L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 2012, 18, 5856–5864. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; LoGrasso, P.V. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J. Biol. Chem. 2011, 286, 16052–16062. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.M.; Gray, N.S.; Zarrinkar, P.P. High-throughput kinase profiling as a platform for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Rahal, R.; Stransky, N.; Lengauer, C.; Hoeflich, K.P. Targeting cancer with kinase inhibitors. J. Clin. Investig. 2015, 125, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Pastro, N.; Zivanovic, A. Kinase inhibitors from marine sponges. Mar. Drugs 2011, 9, 2131–2154. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Mallon, R.; Greenstein, M.; Feldberg, L.R.; Kim, S.C.; Collins, K.; Wojciechowicz, D.; Mangalindan, G.C.; Concepcion, G.P.; Harper, M.K.; et al. Aldisine alkaloids from the Philippine sponge Stylissa massa are potent inhibitors of mitogen-activated protein kinase kinase-1 (MEK-1). J. Med. Chem. 2002, 45, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Segraves, N.L.; Crews, P. A madagascar sponge Batzella sp. As a source of alkylated iminosugars. J. Nat. Prod. 2005, 68, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Kortmansky, J.; Schwartz, G.K. Bryostatin-1: A novel PKC inhibitor in clinical development. Cancer Investig. 2003, 21, 924–936. [Google Scholar] [CrossRef]
- Kinnel, R.B.; Scheuer, P.J. 11-hydroxystaurosporine: A highly cytotoxic, powerful protein kinase C inhibitor from a tunicate. J. Org. Chem. 1992, 57, 6327–6329. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Guzman, E.A.; Pitts, T.P.; Wright, A.E. Early effects of lasonolide A on pancreatic cancer cells. J. Pharmacol. Exp. Ther. 2009, 331, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Alvi, K.A.; Jaspars, M.; Crews, P.; Strulovici, B.; Oto, E. Penazetidine A, an alkaloid inhibitor of protein kinase C. Bioorg. Med. Chem. Lett. 1994, 4, 2447–2450. [Google Scholar] [CrossRef]
- Marion, F.; Williams, D.E.; Patrick, B.O.; Hollander, I.; Mallon, R.; Kim, S.C.; Roll, D.M.; Feldberg, L.; van Soest, R.; Andersen, R.J. Liphagal, a selective inhibitor of PI3 kinase α isolated from the sponge akacoralliphaga: Structure elucidation and biomimetic synthesis. Org. Lett. 2006, 8, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Strangman, W.K.; Marion, F.; Feldberg, L.; Roll, D.; Mallon, R.; Hollander, I.; Andersen, R.J. Synthesis of phosphatidylinositol 3-kinase (PI3K) inhibitory analogues of the sponge meroterpenoid liphagal. J. Med. Chem. 2010, 53, 8523–8533. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Manzaneda, E.; Chahboun, R.; Alvarez, E.; José Cano, M.; Haidour, A.; Alvarez-Manzaneda, R. Enantioselective total synthesis of the selective PI3 kinase inhibitor liphagal. Org. Lett. 2010, 12, 4450–4453. [Google Scholar] [CrossRef] [PubMed]
- Piplani, H.; Rana, C.; Vaish, V.; Vaiphei, K.; Sanyal, S.N. Dolastatin, along with Celecoxib, stimulates apoptosis by a mechanism involving oxidative stress, membrane potential change and PI3-K/AKT pathway down regulation. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 5142–5156. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, M.L.; Rodriguez, J.A.; Jimeno, J.; Kruyt, F.A.E.; Giaccone, G. Kahalalide F induces necrosis-like cell death that involves depletion of ErbB3 and inhibition of Akt signaling. Mol. Pharmacol. 2005, 68, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.H.; Miguel, A.; Zou, Y.; Yuan, Z.; Lu, B.; José, J.; Ana, M.C.; Perez-Soler, R. PM02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death-associated protein kinase. Clin. Cancer Res. 2011, 17, 5353–5366. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, L.F.; Losada, A.; Alcaide, V.; Álvarez, A.M.; Cuadrado, A.; González, L.; Nakayama, K.; Nakayama, K.I.; Fernández-Sousa, J.M.; Muñoz, A.; et al. Aplidin™ induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C δ. Oncogene 2002, 21, 7533–7544. [Google Scholar]
- Lee, K.H.; Nishimura, S.; Matsunaga, S.; Fusetani, N.; Horinouchi, S.; Yoshida, M. Inhibition of protein synthesis and activation of stress-activated protein kinases by onnamide A and theopederin B, antitumor marine natural products. Cancer Sci. 2005, 96, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; Chinnaiyan, A.M. Translating genomics for precision cancer medicine. Annu. Rev. Genomics Hum. Genet. 2014, 15, 395–415. [Google Scholar] [PubMed]
- Encinar, J.A.; Fernandez-Ballester, G.; Galiano-Ibarra, V.; Micol, V. In silico approach for the discovery of new PPARγ modulators among plant-derived polyphenols. Drug Des. Dev. Ther. 2015, 9, 5877–5895. [Google Scholar] [CrossRef] [PubMed]
- Galiano, V.; Garcia-Valtanen, P.; Micol, V.; Encinar, J.A. Looking for inhibitors of the dengue virus NS5 RNA-dependent RNA-polymerase using a molecular docking approach. Drug Des. Dev. Ther. 2016, 10, 3163–3181. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- ADMET Profile for Antineoplasic Drugs. Available online: http://dockingfiles.umh.es/anticancer-drugs/Anticancer_drugslist.asp (accessed on 22 June 2017).
- Marine Natural Library Molecular Docking Site. Available online: http://docking.umh.es/chemlib/mnplib (accessed on 22 June 2017).
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.; Munro, M.H.G. Dictionary of Marine Natural Products, with CD-ROM; Chapman & Hall/CRC: Boca Raton, FL, USA, 2008. [Google Scholar]
- National Institutes of Health (NIH) Chemical Identifier Resolution Service. Available online: https://cactus.nci.nih.gov/chemical/structure (accessed on 22 June 2017).
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Cho, S.Y.; Pak, H.J.; Kim, Y.; Choi, J.Y.; Lee, Y.J.; Gong, B.H.; Kang, Y.S.; Han, T.; Choi, G.; et al. NPCARE: Database of natural products and fractional extracts for cancer regulation. J. Cheminform. 2017, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Developing natural product drugs: Supply problems and how they have been overcome. Pharmacol. Ther. 2016, 162, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on european seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Calado, R.; Sheridan, C.; Alimonti, A.; Osinga, R. Coral aquaculture to support drug discovery. Trends Biotechnol. 2013, 31, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, C.; Francesch, A. Development of yondelis® (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep. 2009, 26, 322–337. [Google Scholar] [CrossRef] [PubMed]
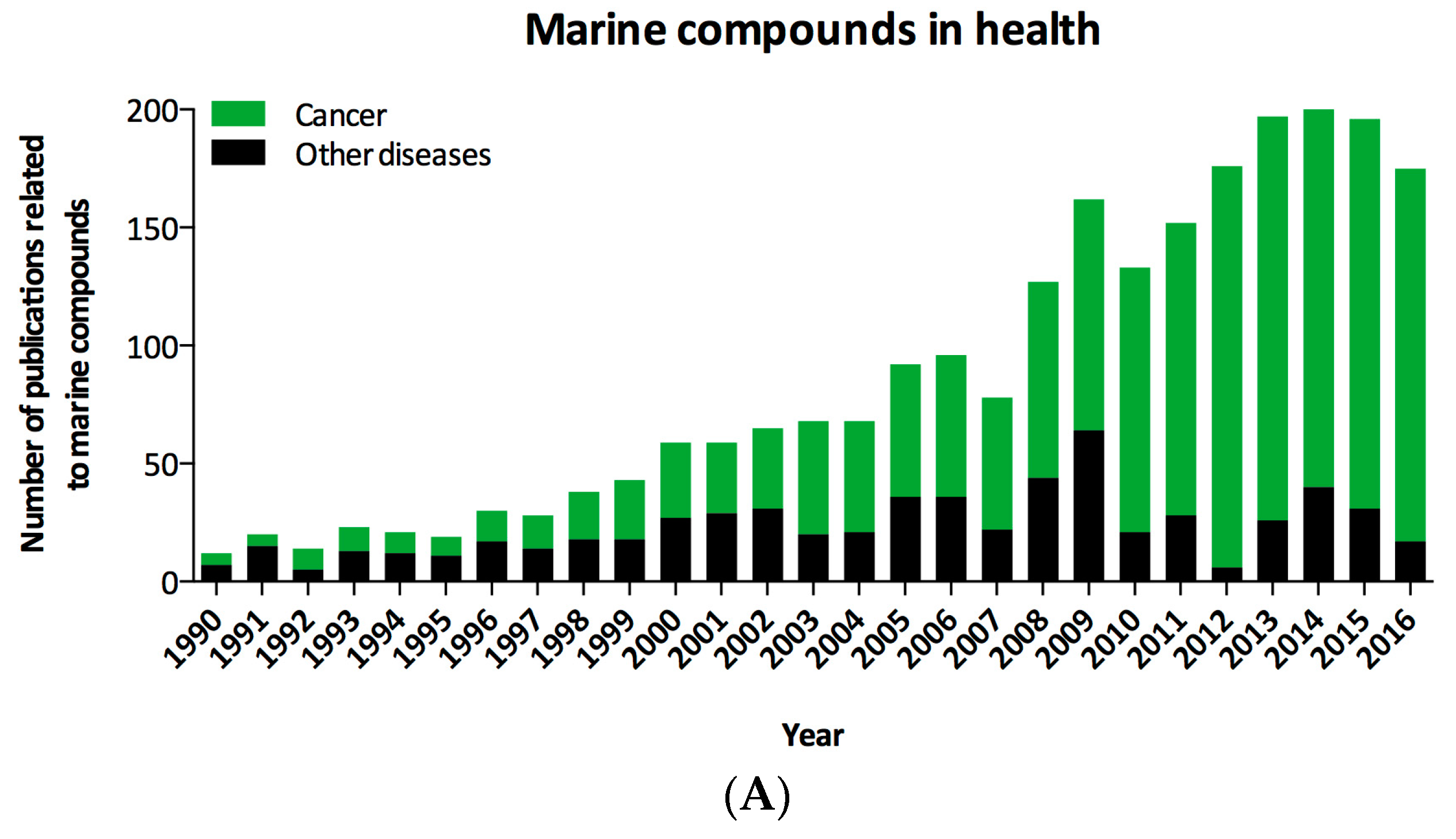
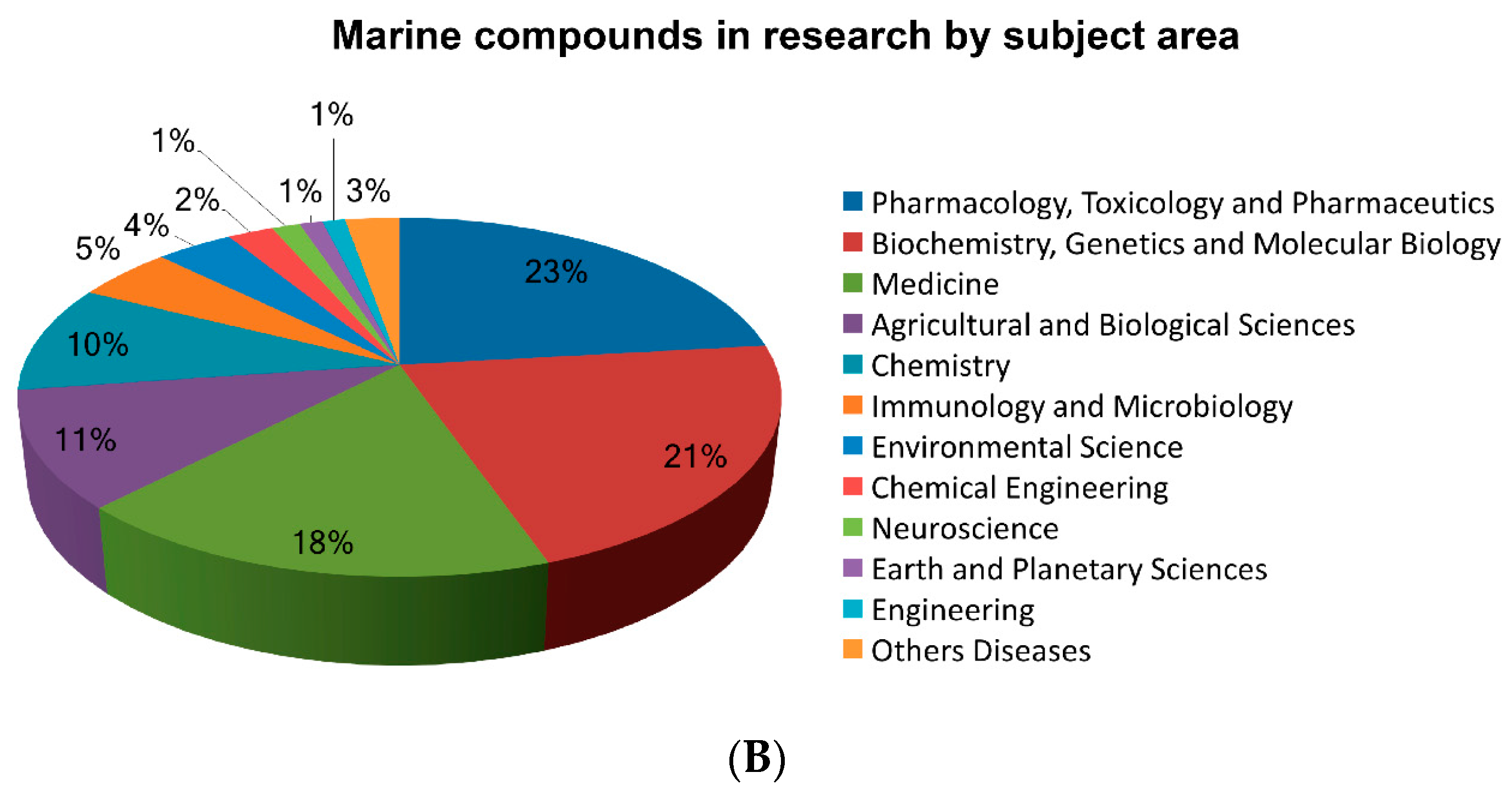

| Organization and Year | Compound Name | Marine Organism | Chemical Class | Disease Area | Mode of Action | Company or Institution | Refs. |
|---|---|---|---|---|---|---|---|
| FDA 1969 | Cytarabine (Ara-C) | Sponge | Nucleoside | Anticancer | DNA polymerase inhibitor | Bedford, Enzon | [37] |
| FDA 2004 | Ziconotide | Cone snail | Peptide | Pain | Modulator of neuronal calcium channels | Neurex Corp | [38] |
| EMEA 2007 | Trabectedin (E7389) | Tunicate | Alkaloid | Anticancer | Inhibits cancer cell growth of and affects the tumor microenvironment | PharmaMar | [39] |
| FDA 2010 | Eribulin mesylate (E7389) | Sponge | Macrolide | Anti-breast cancer | Microtubule interfering agent | Eisai Inc. | [40] |
| FDA 2011 | Brentuximab vedotin (SGN-35) | Mollusk | Antibody-drug conjugate | Lymphoma | CD30-directed antibody-cytotoxic drug conjugate | Seattle Genetics Inc. | [41] |
| Clinical Status | Compound Name | Marine Organism | Chemical Class | Disease Area | Mode of Action | Company or Institution | Refs. |
|---|---|---|---|---|---|---|---|
| Phase III | Plitidepsin | Tunicate | Depsipetide | Anti-cancer | Induces cell cycle arrest or apoptosis | PharmaMar | [76] |
| Gemcitabine (GEM) (Gemzar) | Sponge | Nucleoside | Anti-cancer | Ribonucleotide reductase inhibitor Replaces cytidine during DNA replication | Eli Lilly and Company | [77] | |
| Phase II | Glembatumumab vedotin | Mollusk | Antibody drug conjugate | Breast cancer and melanoma | Targets glycoprotein NMB (a protein overexpressed by multiple tumor types) | Celldex Therapeutics | [78] |
| Elisidepsin | Mollusk | Depsipetide | Anti-cancer | Antineoplastic agent, modifiying lipids from cell membrane | PharmaMar | [79] | |
| PM1004 | Nudibranch | Alkaloid | Anti-cancer | DNA-binding | PharmaMar | [80] | |
| Pseudopterosins | Soft coral | Diterpen glycoside | Wound healing | Eicosanoid metabolism | The Regents Of The University Of California | [81] | |
| IPL576,092 (Contignasterol derivative) | Sponge | Miscellaneous | Anti-inflammatory | Inhibition of leucocyte infiltration and hypersensitivity during allergy | Aventis Pharma | [82] | |
| Phase I/II | PM-10450 (Zalypsis®) | Sponge | Alkaloid | Anti-cancer drug | Transcription inhibitor | PharmaMar | [83] |
| Discodermolide | Sponge | Polyketide | Anti-cancer drug | Microtubule interfering agent | Novartis | [84] | |
| Phase I | Bryostatin-1 | Bryozoa | Polyketide | Anti-cancer drug | Protein kinase C | National Cancer Institute | [85] |
| Pinatuzumab vedotin | Mollusk | Antibody drug conjugate | Non-Hodgkin lymphoma, leukemia | Apoptosis stimulant; Mitosis inhibitor and Tubulin inhibitor | Genentech, Inc. | [86] | |
| Tisotumab Vedotin (HuMax®-TF-ADC) | Mollusk | Antibody drug conjugate | Ovarian, endometrium, cervix and prostate cancer | Antineoplastic, Drug conjugate, Immunotoxin and monoclonal antibodies | Genmab and Seattle Genetics | [87] | |
| HT1286 (Hemiasterlin derivative) | Sponge | Tripeptide | Anti-cancer drug | Microtubule interfering agent | Wyeth | [84] | |
| LAF389 (Bengamide B derivative) | Sponge | Peptide | Anti-cancer drug | Methionine aminopeptidase inhibitor | Novartis | [84] | |
| Hemiasterlin (E7974) | Sponge | Tripeptide | Anti-cancer drug | Microtubule interfering agent | Eisai Inc. | [84] | |
| PM-060184 | Sponge | Polyketide | Anti-cancer drug | Microtubule interfering agent | PharmaMar | [88] | |
| NVP-LAQ824 (Psammaplin derivative, Dacinostat) | Sponge | Miscellaneous | Anti-cancer drug | Histone deacetylase (HDAC) inhibitors or DNA methyltransferases (DNMT) inhibitor | Novartis Pharma | [89] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. https://doi.org/10.3390/molecules22071037
Ruiz-Torres V, Encinar JA, Herranz-López M, Pérez-Sánchez A, Galiano V, Barrajón-Catalán E, Micol V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules. 2017; 22(7):1037. https://doi.org/10.3390/molecules22071037
Chicago/Turabian StyleRuiz-Torres, Verónica, Jose Antonio Encinar, María Herranz-López, Almudena Pérez-Sánchez, Vicente Galiano, Enrique Barrajón-Catalán, and Vicente Micol. 2017. "An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs" Molecules 22, no. 7: 1037. https://doi.org/10.3390/molecules22071037
APA StyleRuiz-Torres, V., Encinar, J. A., Herranz-López, M., Pérez-Sánchez, A., Galiano, V., Barrajón-Catalán, E., & Micol, V. (2017). An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules, 22(7), 1037. https://doi.org/10.3390/molecules22071037