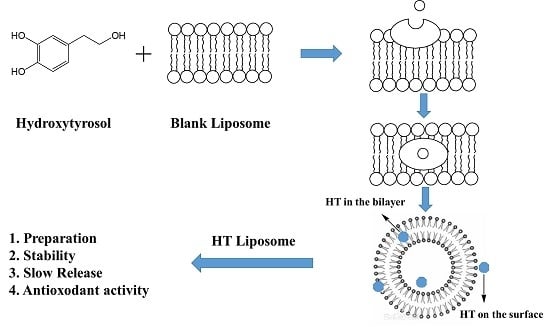
Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of HT Liposomes
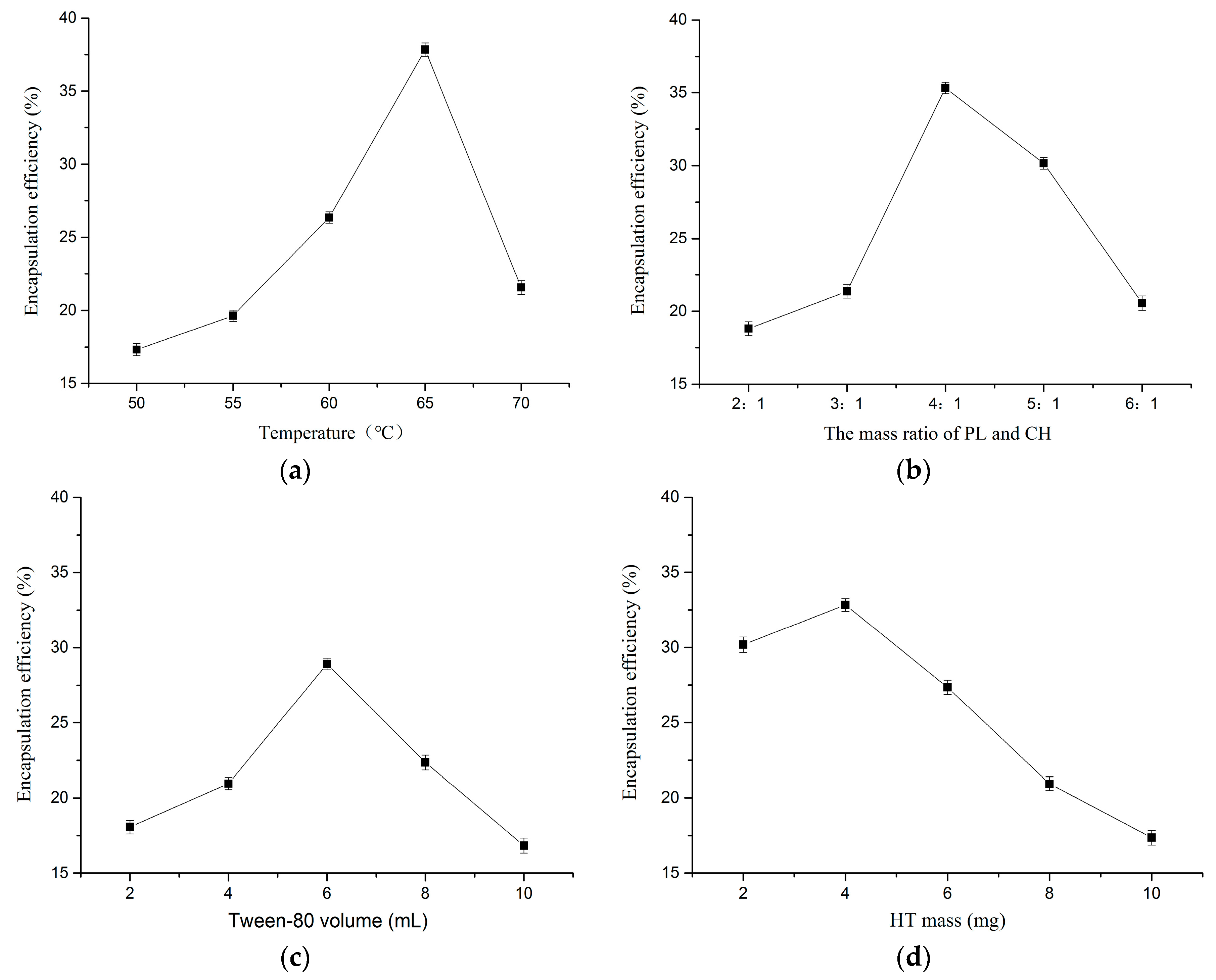
2.1.1. Effect of Temperature
2.1.2. Effect of Mass Ratio of PL and CH
2.1.3. Effect of Tween-80 Volume
2.1.4. Effect of HT Mass
2.1.5. Optimization of HT Liposomes Preparation by RSM
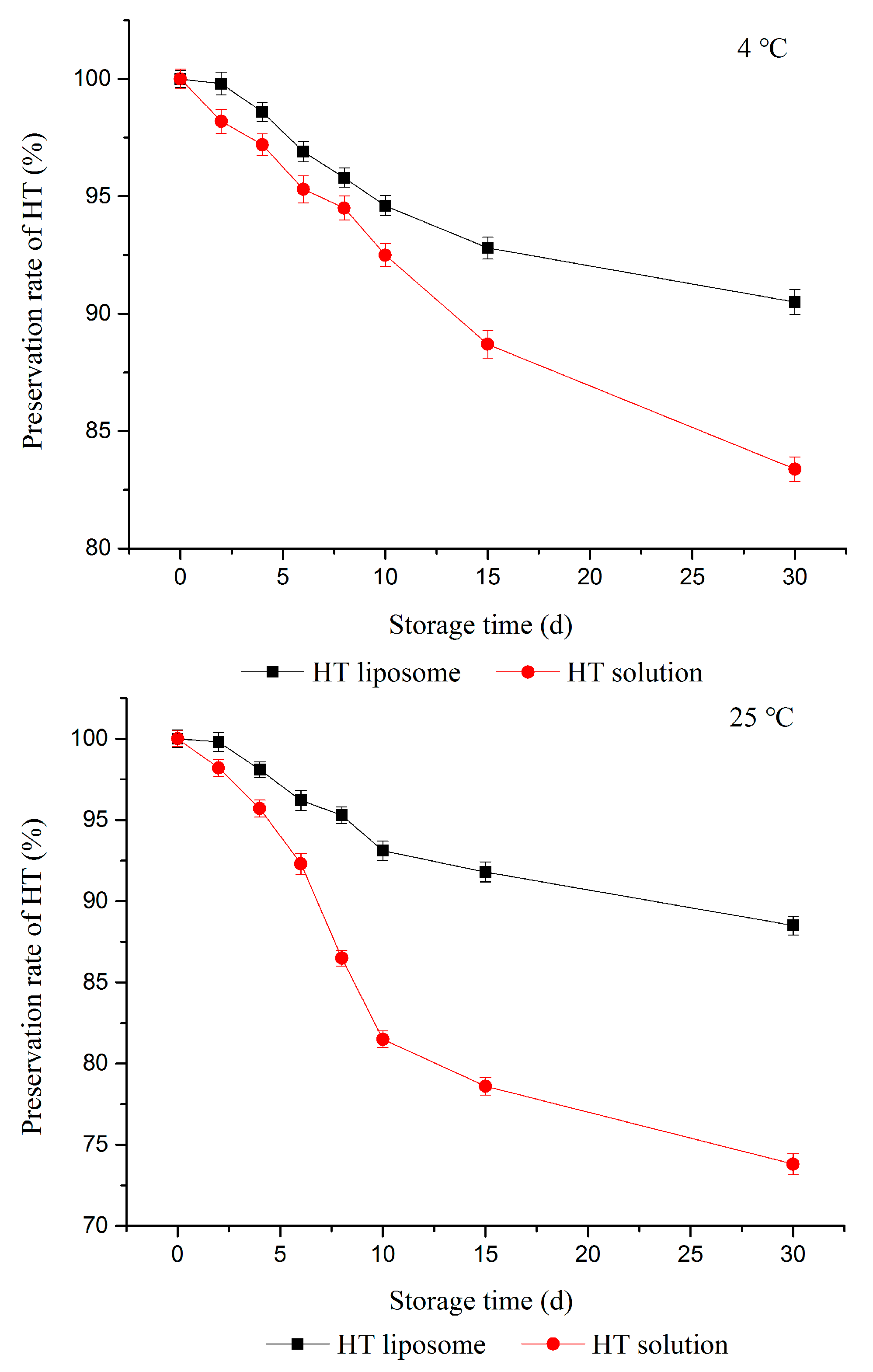
2.2. Mean Particle Size and Stability
2.3. In Vitro Slow Release Study
2.4. DPPH Radical Scavenging Activity of HT Liposomes
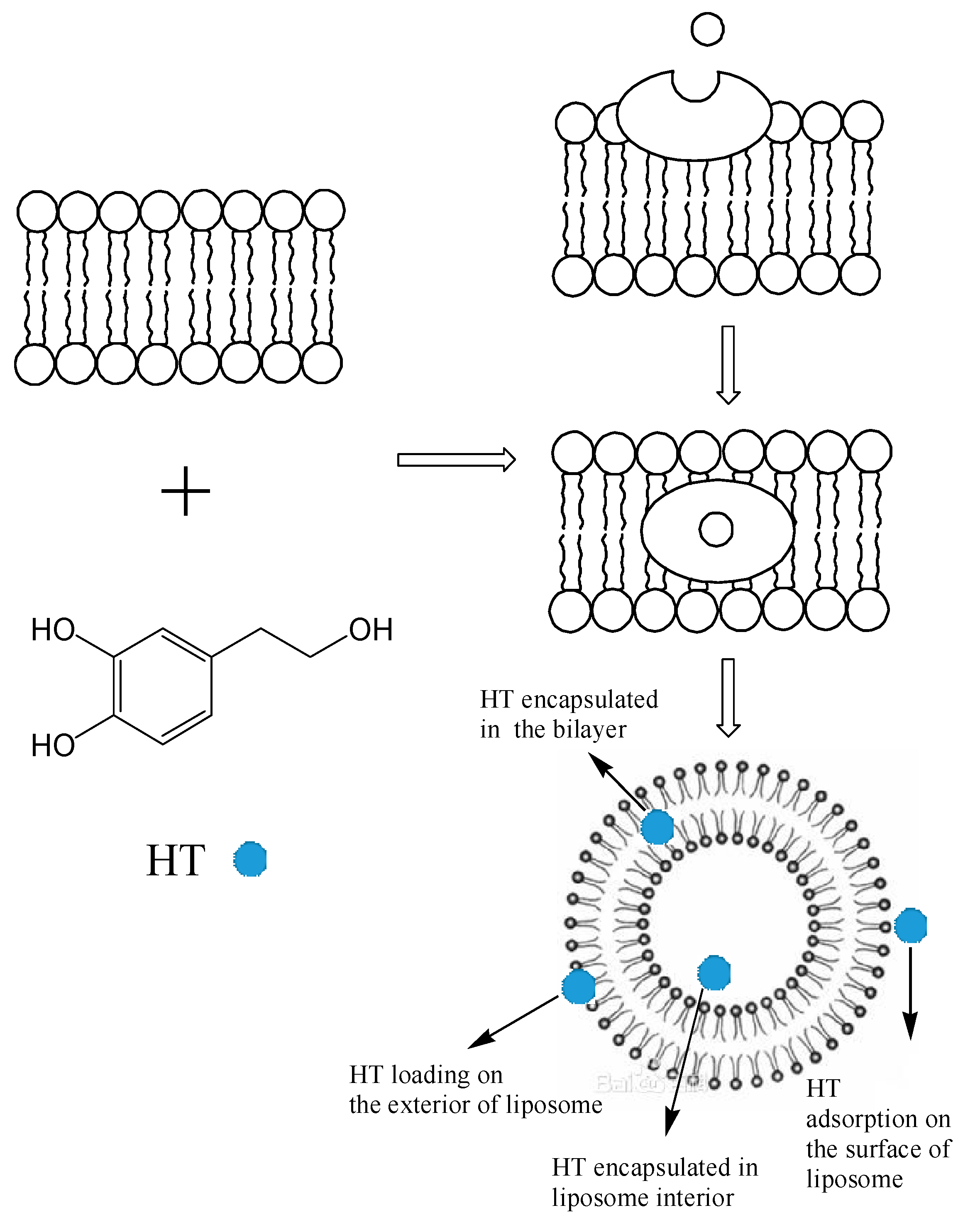
2.5. Encapsulation Mechanism of HT Liposomes
3. Materials and Methods
3.1. Material
3.2. Methods
3.2.1. Preparation of HT Liposomes
3.2.2. High Performance Liquid Chromatography (HPLC) Analysis
3.2.3. The Determination of Encapsulation Efficiency
3.2.4. Single Factor Experimental Design
3.2.5. Box-Behnken Design
3.2.6. Determination of Mean Particle Size and Size Distribution
3.2.7. Stability Tests
- (1)
- Physical stability: HT liposomes were centrifuged 30 min by high speed centrifugation and any phenomena observed was recorded.
- (2)
- Storage stability: EE of prepared liposomes in pH 7.4 PBS solution was measured over a 30-day period at 4 °C and 25 °C storage conditions. Samples were taken at different time intervals (0, 2, 4, 6, 8, 10, 15, 30 days) and EE were calculated immediately. Each experiment was repeated at least three times. The Equation (3) to calculate the preservation rate of HT was shown below:where w is the preservation rate of HT; EEi is the EE of HT liposome at time i; EE0 is the EE of HT liposomes prepared at the beginning.
3.2.8. In Vitro Slow Release Effect Study
3.2.9. DPPH (1,1-diphenyl-2-picrylhydrazyl) Radical Scavenging Assay
3.2.10. Statistical Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HT | hydroxytyrosol |
| RSM | response surface methodology |
| PL | phospholipid |
| CH | cholesterol |
| EE | encapsulation efficiency |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ANOVA | analysis of variance |
| W/O | water-in-oil |
| MWCO | molecular weight cut off |
| DLS | dynamic light scattering |
References
- Adhami, H.R.; Zehl, M.; Dangl, C.; Dorfmeister, D.; Stadler, M.; Urban, E.; Hewitson, P.; Ignatova, S.; Krenn, L. Preparative isolation of oleocanthal, tyrosol, and hydroxytyrosol from olive oil by HPCCC. Food Chem. 2015, 170, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, M.; Biber Muftuler, F.Z.; Yurt Kilcar, A.; Medine, E.I.; Unak, P. Isolation of hydroxytyrosol from olive leaves extract, radioiodination and investigation of bioaffinity using in vivo/in vitro methods. Radiochim. Acta 2013, 101, 585–593. [Google Scholar]
- Visioli, F.; Caruso, D.; Plasmati, E.; Patelli, R.; Mulinacci, N.; Romani, A.; Galli, G.; Galli, C. Hydroxytyrosol, as a component of olive mill waste water, is dose- dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radic. Res. 2001, 34, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Killeen, M.J.; Pontoniere, P.; Crea, R. Hydroxytyrosol: An examination of its potential role in cardiovascular disease, inflammation, and longevity. Agro Food Ind. Hi-Tech 2011, 22, 16–19. [Google Scholar]
- Catalán, Ú.; Rubió, L.; Mc, L.D.L.H.; Herrero, P.; Nadal, P.; Canela, N.; Pedret, A.; Motilva, M.J.; Solà, R. Hydroxytyrosol and its complex forms (secoiridoids) modulate aorta and heart proteome in healthy rats: Potential cardio-protective effects. Mol. Nutr. Food Res. 2016, 60, 2114–2129. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Pinya, S.; Del, M.B.M.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective effects of the polyphenol hydroxytyrosol from olive oil. Curr. Drug Targets 2016, 18, 1–10. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Lo, C.R.; Crisafi, G.; Uccella, N.; Saija, A. On the in vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Medinamartínez, M.S.; Truchado, P.; Castroibáñez, I.; Allende, A. Antimicrobial activity of hydroxytyrosol: A current controversy. Biosci. Biotechnol. Biochem. 2016, 80, 1–10. [Google Scholar]
- Fabiani, R.; De, B.A.; Rosignoli, P.; Servili, M.; Selvaggini, R.; Montedoro, G.F.; Di, S.C.; Morozzi, G. Virgin olive oil phenols inhibit proliferation of human promyelocytic leukemia cells (HL60) by inducing apoptosis and differentiation. J. Nutr. 2006, 136, 614–619. [Google Scholar] [PubMed]
- Bernini, R.; Crisante, F.; Merendino, N.; Molinari, R.; Soldatelli, M.C.; Velotti, F. Synthesis of a Novel Ester of Hydroxytyrosol and α-Lipoic Acid Exhibiting an Antiproliferative Effect on Human Colon Cancer HT-29 Cells. Eur. J. Med. Chem. 2011, 46, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Gallardo, E.; Madrona, A.; Bravo, L.; Sarriá, B.; González-Correa, J.A.; Mateos, R.; Espartero, J.L. Synthesis and antioxidant activity of nitrohydroxytyrosol and its acyl derivatives. J. Agric. Food Chem. 2014, 62, 10297–10303. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Madrona, A.; Pereiracaro, G.; Cert, R.M.; Parrado, J.; Bravo, L.; Espartero, J.L. Synthesis and antioxidant evaluation of isochroman-derivatives of hydroxytyrosol: Structure-activity relationship. Food Chem. 2015, 173, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and Metabolism of Hydroxytyrosol, a Natural Antioxidant from Olive Oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Mcclements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Arik, N.; Monteil, J.; Papadimitriou, V.; Leal-Calderon, F.; Xenakis, A. Microemulsion versus emulsion as effective carrier of hydroxytyrosol. Colloids Surf. B Biointerfaces 2016, 137, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Sun, S.; Li, X.; Lv, S.; Xu, T.; Sun, J.; Feng, W.; Zhang, L.; Li, Y. Preparation, in vitro and in vivo evaluation of mPEG-PLGA nanoparticles co-loaded with syringopicroside and hydroxytyrosol. J. Mater. Sci. 2016, 27, 24. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Brusa, P.; Rocco, F.; Arpicco, S.; Ceruti, M.; Cattel, L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J. Control. Release 2004, 100, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, N.R.; Vodovozova, E.L. Differential binding of plasma proteins by liposomes loaded with lipophilic prodrugs of methotrexate and melphalan in the bilayer. Biochem. Biokhimiia 2014, 79, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, N.R.; Svirshchevskaya, E.V.; Sitnikov, N.S.; Abodo, L.; Sutorius, H.; Zapke, J.; Velder, J.; Thomopoulou, P.; Oschkinat, H.; Prokop, A. Lipophilic prodrugs of a triazole-containing colchicine analogue in liposomes: Biological effects on human tumor cells. Russ. J. Bioorg. Chem. 2013, 39, 543–552. [Google Scholar] [CrossRef]
- Kaess, K.; Fahr, A. Liposomes as solubilizers for lipophilic parenteral drugs: Transfer of drug and lipid marker to plasma proteins. Eur. J. Lipid Sci. Technol. 2015, 116, 1137–1144. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, H.; Maruyama, K.; Okinaga, K.; Sasaki, K.; Sekine, T.; Ishida, O.; Ogiwara, N.; Johkura, K.; Yonemura, Y. Intracellular targeting therapy of cisplatin-encapsulated transferrin-polyethylene glycol liposome on peritoneal dissemination of gastric cancer. Int. J. Cancer 2002, 99, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Polanska, H.; Rodrigo, M.A.M.; Guran, R.; Kulich, P.; Kopel, P.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Kizek, R. Prostate tumor attenuation in the nu/nu murine model due to anti-sarcosine antibodies in folate-targeted liposomes. Sci. Rep. 2016, 6, 33379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jia, L.J.; Gong, J.G.; Li, G.Z.; Kang, B. Preparation of long-circulating paclitaxel-containing liposomes and its pharmacokinetics. Food Drug 2009, 11, 14–17. [Google Scholar]
- Minato, S.; Iwanaga, K.; Kakemi, M.; Yamashita, S.; Oku, N. Application of polyethyleneglycol (PEG)-modified liposomes for oral vaccine: Effect of lipid dose on systemic and mucosal immunity. J. Control. Release 2003, 89, 189–197. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Mcgurk, S.L.; Liversidge, G.G. Insulin nanoparticles: A novel formulation approach for poorly water soluble Zn-insulin. Pharm. Res. 2004, 21, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Gumulec, J.; Fojtu, M.; Raudenska, M.; Sztalmachova, M.; Skotakova, A.; Vlachova, J.; Skalickova, S.; Nejdl, L.; Kopel, P.; Knopfova, L. Modulation of induced cytotoxicity of doxorubicin by using apoferritin and liposomal cages. Int. J. Mol. Sci. 2014, 15, 22960–22977. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Mateos-Diaz, E.; Leal-Calderon, F.; Xenakis, A.; Carrière, F. Water-in-oil microemulsions versus emulsions as carriers of hydroxytyrosol: An in vitro gastrointestinal lipolysis study using the pHstat technique. Food Funct. 2016, 7, 2258. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, S.J.; Bast, A.; De, V.J.; Haenen, G.R. The olive oil antioxidant hydroxytyrosol efficiently protects against the oxidative stress-induced impairment of the NObullet response of isolated rat aorta. AJP Heart Circ. Physiol. 2007, 292, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Cao, G.Q.; Chen, T.T. Study on preparation of vitamin C liposome. Soybean Sci. 2007, 26, 270–272. [Google Scholar]
- Yuan, J.J.; Wang, C.Z.; Ye, J.Z.; Tao, R.; Zhang, Y.S. Enzymatic hydrolysis of oleuropein from Olea europea (olive) leaf extract and antioxidant activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Du, J. Revisiting the time for removing the unloaded drug by dialysis method based on a biocompatible and biodegradable polymer vesicle. Polymer 2012, 53, 2068–2073. [Google Scholar] [CrossRef]
- Nallamothu, R.; Wood, G.C.; Pattillo, C.B.; Scott, R.C.; Kiani, M.F.; Moore, B.M.; Thoma, L.A. A tumor vasculature targeted liposome delivery system for combretastatin A4: Design, characterization, and in vitro evaluation. AAPS PharmSciTech 2006, 7, 7–16. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
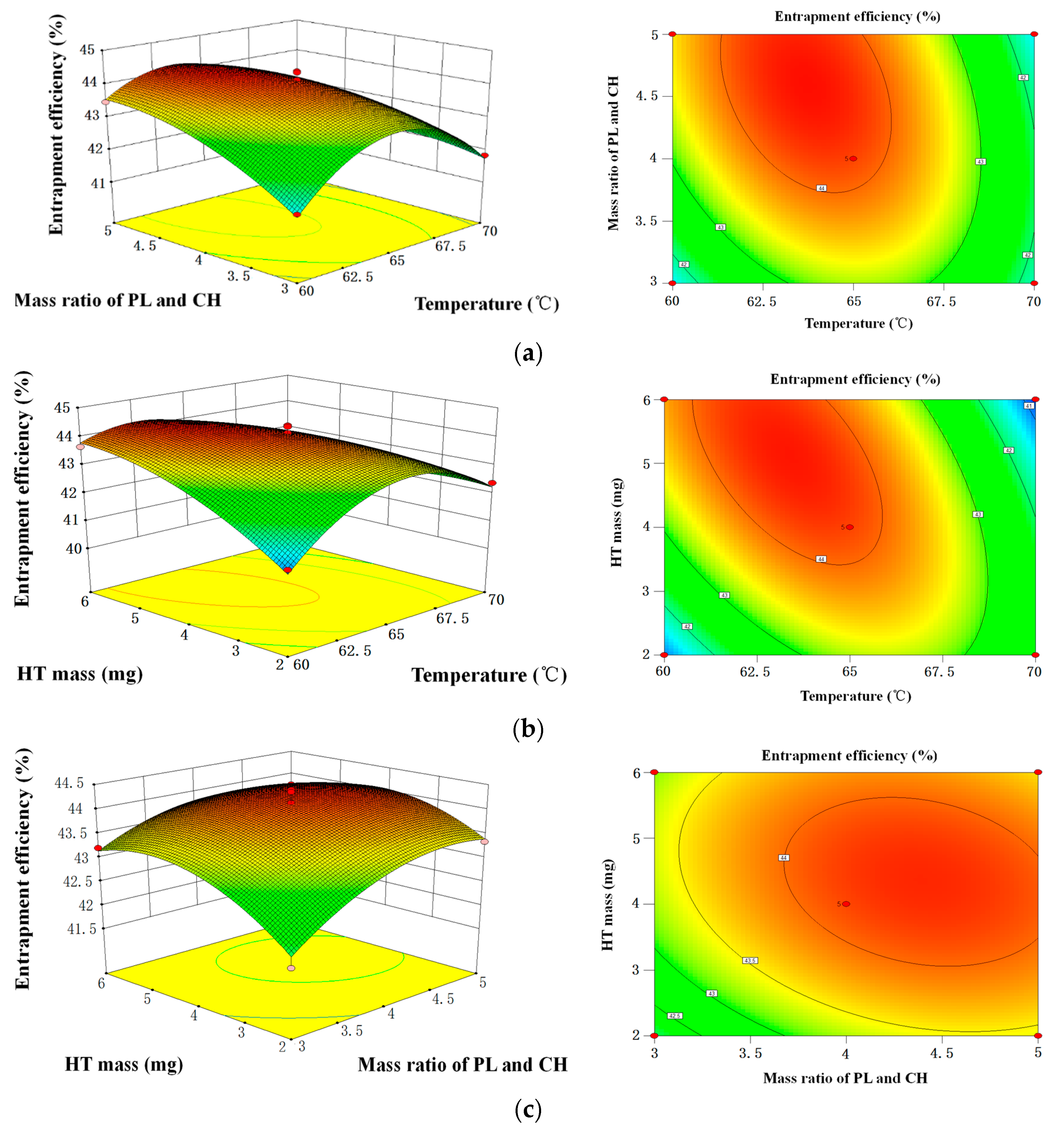


| No. | X1 Temperature/°C | X2 Mass Ratio of PL and CH | X3 HT Mass/mg | Y EE/% |
|---|---|---|---|---|
| 1 | −1 | 0 | 1 | 43.65 |
| 2 | 0 | −1 | −1 | 41.91 |
| 3 | −1 | 1 | 0 | 43.47 |
| 4 | 1 | −1 | 0 | 41.83 |
| 5 | 1 | 0 | −1 | 42.38 |
| 6 | 0 | 0 | 0 | 43.86 |
| 7 | −1 | −1 | 0 | 41.65 |
| 8 | 0 | 0 | 0 | 43.92 |
| 9 | 0 | 1 | −1 | 43.34 |
| 10 | 0 | 0 | 0 | 44.35 |
| 11 | 1 | 0 | 1 | 40.56 |
| 12 | 0 | 0 | 0 | 44.39 |
| 13 | -1 | 0 | −1 | 41.25 |
| 14 | 0 | 0 | 0 | 44.13 |
| 15 | 1 | 1 | 0 | 41.32 |
| 16 | 0 | 1 | 1 | 43.75 |
| 17 | 0 | −1 | 1 | 43.21 |
| Sources of Variation | Sum of Squares | df | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| model | 23.90 | 9 | 2.66 | 41.57 | <0.0001 | *** |
| X1 | 1.93 | 1 | 1.93 | 30.23 | 0.0009 | *** |
| X2 | 1.34 | 1 | 1.34 | 21.06 | 0.0025 | ** |
| X3 | 0.66 | 1 | 0.66 | 10.26 | 0.0150 | * |
| X1X2 | 1.36 | 1 | 1.36 | 21.25 | 0.0025 | ** |
| X1X3 | 4.45 | 1 | 4.45 | 69.71 | <0.0001 | *** |
| X2X3 | 0.20 | 1 | 0.20 | 3.10 | 0.1217 | |
| X12 | 10.48 | 1 | 10.48 | 164.06 | <0.0001 | *** |
| X22 | 0.99 | 1 | 0.99 | 15.51 | 0.0056 | ** |
| X32 | 1.48 | 1 | 1.48 | 23.14 | 0.0019 | ** |
| residual | 0.45 | 7 | 0.064 | |||
| lack of fit | 0.21 | 3 | 0.071 | 1.23 | 0.4096 | no |
| pure error | 0.23 | 4 | 0.058 | |||
| total | 24.34 | 16 | ||||
| R2 | 0.9816 | |||||
| Radj2 | 0.9580 | |||||
| Adeq precision | 17.696 |
| Concentration (μg/mL) | DPPH Scavenging Capacity of HT Solution (%) | DPPH Scavenging Capacity of HT Liposome (%) |
|---|---|---|
| 0.25 | 0.17 ± 0.08 eA | 0.19 ± 0.06 eA |
| 0.5 | 0.25 ± 0.13 dA | 0.31 ± 0.93 dA |
| 1 | 0.47 ± 0.16 cA | 0.51 ± 0.43 cA |
| 1.5 | 0.65 ± 0.12 bA | 0.68 ± 0.29 bA |
| 2 | 0.88 ± 0.18 aA | 0.91 ± 0.61 aA |
| Independent Variables | Symbol | Variable Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | X1 | 60 | 65 | 70 |
| Mass ratio of PLand CH | X2 | 3:1 | 4:1 | 5:1 |
| HT mass (mg) | X3 | 2 | 4 | 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.-J.; Qin, F.G.F.; Tu, J.-L.; Li, B. Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive. Molecules 2017, 22, 870. https://doi.org/10.3390/molecules22060870
Yuan J-J, Qin FGF, Tu J-L, Li B. Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive. Molecules. 2017; 22(6):870. https://doi.org/10.3390/molecules22060870
Chicago/Turabian StyleYuan, Jiao-Jiao, Frank G.F. Qin, Jun-Ling Tu, and Bing Li. 2017. "Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive" Molecules 22, no. 6: 870. https://doi.org/10.3390/molecules22060870
APA StyleYuan, J.-J., Qin, F. G. F., Tu, J.-L., & Li, B. (2017). Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive. Molecules, 22(6), 870. https://doi.org/10.3390/molecules22060870