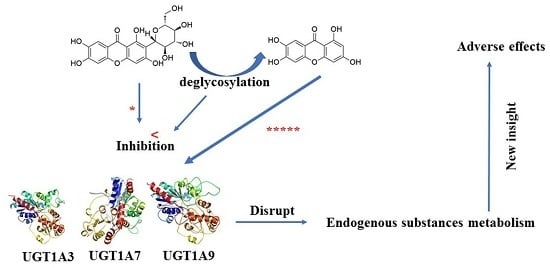
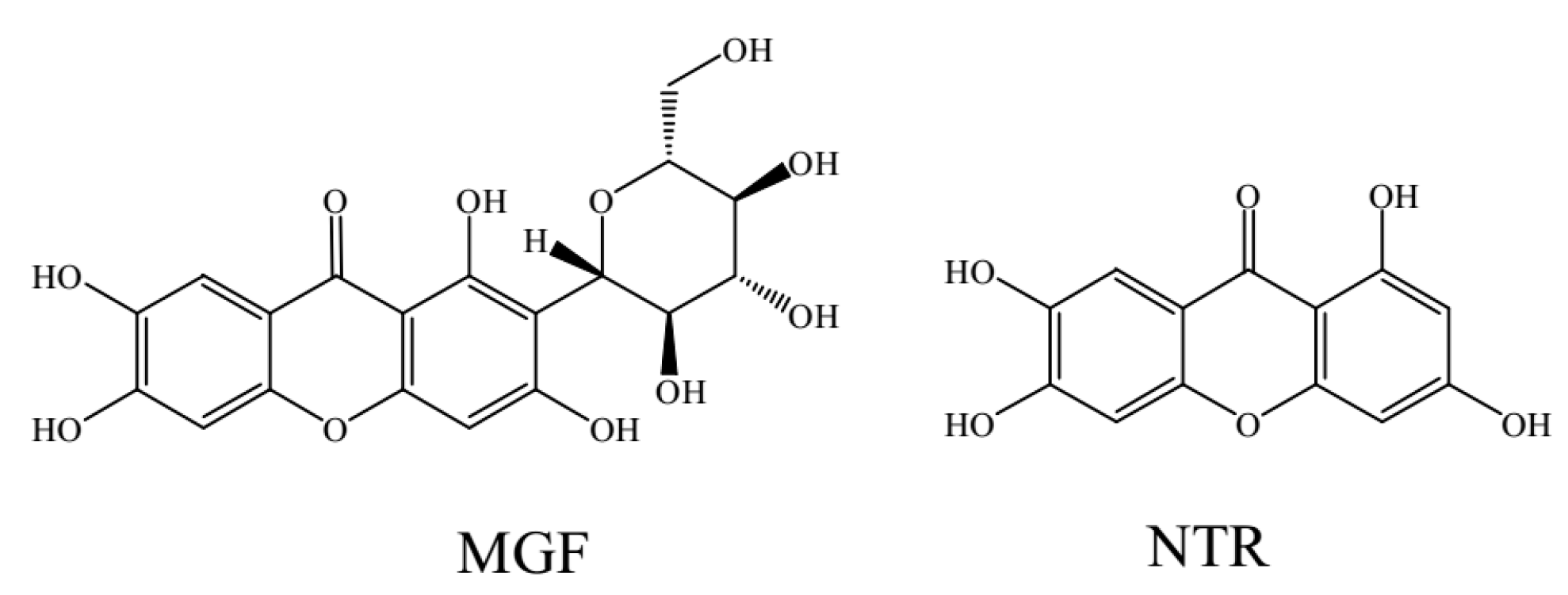
In Vitro Comparative Study of the Inhibitory Effects of Mangiferin and Its Aglycone Norathyriol towards UDP-Glucuronosyl Transferase (UGT) Isoforms
Abstract
:1. Introduction
2. Results
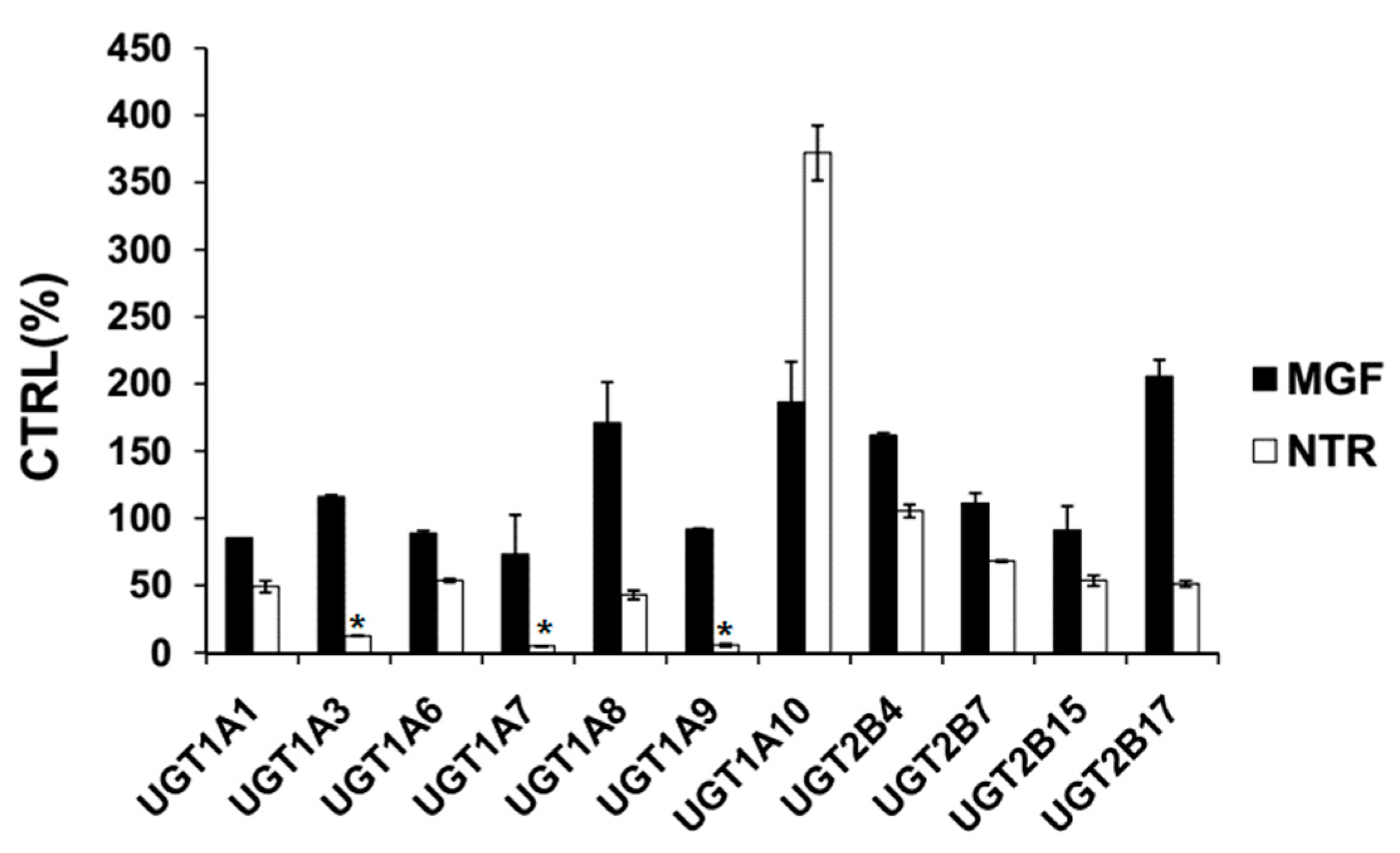
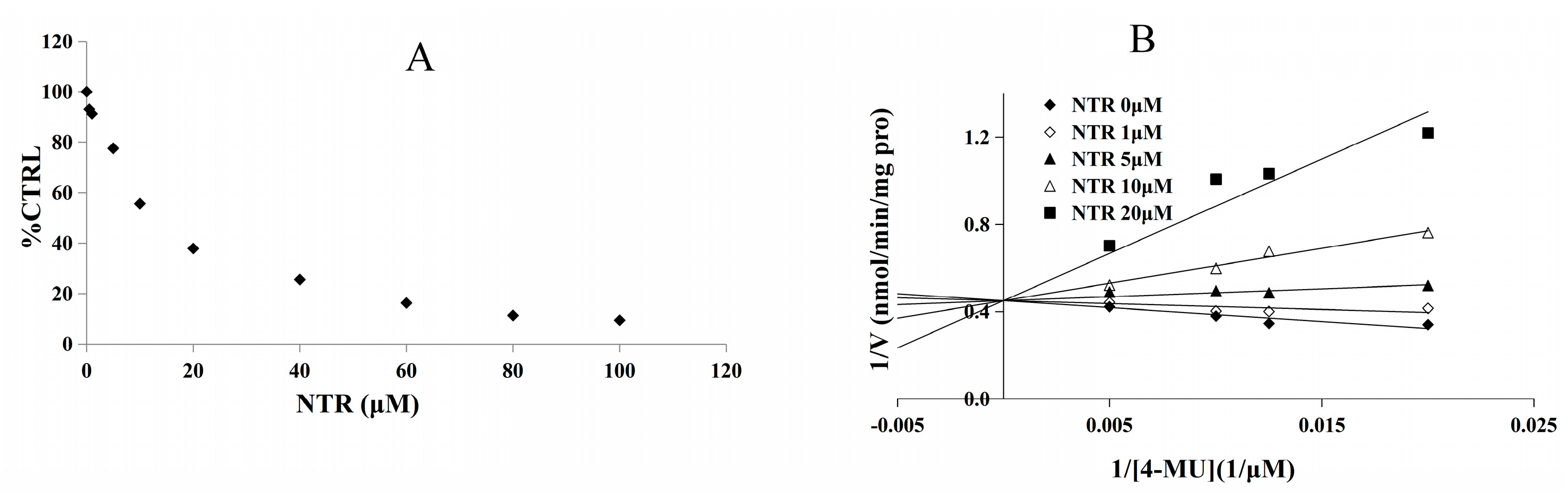
2.1. Comparison of Inhibition Effect of MGF and NTR towards Various Important UGT Isoforms
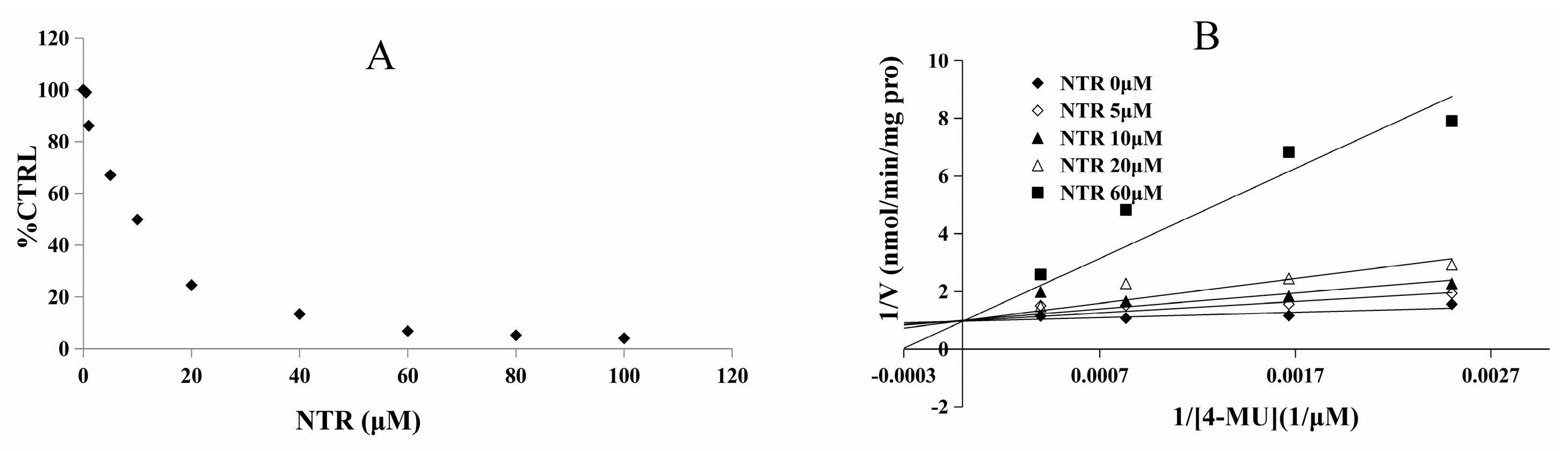
2.2. Inhibition Type and Kinetics of NTR towards UGT1A3, UGT1A7 and UGT1A9
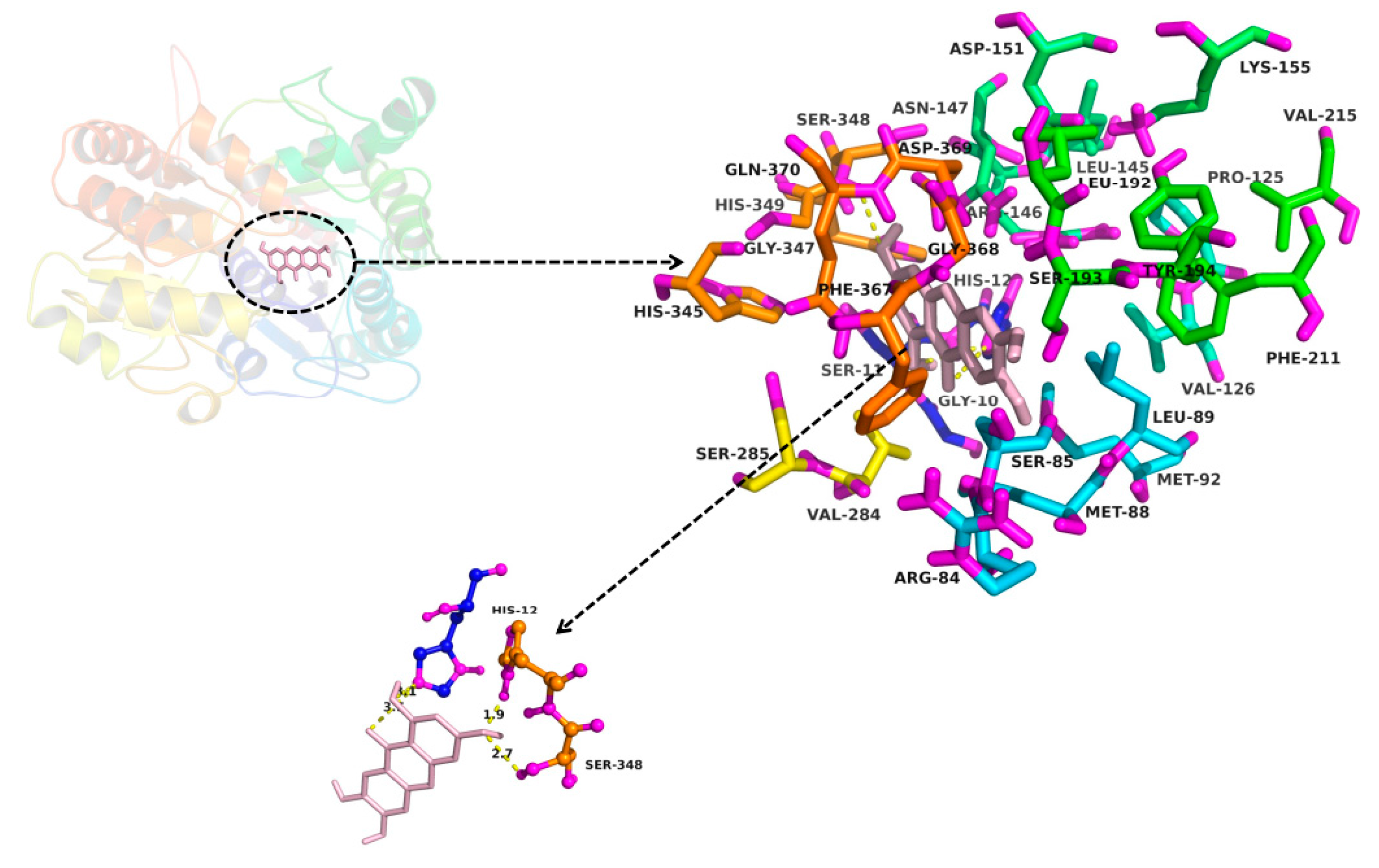
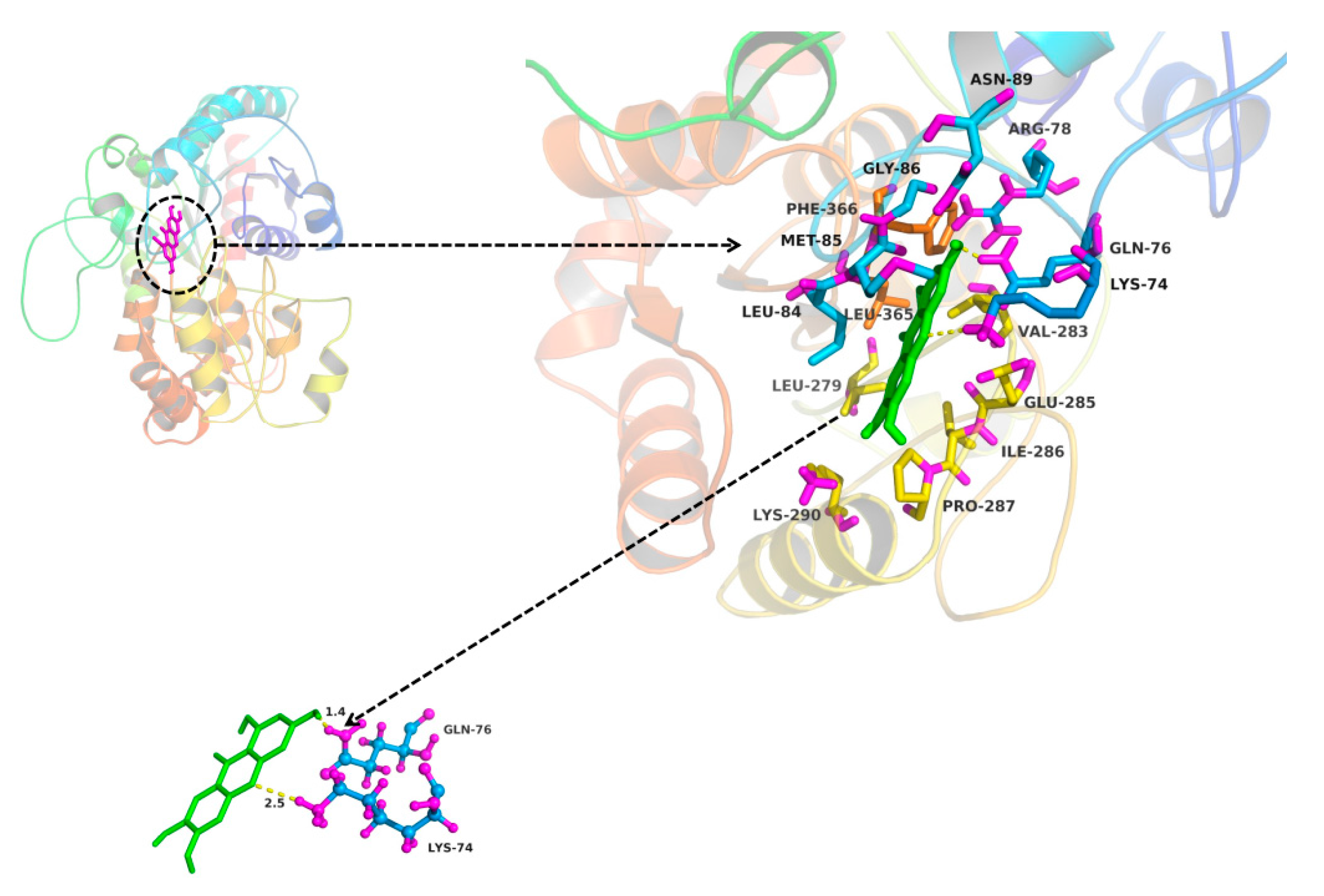
2.3. In Silico Docking to Explain the Inhibition of NTR towards UGT1A3, UGT1A7 and UGT1A9
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Investigation of the Inhibition Potential of MGF and NTR on UGT Isoforms
4.3. In Silico Docking to Explain the Inhibition of NTR towards UGT1A3, UGT1A7 and UGT1A9
4.4. Inhibition Kinetic Analysis and In Vitro–In Vivo Extrapolation (IVIVE)
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, F.; Xu, W.; Wei, H.; Sun, L.N.; Gao, S.H.; Yang, Q.; Feng, J.; Zhang, F.; Chen, W.S. Simultaneous determination of active xanthone glycosides, timosaponins and alkaloids in rat plasma after oral administration of Zi-Shen Pill extract for the pharmacokinetic study by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2010, 878, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nageshwar, R.B.; Satish, R.B.S. Mangiferin attenuates methylmercury induced cytotoxicity against IMR-32, human neuroblastoma cells by the inhibition of oxidative stress and free radical scavenging potential. Chem.-Biol. Interact. 2011, 193, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Duang, X.Y.; Wang, Q.; Zhou, X.D.; Huang, D.M. Mangiferin: A possible strategy for periodontal disease to therapy. Med. Hypotheses 2011, 76, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Girón, M.D.; Sevillano, N.; Salto, R.; Haidour, A.; Manzano, M.; Jiménez, M.L.; Rueda, R.; López-Pedrosa, J.M. Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6rat myotubes: Role of mangiferin. Clin. Nutr. 2009, 28, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Apontes, P.; Liu, Z.B.; Su, K.; Benard, O.; Youn, D.Y.; Li, X.S.; Li, W.; Mirza, R.H.; Bastie, C.C.; Jelicks, L.A.; et al. Mangiferin stimulates carbohydrate oxidation and protects against metabolic disorders induced by high-fat diets. Diabetes 2014, 63, 3626–3636. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Tsubaki, M.; Yamazoe, Y.; Satou, T.; Itoh, T.; Kidera, Y.; Tanimori, Y.; Yanae, M.; Matsuda, H.; Taga, A.; et al. Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP expressions and nuclear entry of NF-ĸB in HL-60 cells. Arch. Pharm. Res. 2011, 34, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Savikin, K.; Menković, N.; Zdunić, G.; Stević, T.; Radanović, D.; Janković, T. Antimicrobial activity of Gentiana lutea L. extracts. Z. Naturforschung C 2009, 64, 339–342. [Google Scholar] [CrossRef]
- Wang, R.R.; Gao, Y.D.; Ma, C.H.; Zhang, X.J.; Huang, C.G.; Huang, J.F.; Zheng, Y.T. Mangiferin, an anti-HIV-1 agent targeting protease and effective against resistant strains. Molecules 2011, 16, 4264–4277. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Baliga, M.S. Radioprotection by mangiferin in DBAxC57BL mice: A preliminary study. Phytomedicine 2005, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Barbary, M.A.; El-Ghorab, D.M.H.; Bohlin, L.; Borg-Karlson, A.K.; Goransson, U.; Verpoorte, R. Recent insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 2010, 17, 854–901. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Ghorab, D.M.; El-Barbary, M.A.; Zayed, M.F.; Goransson, U.; Larsson, S.; Verpoorte, R. Naturally occurring xanthones; latest investigations: isolation, structure elucidation and chemosystematic significance. Curr. Med. Chem. 2009, 16, 2581–2626. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Xu, F.P.; Zeng, X.; Yang, L.; Deng, Y.H.; Wu, Z.F.; Feng, Y.; Li, X. Application of a liquid chromatography/tandem mass spectrometry method to pharmacokinetic study of mangiferin in rats. J. Chromatogr. B 2010, 878, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Han, D.D.; Chen, C.J.; Cong, Z.; Zhang, Y.; Tang, X. Determination of mangiferin in rat plasma by liquid–liquid extraction with UPLC–MS/MS. J. Pharm. Biomed. Anal. 2010, 51, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M. Influence of route of administration on drug availability. J. Pharm. Sci. 1972, 61, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Pond, S.M.; Tozer, T.N. First-pass elimination basic concepts and clinical consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Wu, B.; Pan, G.Y.; He, L.; Li, Z.X.; Fan, M.S.; Chen, M.C.; Wang, K.; Huang, C.G. Metabolism and pharmacokinetics of mangiferin in conventional rats, pseudo-germ-free rats, and streptozotocin-induced diabetic rats. Drug Metab. Dispos. 2012, 40, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Wang, L.X.; Tang, Y.H.; Fan, M.B.; Xiao, H.B.; Huang, C.G. Identification of major xanthones and steroidal saponins in rat urine by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry technology following oral administration of Rhizoma Anemarrhenae decoction. Biomed. Chromatogr. 2008, 22, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Sanugul, K.; Akao, T.; Li, Y.; Kakiuchi, N.; Nakamura, N.; Hattori, M. Isolation of a human intestinal bacterium that transforms mangiferin to norathyriol and inducibility of the enzyme that cleaves a C-glucosyl bond. Biol. Pharm. Bull. 2005, 28, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Blaut, M. Deglycosylation of puerarin and other aromatic C-glucosides by a newly isolated human intestinal bacterium. Environ. Microbiol. 2011, 13, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Chieli, E.; Romiti, N.; Rodeiro, I.; Garrido, G. In vitro effects of Mangiferaindica and polyphenols derived on ABCB1/P-glycoprotein activity. Food Chem. Toxicol. 2009, 47, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.F.; Lin, C.N.; Lu, M.C.; Wang, J.P. Inhibition of the arachidonic acid cascade by norathyriol via blockade of cyclooxygenase and lipoxygenase activity in neutrophils. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Z.; Lin, W.C.; Yeh, F.T.; Lin, C.N.; Wu, C.H. Decreased protein kinase C activation mediates inhibitory effect of norathyriol on serotonin-mediated endothelial permeability. Eur. J. Pharmacol. 1998, 353, 303–313. [Google Scholar] [CrossRef]
- Li, J.X.; Malakhova, M.; Mottamal, M.; Reddy, K.; Kurinov, I.; Carper, A.; Langfald, A.; Oi, N.; Kim, O.M.; Zhu, F.; et al. Norathyriol suppresses skin cancers induced by solar ultraviolet radiation by targeting ERK kinases. Cancer Res. 2012, 72, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yan, J.M.; Niu, Y.F.; Li, Y.; Lin, H.; Liu, X.; Liu, J.K.; Li, L. Mangiferin and its aglycone, norathyriol, improve glucose metabolism by activation of AMP activated protein kinase. Pharm. Biol. 2014, 52, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Kirankumar, G.V.; Sekhar, H.S.; Nagaraj, K.; Nagaraju, B.; Ravi, C.M.; Hossein, N. Drug Interaction of Repaglinide and Mangiferin in Rats. Int. J. Pharm. Chem. Sci. 2013, 2, 1218–1226. [Google Scholar]
- Oda, S.; Fukami, T.; Yokoi, T.; Nakajima, M. A comprehensive review of UDP-glucuronosyltransferase and esterases for drug development. Drug Metab. Pharmacokinet. 2015, 30, 30–51. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Ensom, M.H.; Chang, T.K. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharm. Ther. 2005, 106, 97–132. [Google Scholar] [CrossRef] [PubMed]
- Tukey, R.H.; Strassburg, C.P. Human UDP-Glucuronosyltransferases: Metabolism, Expression, and Disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.; Miners, J.; Mackenzie, P.I. The UDP-glucuronosyltransferases: Their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013, 45, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett. 2009, 583, 3303–3309. [Google Scholar] [CrossRef] [PubMed]
- Judith, M.J.; Quincy, C.S.C.; Prabhu, R.; Soma, D.; Ruslan, S.; Michael, B.S. UGT1A1 polymorphism and hyperbilirubinemia in a patient who received sorafenib. Cancer Chemother. Pharmacol. 2009, 65, 1–4. [Google Scholar]
- Bourcier, K.; Hyland, R.; Kempshall, S.; Jones, R.; Mximilien, J.; Irvine, N.; Jones, B. Investigation into UDP-Glucuronosyltransferase (UGT) enzyme kinetics of imidazole- and triazole-containing antifungal drugs in human Liver microsomes and recombinant UGT enzymes. Drug. Metab. Dispos. 2010, 38, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.X.; Zhang, C.Z.; Li, R.S.; Chen, B.W.; Zhang, W.H.; Zhao, Z.Y.; Fu, Z.W.; Du, Z.; Du, X.L.; Yang, X.L.; et al. Evaluation and Comparison of the Inhibition Effect of Astragaloside IV and Aglycone Cycloastragenol on Various UDP-Glucuronosyltransferase (UGT) Isoforms. Molecules 2016, 21, 1616. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wu, J.; Liu, D.; Liu, M.; Zhang, H.; Huang, S.; Xiong, Y.; Xia, C. In vitro inhibition of UGT1A3, UGT1A4 by ursolic and oleanolic acid and drug–drug interaction risk prediction. Xenobiotica 2016, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Zheng, Y.F.; Min, J.S.; Park, J.B.; Bae, S.H.; Yoon, K.D.; Chin, Y.W.; Oh, E.; Bae, S.K. In vitro, Stereoselective Inhibition of Ginsenosides toward UDP-glucuronosyltransferase (UGT) Isoforms. Toxicol. Lett. 2016, 259, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Li, B.M.; Li, S.; Li, W.P. Deglycosylation of glucoaurantio-obtusin affects its inhibition capability towards drug metabolizing enzymes (DMEs). Lat. Am. J. Pharm. 2013, 32, 1249–1251. [Google Scholar]
- Guo, B.; Fan, X.R.; Fang, Z.Z.; Cao, Y.F.; Hu, C.M.; Yang, I.; Zhang, Y.Y.; He, R.R.; Zhu, X.; Yu, Z.W.; et al. Deglycosylation of liquiritin strongly enhances its inhibitory potential towards UDP-glucuronosyltransferase (UGT) isoforms. Phytother. Res. 2013, 27, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.F.; He, R.R.; Cao, J.; Chen, J.X.; Huang, T.; Liu, Y. Drug–Drug Interactions Potential of Icariin and Its Intestinal Metabolites via Inhibition of Intestinal UDP-Glucuronosyltransferases. Evid.-Based Complement. Altern. Med. 2012, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.Y.; Wang, T.; Wang, Y.J.; Cui, X.; Zhang, H.J.; Fang, Z.Z. Inhibition of UDP-Glucuronosyl transferase (UGT) Isoforms by Arctiin and Arctigenin. Phytother. Res. 2016, 30, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.Y.; Cao, Y.F.; Hu, C.M.; Fang, Z.Z.; Sun, X.Y.; Hong, M.; Zhu, Z.T. Comparison of inhibition capability of scutellarin and scutellarin towards important liver UDP-glucuronosyltransferase (UGT) isoforms. Photother. Res. 2014, 28, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.G.; Mackenzie, P.I.; Chowdhury, J.R. Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab. Dispos. Biol. Fate Chem. 2004, 32, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Trottier, J.; Verreault, M.; Grepper, S.; Monte, D.; Belanger, J.; Kaeding, J.; Inaba, T.T.; Barbier, O. Human UDP-glucuronosyltransferase (UGT)1A3 enzyme conjugates chenodeoxycholic acid in the liver. Hepatology 2006, 44, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Mojarrabi, B.; Butler, R.; Mackenzie, P.I. cDNA cloning and characterization of the human UDP glucuronosyltransferase, UGT1A3. Biochem. Biophys. Res. Commun. 1996, 225, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Green, M.D.; King, C.D.; Mojarrabi, B.; Mackenzie, P.I.; Teply, T.R. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP glucuronosyl transferase 1A3. Drug Metab. Dispos. 1998, 26, 507–512. [Google Scholar] [PubMed]
- Kuehl, G.E.; Lampe, J.W.; Potter, J.D.; Bigler, J. Glucuronidation of nonsteroidal anti-inflammatory drugs: Identifying the enzymes responsible in human liver microsomes. Drug Metab. Dispos. 2005, 33, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Prueksaritanont, T.; Zhao, J.J.; Ma, B.; Roadcap, B.A.; Tang, C.; Qiu, Y.; Liu, L.; Pearson, P.G.; Baillie, T.A. Mechanistic studies on metabolic interactions between gemfibrozil and statins. J. Pharmacol. Exp. Ther. 2002, 301, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, C.P.; Oldhafer, K.; Manns, M.P.; Tukey, R.H. Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: Identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissues. Mol. Pharmacol. 1997, 52, 212–220. [Google Scholar] [PubMed]
- Ritter, J.K. Roles of glucuronidation and UDP-glucuronosyltransferases in xenobiotic bioactivation reactions. Chem. Biol. Interact. 2000, 129, 171–193. [Google Scholar] [CrossRef]
- Yang, S.Y.; Qiu, Z.X.; Zhang, Q.Y.; Chen, J.Y.; Chen, X.J. Inhibitory Effects of Calf Thymus DNA on Metabolism Activity of CYP450 Enzyme in Human Liver Microsomes. Drug Metab. Pharmacokinet. 2014, 29, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, G.; Tang, Y.H.; Zhu, H.Y.; Ma, R.R.; Sun, Z.L.; Huang, C.G. High-performance liquid chromatographic method for the determination of mangiferin in rat plasma and urine. Biomed. Chromatogr. 2006, 20, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, F.; Li, Y.; Li, Y.; Wang, M.; Sun, D.; Sun, C. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012, 132, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.T.; Gao, Y.; Xu, Z.; Lian, S.; Ma, Y.J.; Guo, X.Z.; Hu, P.; Li, Z.X.; Huang, C.G. Pharmacokinetics of mangiferin and its metabolite—Norathyriol, Part 1: Systemic evaluation of hepatic first-pass effect in vitro, and in vivo. Biofactors 2016, 42, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, Y.F.; Ran, R.X.; Li, R.S.; Wu, X.; Dong, P.P.; Zhang, Y.Y.; Hu, C.M.; Wang, W.M. Strong specific inhibition of UDP-glucuronosyltransferase 2B7 by atractylenolide I and III. Phytother. Res. 2016, 30, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Z.; Wang, H.N.; Cao, Y.F.; Sun, D.X.; Wang, L.X.; Hong, M.; Huang, T.; Chen, J.X.; Zeng, A.J. Enantioselective Inhibition of Carprofen Towards UDPglucuronosyltransferase (UGT) 2B7. Chirality 2015, 27, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Z.; Cao, Y.F.; Hu, C.M.; Hong, M.; Sun, X.Y.; Ge, G.B.; Liu, Y.; Zhang, Y.Y.; Yang, L.; Sun, H.Z. Structure-inhibition relationship of ginsenosides towards UDP-glucuronosyltransferases (UGTs). Toxicol. Appl. Pharmacol. 2013, 267, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.W.; Wu, X.; Zhao, Z.Y.; Fu, Z.W.; Huang, C.T.; Ye, L.X.; Du, Z.; Yu, Y.; Fang, Z.Z.; et al. The Inhibition of UDP-Glucuronosyltransferase (UGT) Isoforms by Praeruptorin A and B. Phytother. Res. 2016, 30, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, Y.F.; Ran, R.X.; Dong, P.P.; Gonzalez, F.J.; Wu, X.; Huang, T.; Chen, J.X.; Fu, Z.W.; Li, R.S.; et al. New insights into the risk of phthalates: Inhibition of UDP-glucuronosyltransferases. Chemosphere 2016, 144, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.M.; Lin, P.; Dai, T.; Zhang, C.H.; Ma, R.P. Docking study of cardiovascular disease treatment drug salvianolic acid A and C into the activity cavity of intestinal UDP-Glucuronosyltransferase(UGT) 1A8. Lat. Am. J. Pharm. 2017, 36, 179–183. [Google Scholar]
- Liu, X.; Cao, Y.F.; Dong, P.P.; Zhu, L.L.; Zhao, Z.Y.; Wu, X.; Fu, Z.W.; Huang, C.T.; Fang, Z.Z.; Sun, H.Z. The inhibition of UDP-glucuronosyltransferases (UGTs) by vitamin A. Xenobiotica 2017, 47, 376–381. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Zhang, C.-Z.; Ran, R.-X.; Cao, Y.-F.; Du, Z.; Fu, Z.-W.; Huang, C.-T.; Zhao, Z.-Y.; Zhang, W.-H.; Fang, Z.-Z. In Vitro Comparative Study of the Inhibitory Effects of Mangiferin and Its Aglycone Norathyriol towards UDP-Glucuronosyl Transferase (UGT) Isoforms. Molecules 2017, 22, 1008. https://doi.org/10.3390/molecules22061008
Sun D, Zhang C-Z, Ran R-X, Cao Y-F, Du Z, Fu Z-W, Huang C-T, Zhao Z-Y, Zhang W-H, Fang Z-Z. In Vitro Comparative Study of the Inhibitory Effects of Mangiferin and Its Aglycone Norathyriol towards UDP-Glucuronosyl Transferase (UGT) Isoforms. Molecules. 2017; 22(6):1008. https://doi.org/10.3390/molecules22061008
Chicago/Turabian StyleSun, Dan, Chun-Ze Zhang, Rui-Xue Ran, Yun-Feng Cao, Zuo Du, Zhi-Wei Fu, Chun-Ting Huang, Zhen-Ying Zhao, Wei-Hua Zhang, and Zhong-Ze Fang. 2017. "In Vitro Comparative Study of the Inhibitory Effects of Mangiferin and Its Aglycone Norathyriol towards UDP-Glucuronosyl Transferase (UGT) Isoforms" Molecules 22, no. 6: 1008. https://doi.org/10.3390/molecules22061008
APA StyleSun, D., Zhang, C.-Z., Ran, R.-X., Cao, Y.-F., Du, Z., Fu, Z.-W., Huang, C.-T., Zhao, Z.-Y., Zhang, W.-H., & Fang, Z.-Z. (2017). In Vitro Comparative Study of the Inhibitory Effects of Mangiferin and Its Aglycone Norathyriol towards UDP-Glucuronosyl Transferase (UGT) Isoforms. Molecules, 22(6), 1008. https://doi.org/10.3390/molecules22061008