The Phenolic Fraction of Mentha haplocalyx and Its Constituent Linarin Ameliorate Inflammatory Response through Inactivation of NF-κB and MAPKs in Lipopolysaccharide-Induced RAW264.7 Cells
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Chemical Composition of the Phenolic Fraction of Mentha haplocalyx by HPLC-LTQ-Orbitrap MS
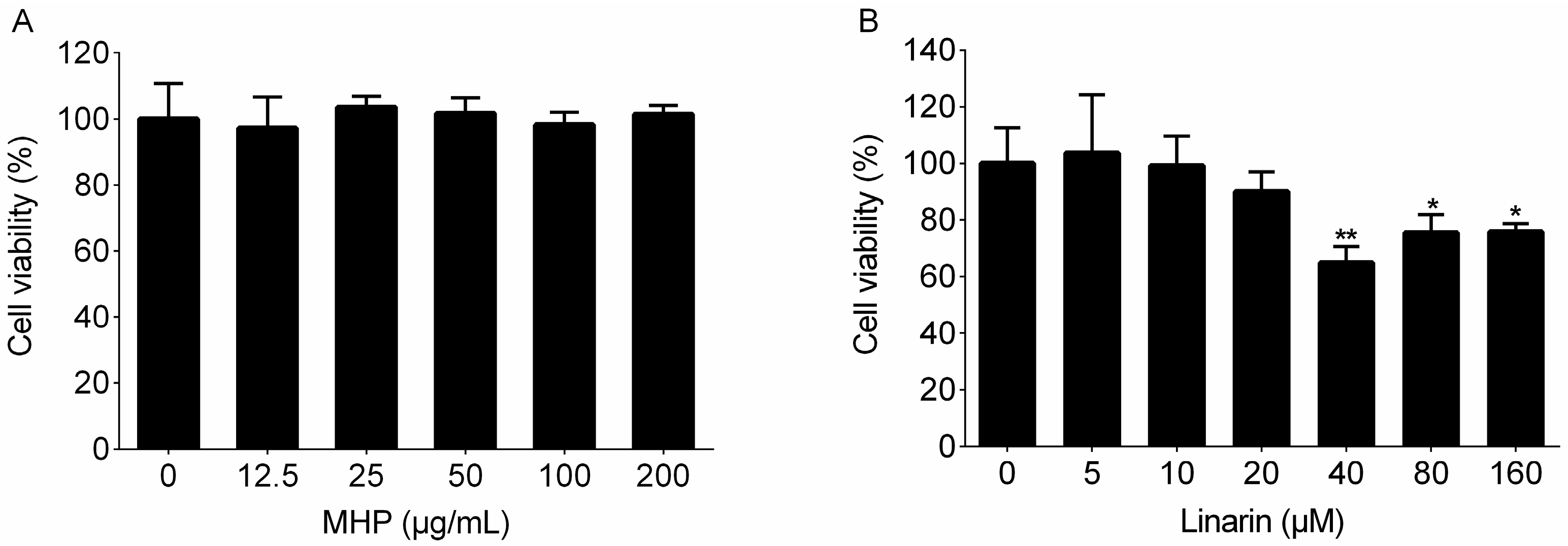
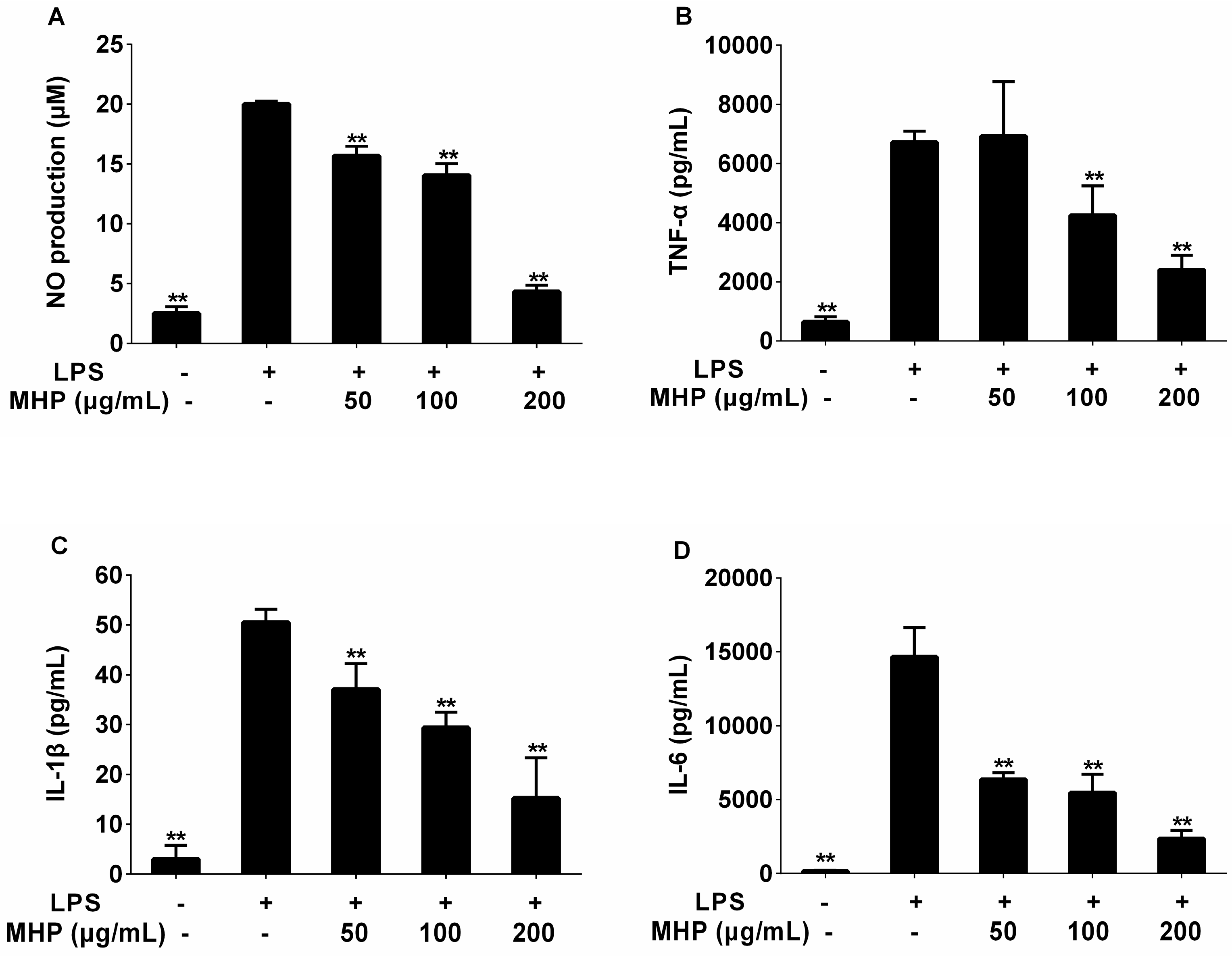
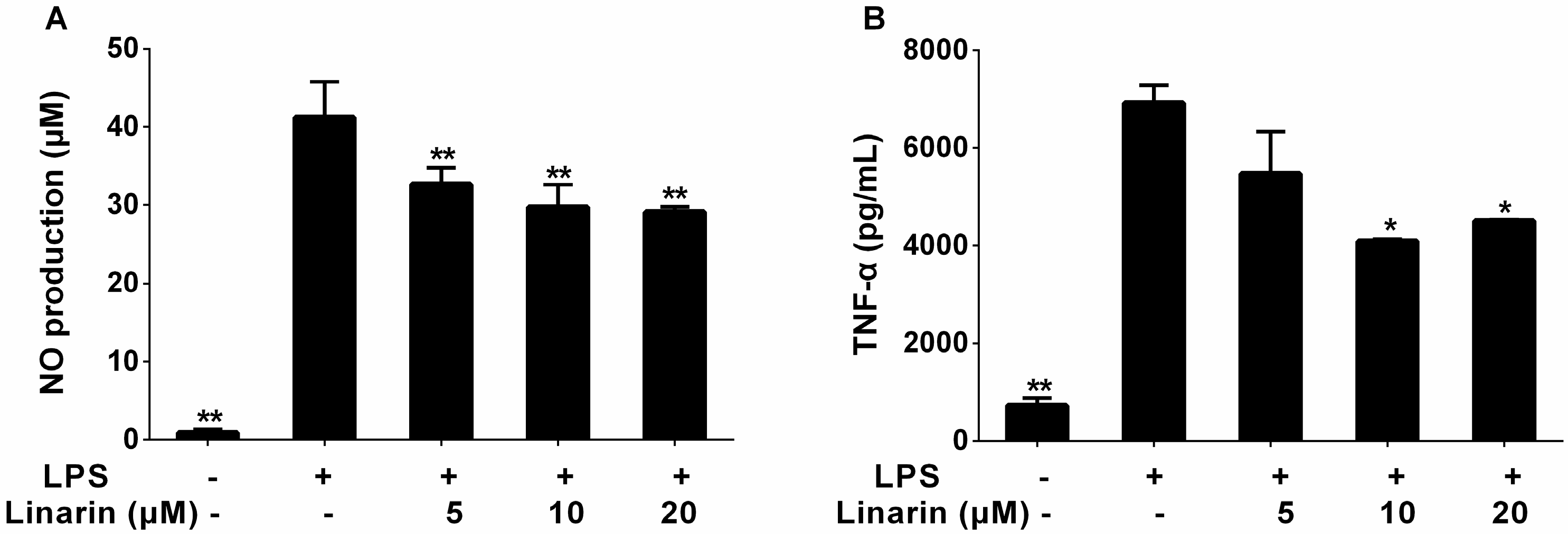
2.2. Effects of the Phenolic Fraction of Mentha haplocalyx and Linarin on NO Production and iNOS Expression Level in LPS-Induced RAW264.7 Cells
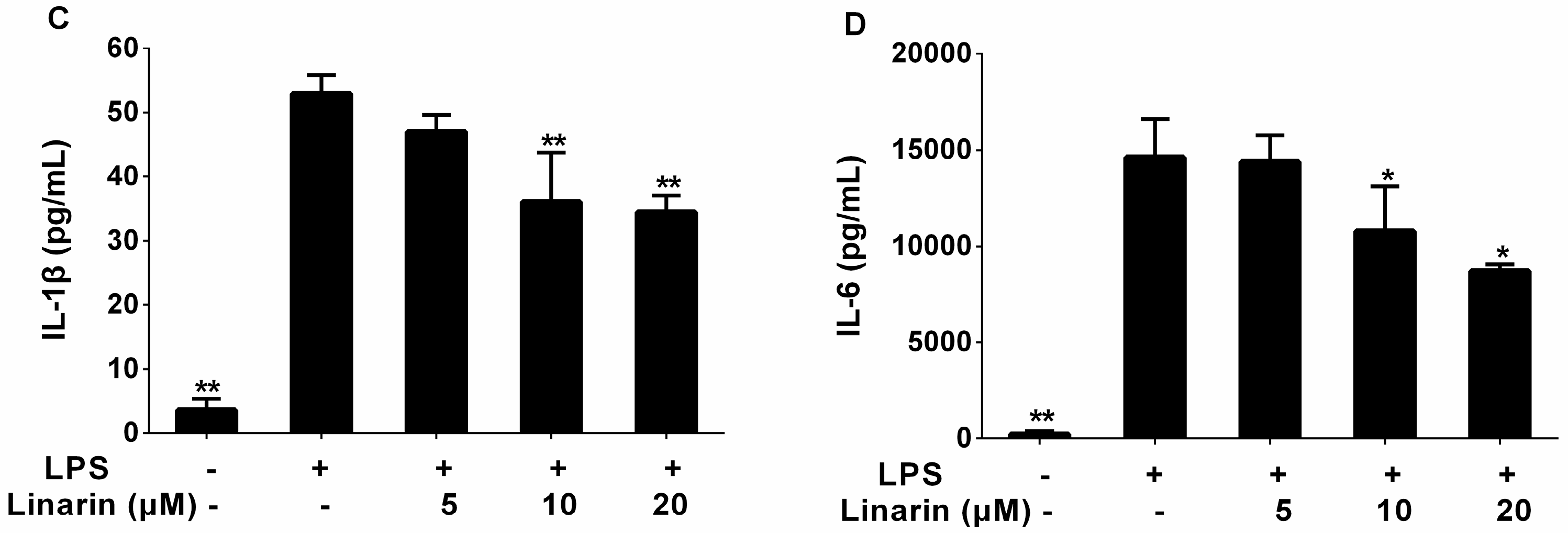
2.3. Effects of the Phenolic Fraction of Mentha haplocalyx and Linarin on the Production of TNF-α, IL-1β, and IL-6 in LPS-Induced RAW264.7 Cells
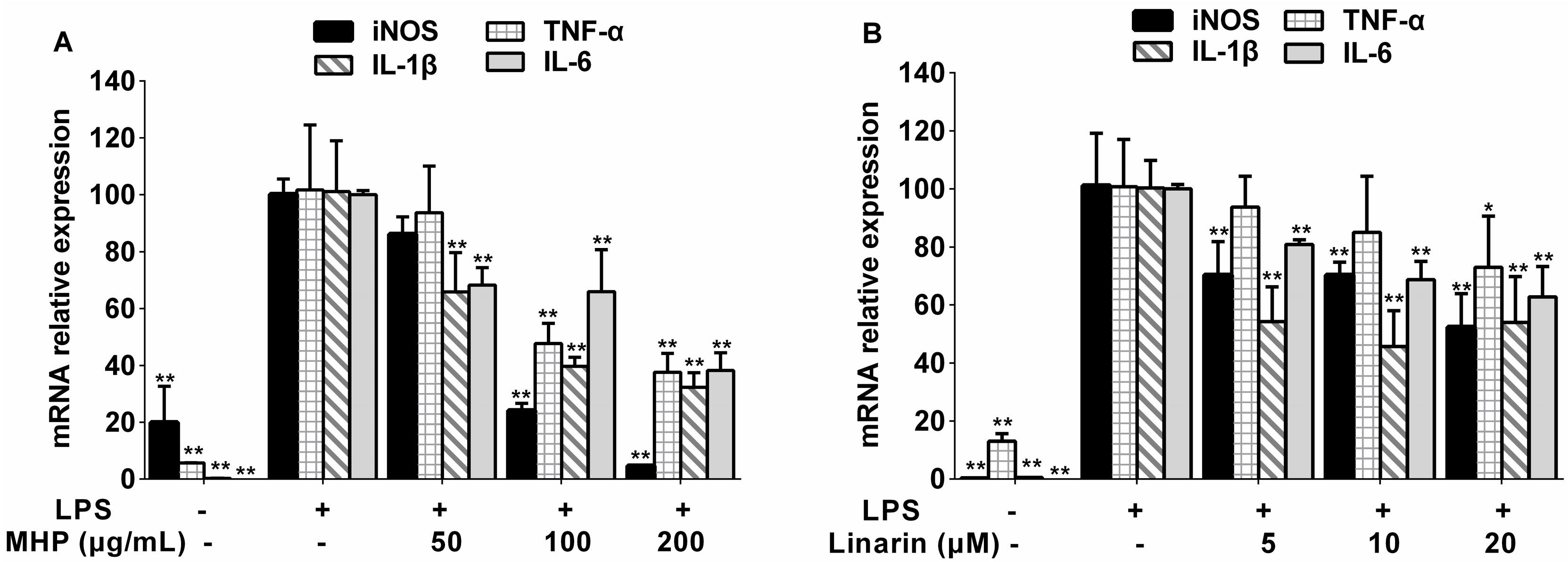
2.4. Effects of the Phenolic Fraction of Mentha haplocalyx and Linarin on TNF-α, IL-1β, and IL-6 mRNA Expression in LPS-Induced RAW264.7 Cells
2.5. Effects of the Phenolic Fraction of Mentha haplocalyx and Linarin on NF-κB p65 and IκBα in LPS-Induced RAW264.7 Cells
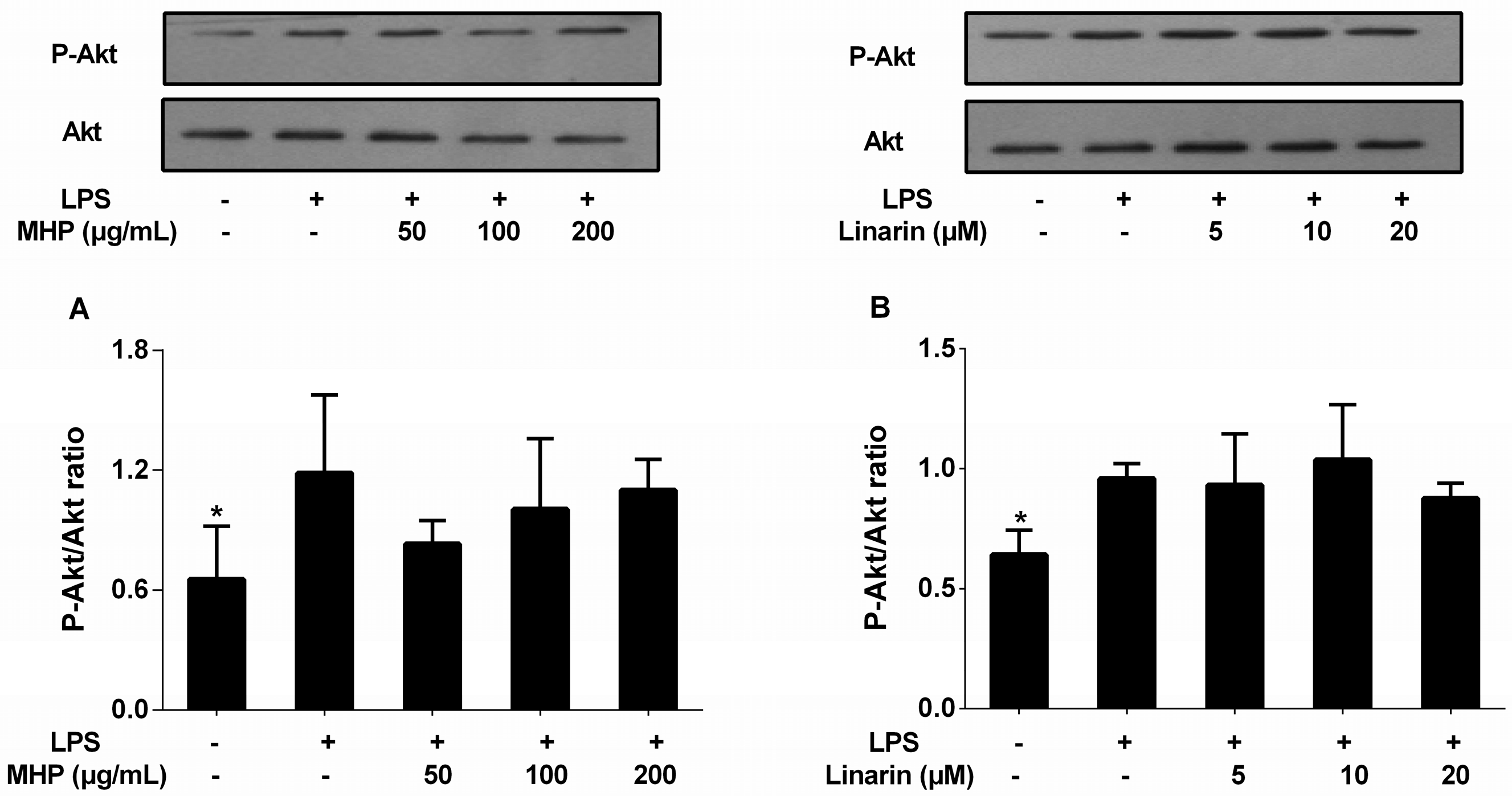
2.6. Effects of the Phenolic Fraction of Mentha haplocalyx and Linarin on Akt in LPS-Induced RAW264.7 Cells
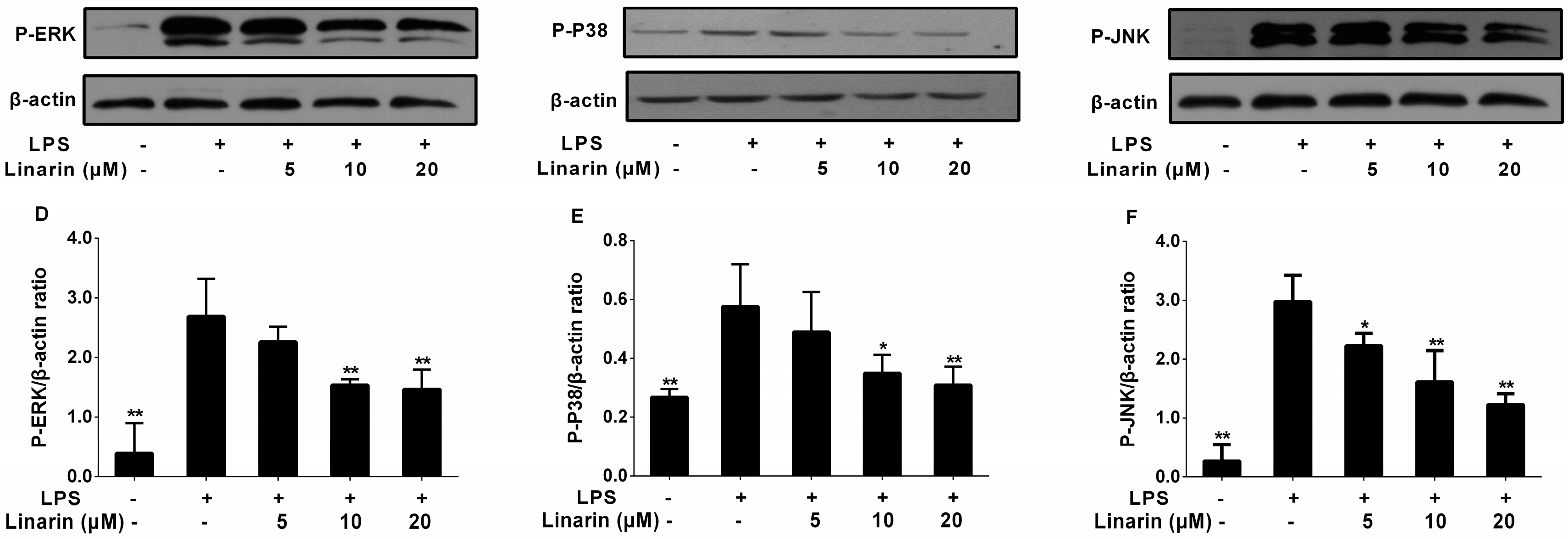
2.7. Effects of the Phenolic Fraction of Mentha haplocalyx and Linarin on MAPKs in LPS-Induced RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of the Phenolic Fraction of Mentha haplocalyx Extract
4.3. HPLC-MS/MS Analysis of MHP
4.4. Cell Culture Conditions and Treatment
4.5. Cell Viability Assay
4.6. Effect on NO Production
4.7. Cytokines Measurement
4.8. RNA Extraction and qRT-PCR
4.9. Western Blot Analysis
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, D.H.; Yun, C.H.; Kim, M.H.; Naveen Kumar, C.; Yun, B.H.; Shin, J.S.; An, H.J.; Lee, Y.H.; Yun, Y.D.; Rim, H.K.; et al. 4′-Bromo-5,6,7-trimethoxyflavone represses lipopolysaccharide-induced iNOS and COX-2 expressions by suppressing the NF-κB signaling pathway in RAW 264.7 macrophages. Bioorg. Med. Chem. Lett. 2012, 22, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Yu, K.; Chen, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell. Immunol. 2013, 285, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Pan, L.L.; Jia, Y.L.; Wu, D.; Xiong, Q.H.; Wang, Y.; Zhu, Y.Z. A novel compound DSC suppresses lipopolysaccharide-induced inflammatory responses by inhibition of Akt/NF-κB signalling in macrophages. Eur. J. Pharmacol. 2013, 708, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.O.; Hamilton, T.A. The cell biology of macrophage activation. Annu. Rev. Immunol. 1984, 2, 283–318. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kwon, O.K.; Yuniato, P.; Marwoto, B.; Lee, J.; Oh, S.R.; Kim, J.H.; Ahn, K.S. Amelioration of an LPS-induced inflammatory response using a methanolic extract of Lagerstroemia ovalifolia to suppress the activation of NF-κB in RAW264.7 macrophages. Int. J. Mol. Med. 2016, 38, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Moon, T.C.; Quan, Z.; Lee, E.; Kim, Y.K.; Yang, J.H.; Suh, S.J.; Jeong, T.C.; Lee, S.H.; Kim, C.H.; et al. The naturally occurring flavolignan, deoxypodophyllotoxin, inhibits lipopolysaccharide-induced iNOS expression through the NF-κB activation in RAW264. 7 macrophage cells. Biol. Pharm. Bull. 2008, 31, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Jung, M.; Bang, M.H.; Chung, D.K.; Kim, J. Inhibitory effects of a spinasterol glycoside on lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines via down-regulating MAP kinase pathways and NF-κB activation in RAW264.7 macrophage cells. Int. Immunopharmacol. 2012, 13, 264–270. [Google Scholar] [CrossRef]
- May, M.J.; Ghosh, S. Signal transduction through NF-κB. Immunol. Today 1998, 19, 80–88. [Google Scholar] [CrossRef]
- Fan, G.W.; Zhang, Y.; Jiang, X.; Zhu, Y.; Wang, B.; Su, L.; Cao, W.; Zhang, H.; Gao, X. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation 2013, 36, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, H.; Mi, C.; Li, H.L.; Lee, J.J.; Jin, X. Malloapelta B suppresses LPS-induced NF-κB activation and NF-κB-regulated target gene products. Int. Immunopharmacol. 2015, 24, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Bilehal, D.; Lee, G.-S.; Ryu, D.-S.; Kim, H.-K.; Suk, D.-H.; Lee, D.-S. Anti-inflammatory effect of the hexane fraction from Orostachys japonicus in RAW 264.7 cells by suppression of NF-κB and PI3K-Akt signaling. J. Funct. Foods 2013, 5, 1217–1225. [Google Scholar] [CrossRef]
- Pan, X.; Cao, X.; Li, N.; Xu, Y.; Wu, Q.; Bai, J.; Yin, Z.; Luo, L.; Lan, L. Forsythin inhibits lipopolysaccharide-induced inflammation by suppressing JAK-STAT and p38 MAPK signalings and ROS production. Inflamm. Res. 2014, 63, 597–608. [Google Scholar] [CrossRef] [PubMed]
- She, G.M.; Xu, C.; Liu, B. New monocyclic monoterpenoid glycoside from Mentha haplocalyx Briq. Chem. Cent. J. 2012, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Baskar, T.B.; Yeo, S.K.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, S.S.; Park, S.U. Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus 2016, 5, 1628. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Shan, Q.; Li, X.; Cong, X.; Zhang, Y.; Cai, H.; Cai, B. Analysis of fresh Mentha haplocalyx volatile components by comprehensive two-dimensional gas chromatography and high-resolution time-of-flight mass spectrometry. Analyst 2011, 136, 4653–4661. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Abbas, K.; Qadir, M.I. Gastro-protective effect of ethanolic extract of Mentha longifolia in alcohol- and aspirin-induced gastric ulcer models. Bangladesh J. Pharmacol. 2015, 10. [Google Scholar] [CrossRef]
- Estrada-Soto, S.; Gonzalez-Maldonado, D.; Castillo-Espana, P.; Aguirre-Crespo, F.; Sanchez-Salgado, J.C. Spasmolytic effect of Mentha pulegium L. involves ionic flux regulation in rat ileum strips. J. Smooth Muscle Res. 2010, 46, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Baliga, M.S. Influence of the leaf extract of Mentha arvensis linn. (mint) on the survival of mice exposed to different doses of gamma radiation. Strahlenther. Onkol. 2002, 178, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Samarth, R.M.; Panwar, M.; Kumar, A. Modulatory effects of Mentha piperita on lung tumor incidence, genotoxicity, and oxidative stress in benzo[a]pyrene-treated Swiss albino mice. Environ. Mol. Mutagen. 2006, 47, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Alam, A.; Sultana, S. Attenuation of benzoyl peroxide-mediated cutaneous oxidative stress and hyperproliferative response by the prophylactic treatment of mice with spearmint (Mentha spicata). Food Chem. Toxicol. 2000, 38, 939–948. [Google Scholar] [CrossRef]
- Orhan, I.E.; Ozcelik, B.; Kartal, M.; Kan, Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar] [CrossRef]
- Amzazi, S.; Ghoulami, S.; Bakri, Y.; Idrissi, A.; Fkih-Tétouani, S.; Benjouad, A. Human immunodeficiency virus type 1 inhibitory activity of Mentha longifolia. Thérapie 2003, 58, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukic, N.; Bozin, B.; Mentha, L. Species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharm. Des. 2008, 14, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ni, Y.; Kokot, S. Differentiation of mint (Mentha haplocalyx Briq.) from different regions in China using gas and liquid chromatography. J. Sep. Sci. 2015, 38, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.H.; Shi, R.B. Studies on the chemixcal constituents in herb of Mentha haplocalyx. China J. Chin. Mater. Med. 2005, 30, 1086–1088. [Google Scholar] [CrossRef]
- Hao, L.L.; Li, X.R.; Cao, L.; Chen, S.H. The interaction between exterior releasing herbs and anti-infection. Mod. Med. J. China 2006, 8, 84–85. [Google Scholar] [CrossRef]
- Liao, H.; Banbury, L.K.; Leach, D.N. Elucidation of Danzhixiaoyao Wan and its constituent herbs on antioxidant activity and inhibition of nitric oxide production. Evid. Based Complement. Altern. Med. 2007, 4, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Xu, L.Y.; Li, Z.L.; Qian, S.H.; Qin, M.J. Flavonoids from Mentha haplocalyx. Chem. Nat. Compd. 2014, 50, 124–125. [Google Scholar] [CrossRef]
- She, G.M.; Xu, C.; Liu, B.; Shi, R.B. Polyphenolic acids from mint (the aerial of Mentha haplocalyx Briq.) with DPPH radical scavenging activity. J. Food Sci. 2010, 75, C359–C362. [Google Scholar] [CrossRef] [PubMed]
- She, G.M.; Xu, C.; Liu, B. Phenylpropanoids from Mentha haplocalyx. Chem. Nat. Compd. 2013, 48, 1083–1084. [Google Scholar] [CrossRef]
- Farnad, N.; Heidari, R.; Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of peppermint (Mentha piperita). J. Food Meas. Charact. 2014, 8, 113–121. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Zhang, F.; Liu, B. Optimization of purification technology for total phenolic acid from Mentha haplocalyx by macroporous resin. Chin. J. Exp. Tradit. Med. Form. 2012, 18, 9–11. [Google Scholar]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Lee, J.A.; Seo, C.S.; Ha, H.; Lee, N.H.; Shin, H.K. Protective effects of Mentha haplocalyx ethanol extract (MH) in a mouse model of allergic asthma. Phytother. Res. 2011, 25, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Priya, N.G.; Subathra, M.; Ramesh, A. Anti-inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environ. Toxicol. Pharmacol. 2008, 26, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.F.; Muhammad, J.S.; Shahryar, S.; Usmanghani, K.; Gilani, A.H.; Jafri, W.; Sugiyama, T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol. 2012, 141, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Salin, O.; Tormakangas, L.; Leinonen, M.; Saario, E.; Hagstrom, M.; Ketola, R.A.; Saikku, P.; Vuorela, H.; Vuorela, P.M. Corn mint (Mentha arvensis) extract diminishes acute chlamydia pneumoniae infection in vitro and in vivo. J. Agric. Food Chem. 2011, 59, 12836–12842. [Google Scholar] [CrossRef] [PubMed]
- Karimian, P.; Kavoosi, G.; Amirghofran, Z. Anti-inflammatory effect of Mentha longifolia in lipopolysaccharide-stimulated macrophages reduction of nitric oxide production through inhibition of inducible nitric oxide synthase. J. Immunotoxicol. 2013, 10, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Guan, Y.; Yin, Y.; Duan, J.; Zhou, D.; Zhu, Y.; Quan, W.; Xi, M.; Wen, A. Anti-inflammatory effect of protocatechuic aldehyde on myocardial ischemia/reperfusion injury in vivo and in vitro. Inflammation 2013, 36, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; Kim, D.H.; An, H.J.; Kim, M.K.; Kim, N.D.; Chung, H.Y. Caffeic acid regulates LPS-induced NF-κB activation through NIK/IKK and c-Src/ERK signaling pathways in endothelial cells. Arch. Pharm. Res. 2014, 37, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.; Iqbal, M.; Anwer, M.K.; Al Hoshani, A.R.; Attia, S.M.; Ahmad, S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Zhang, J.; Li, R. Salvianolic acid B attenuates lung inflammation induced by cigarette smoke in mice. Eur. J. Pharmacol. 2015, 761, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Chen, X.G.; Qu, G.W.; Yue, X.D.; Zhu, H.B.; Tian, J.W.; Fu, F.H. Rosmarinic acid protects against experimental sepsis by inhibiting proinflammatory factor release and ameliorating hemodynamics. Shock 2009, 32, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Oinonen, P.P.; Jokela, J.K.; Hatakka, A.I.; Vuorela, P.M. Linarin, a selective acetylcholinesterase inhibitor from Mentha arvensis. Fitoterapia 2006, 77, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vazquez, M.; Ramirez Apan, T.O.; Aguilar, H.; Bye, R. Analgesic and antipyretic activities of an aqueous extract and of the flavone linarin of Buddleia cordata. Planta Med. 1996, 62, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Fan, P.; Perez, R.G.; Lou, H. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorg. Med. Chem. 2011, 19, 4021–4027. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cho, H.I.; Kim, S.J.; Park, J.H.; Kim, J.S.; Kim, Y.H.; Lee, S.K.; Kwak, J.H.; Lee, S.M. Protective effect of linarin against d-galactosamine and lipopolysaccharide-induced fulminant hepatic failure. Eur. J. Pharmacol. 2014, 738, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, Y.; Wang, X.; Di, X. A rapid and sensitive LC-MS/MS method for the determination of linarin in small-volume rat plasma and tissue samples and its application to pharmacokinetic and tissue distribution study. Biomed. Chromatogr. 2016, 30, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.H.; Seo, M.-J.; Eom, S.H.; Kim, W. Linarin down-regulates phagocytosis, pro-inflammatory cytokine production, and activation marker expression in RAW264.7 macrophages. Food Sci. Biotechnol. 2016, 25, 1437–1442. [Google Scholar] [CrossRef]
- Chen, G.; Li, K.K.; Fung, C.H.; Liu, C.L.; Wong, H.L.; Leung, P.C.; Ko, C.H. Er-Miao-San, a traditional herbal formula containing Rhizoma Atractylodis and Cortex Phellodendri inhibits inflammatory mediators in LPS-stimulated RAW264.7 macrophages through inhibition of NF-κB pathway and MAPKs activation. J. Ethnopharmacol. 2014, 154, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S. Production of nitric oxide by glial cells regulation and potential roles in the CNS. Glia 2000, 29, 1–13. [Google Scholar] [CrossRef]
- Sclavons, C.; Burtea, C.; Boutry, S.; Laurent, S.; Vander Elst, L.; Muller, R.N. Phage display screening for tumor necrosis factor-α-binding peptides: Detection of inflammation in a mouse model of hepatitis. Int. J. Pept. 2013, 2013, 348409. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.J.; Chung, T.W.; Son, M.J.; Kim, S.H.; Moon, T.C.; Son, K.H.; Kim, H.P.; Chang, H.W.; Kim, C.H. The naturally occurring biflavonoid, ochnaflavone, inhibits LPS-induced iNOS expression, which is mediated by ERK1/2 via NF-κB regulation, in RAW264.7 cells. Arch. Biochem. Biophys. 2006, 447, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, Q. Calcineurin stimulates the expression of inflammatory factors in RAW 264.7 cells by interacting with proteasome subunit alpha type 6. Biochem. Biophys. Res. Commun. 2011, 407, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, H.; Meng, X.; Wang, F.; Wang, P. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. J. Ethnopharmacol. 2014, 153, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; Lim, M.H.; Chang, W.; Lim, S.; Choi, S.; et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007, 114, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Wang, Y.J.; Ho, C.T. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem. Pharmacol. 2006, 72, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.S.; Park, D. Rosmarinic acid induces melanogenesis through protein kinase a activation signaling. Biochem. Pharmacol. 2007, 74, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Cui, X.; Lee, D.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-κB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules 2013, 18, 13245–13259. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.-J.; Bae, Y.-S.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Salicortin suppresses lipopolysaccharide-stimulated inflammatory responses via blockade of NF-κB and JNK activation in RAW 264.7 macrophages. BMB Rep. 2014, 47, 318–323. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the phenolic fraction from Mentha haplocalyx are available from the authors. |
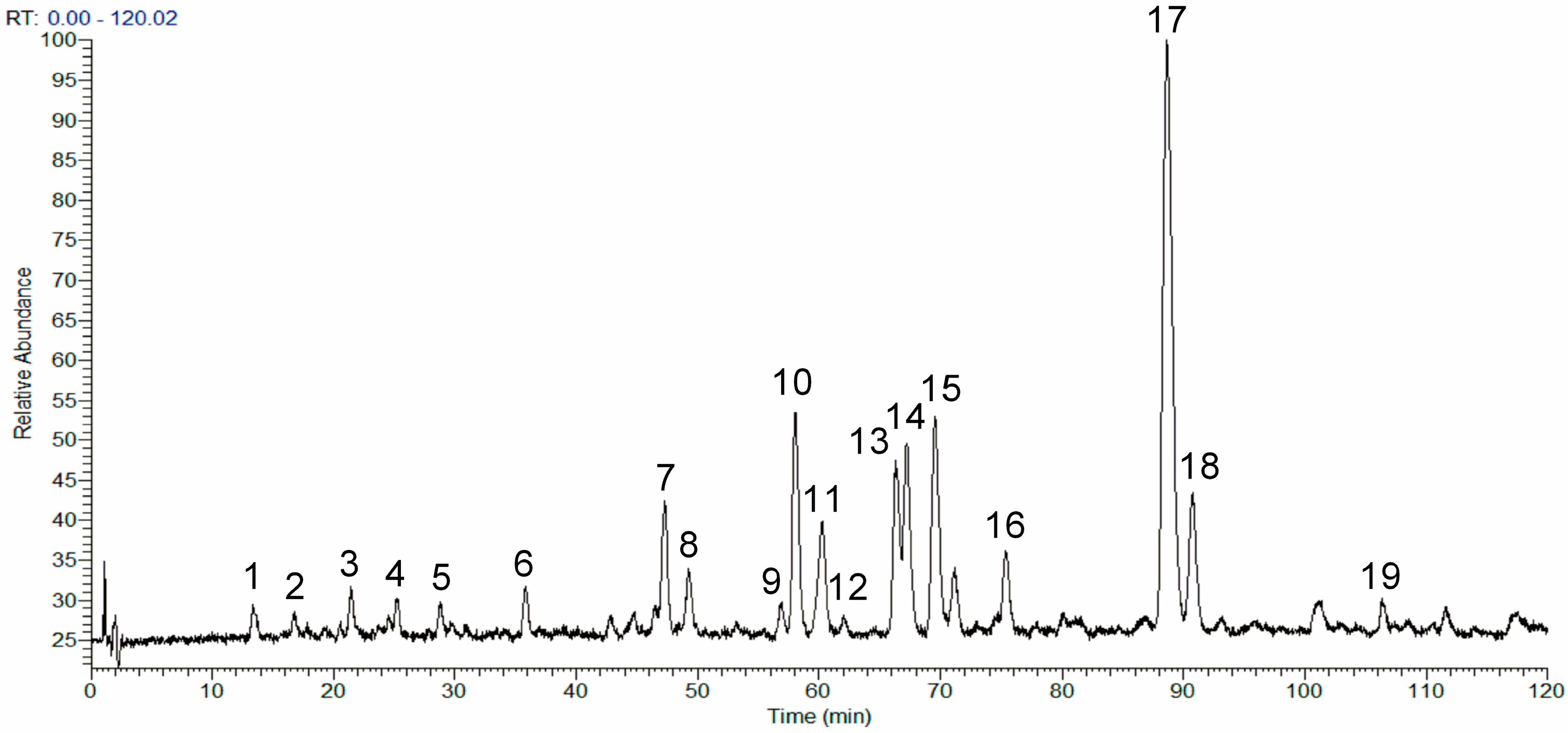



| Peak | tR (min) | [M + H]+/[M + Na]+ (m/z) | [M − H]− (m/z) | Formula | MS2 Ions (m/z) | Identification |
|---|---|---|---|---|---|---|
| 1 | 13.38 | − | 137.0246 | C7H6O3 | 118.7616, 108.8020, 91.9109 | protocatechuic aldehyde |
| 2 | 16.63 | − | 163.0401 | C9H8O3 | 163.0300, 118.9575, 94.9441 | p-coumaric acid |
| 3 | 21.38 | − | 179.0350 | C9H8O4 | 160.8768, 134.9199, 82.7767 | caffeic acid |
| 4 | 25.19 | − | 387.1655 | C20H20O8 | 369.2120, 340.9894, 207.0837, 163.0436, 119.0394 | ethyl rosmarinate |
| 5 | 28.81 | − | 337.0923 | C16H18O8 | 172.9905, 162.8953, 154.9699, 136.9224 | p-coumaroyl quinic acid |
| 6 | 35.79 | − | 514.3244 | − | 466.0504, 412.9174, 378.0275, 251.9538 | unknown |
| 7 | 47.24 | 588.4072 | − | − | 570.5060, 475.3133, 362.3077, 249.0771 | unknown |
| 8 | 49.10 | − | 537.1034 | C27H22O12 | 493.1606, 383.1935, 313.1821, 295.0162, 202.9409 | lithospermic acid |
| 9 | 56.90 | − | 577.1559 | C27H30O14 | 311.0969, 269.0196, 241.0736 | apigenin-7-O-rutinoside |
| 10 | 58.02 | − | 740.4911 | − | 717.5318, 693.6151, 603.9049, 536.1751 | unknown |
| 11 | 60.17 | 633.1774, 611.1957 | − | C28H34O15 | 615.2299, 483.1602, 331.0648 | hesperidin |
| 12 | 61.90 | 631.1624, 609.1804 | − | C28H32O15 | 463.1456, 447.0624, 301.0494, 286.0661, 191.0885 | diosmin |
| 13 | 66.44 | − | 359.0767 | C18H16O8 | 341.1331, 160.9566, 132.9843 | rosmarinic acid |
| 14 | 66.91 | 418.9493 | − | C20H18O10 | 400.2057, 362.4225, 344.5225, 220.0517 | salvianolic acid D |
| 15 | 70.48 | 741.1419 | − | C36H30O16 | 561.0992, 543.1505, 451.1976, 363.1163, 319.0901 | salvianolic acid B |
| 16 | 75.29 | 475.3232 | − | 457.2657, 317.2458, 249.2445, 167.2896 | unknown | |
| 17 | 88.55 | 593.1849, 615.1667 | − | C28H32O14 | 489.1708, 447.1117, 285.0378, 270.0775, 242.1294 | linarin |
| 18 | 90.61 | 797.2488 | − | − | 651.1746, 447.0908, 285.0492, 24.9773 | unknown |
| 19 | 107.36 | 361.0909 | − | C18H16O8 | 346.0471, 328.0377, 300.1852, 213.0013, 150.7952 | thymonin |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, S.; Xuan, Z.; Ge, D.; Chen, X.; Zhang, J.; Wang, Q.; Wu, Y.; Liu, B. The Phenolic Fraction of Mentha haplocalyx and Its Constituent Linarin Ameliorate Inflammatory Response through Inactivation of NF-κB and MAPKs in Lipopolysaccharide-Induced RAW264.7 Cells. Molecules 2017, 22, 811. https://doi.org/10.3390/molecules22050811
Chen X, Zhang S, Xuan Z, Ge D, Chen X, Zhang J, Wang Q, Wu Y, Liu B. The Phenolic Fraction of Mentha haplocalyx and Its Constituent Linarin Ameliorate Inflammatory Response through Inactivation of NF-κB and MAPKs in Lipopolysaccharide-Induced RAW264.7 Cells. Molecules. 2017; 22(5):811. https://doi.org/10.3390/molecules22050811
Chicago/Turabian StyleChen, Xiangyang, Shujing Zhang, Zinan Xuan, Dongyu Ge, Xiaoming Chen, Junjie Zhang, Qian Wang, Ying Wu, and Bin Liu. 2017. "The Phenolic Fraction of Mentha haplocalyx and Its Constituent Linarin Ameliorate Inflammatory Response through Inactivation of NF-κB and MAPKs in Lipopolysaccharide-Induced RAW264.7 Cells" Molecules 22, no. 5: 811. https://doi.org/10.3390/molecules22050811
APA StyleChen, X., Zhang, S., Xuan, Z., Ge, D., Chen, X., Zhang, J., Wang, Q., Wu, Y., & Liu, B. (2017). The Phenolic Fraction of Mentha haplocalyx and Its Constituent Linarin Ameliorate Inflammatory Response through Inactivation of NF-κB and MAPKs in Lipopolysaccharide-Induced RAW264.7 Cells. Molecules, 22(5), 811. https://doi.org/10.3390/molecules22050811





