Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves
Abstract
:1. Introduction
2. Results and Discussion
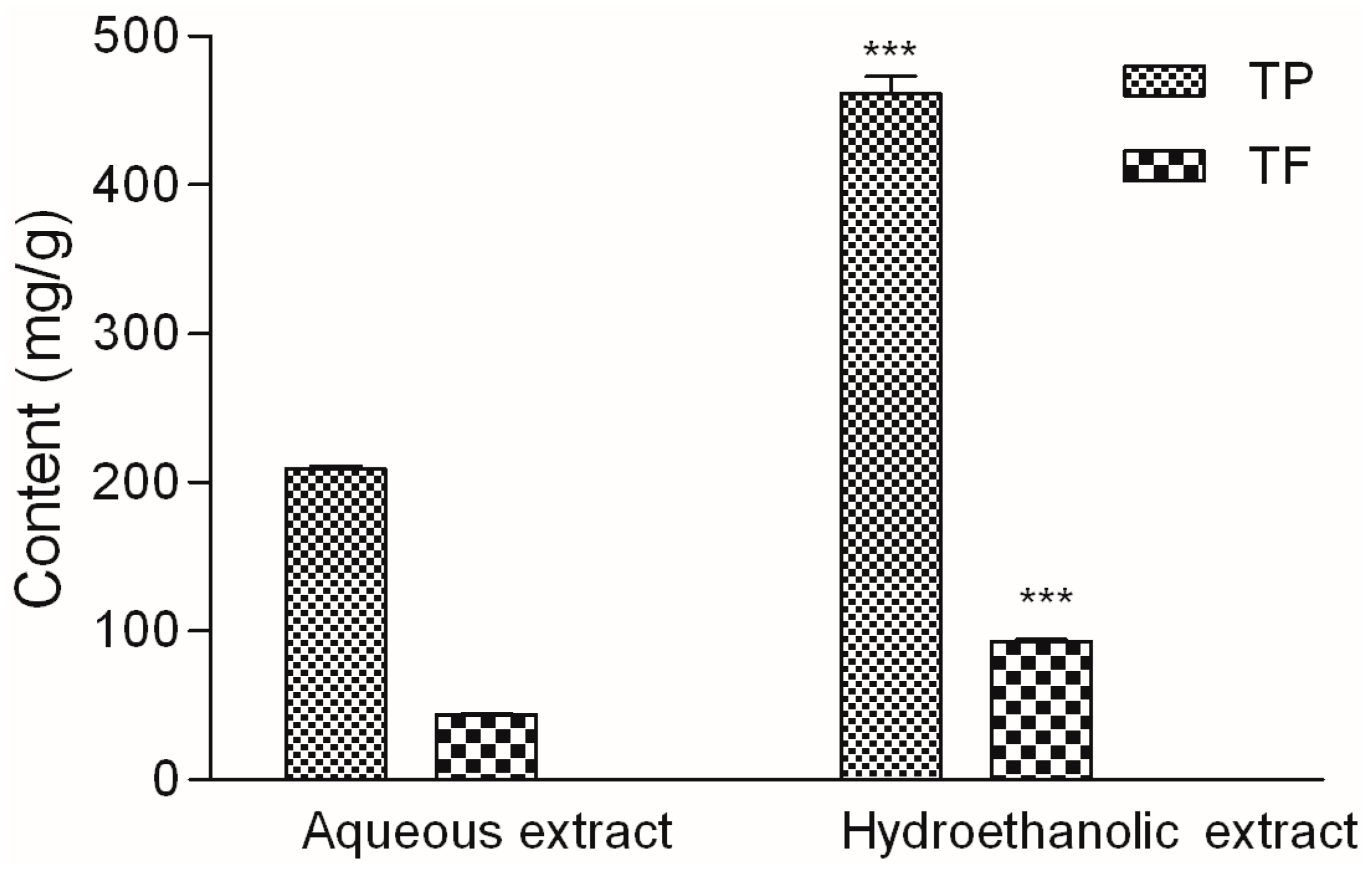
2.1. Phytochemical Analysis
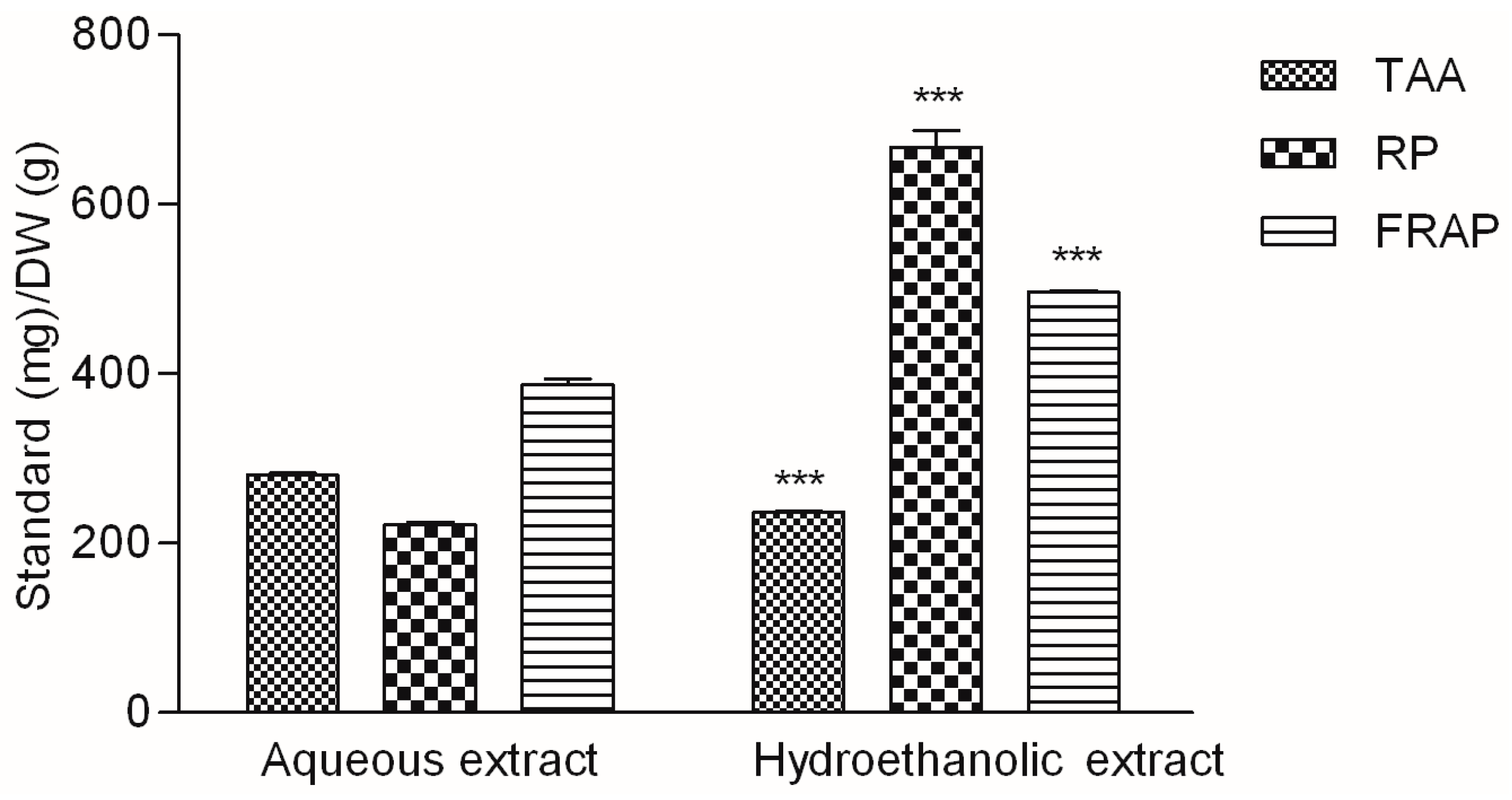
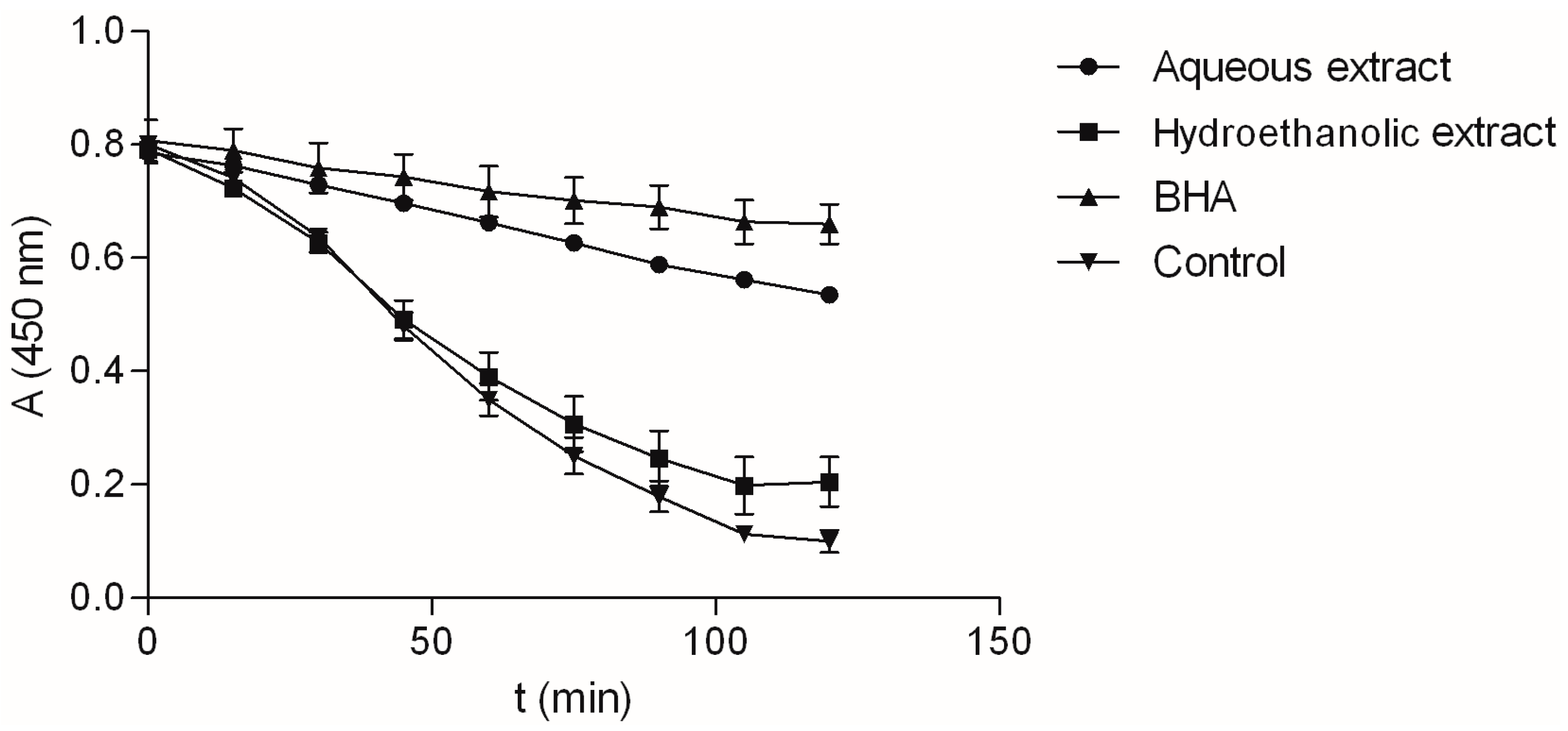
2.2. Antioxidant Activity of the Extracts
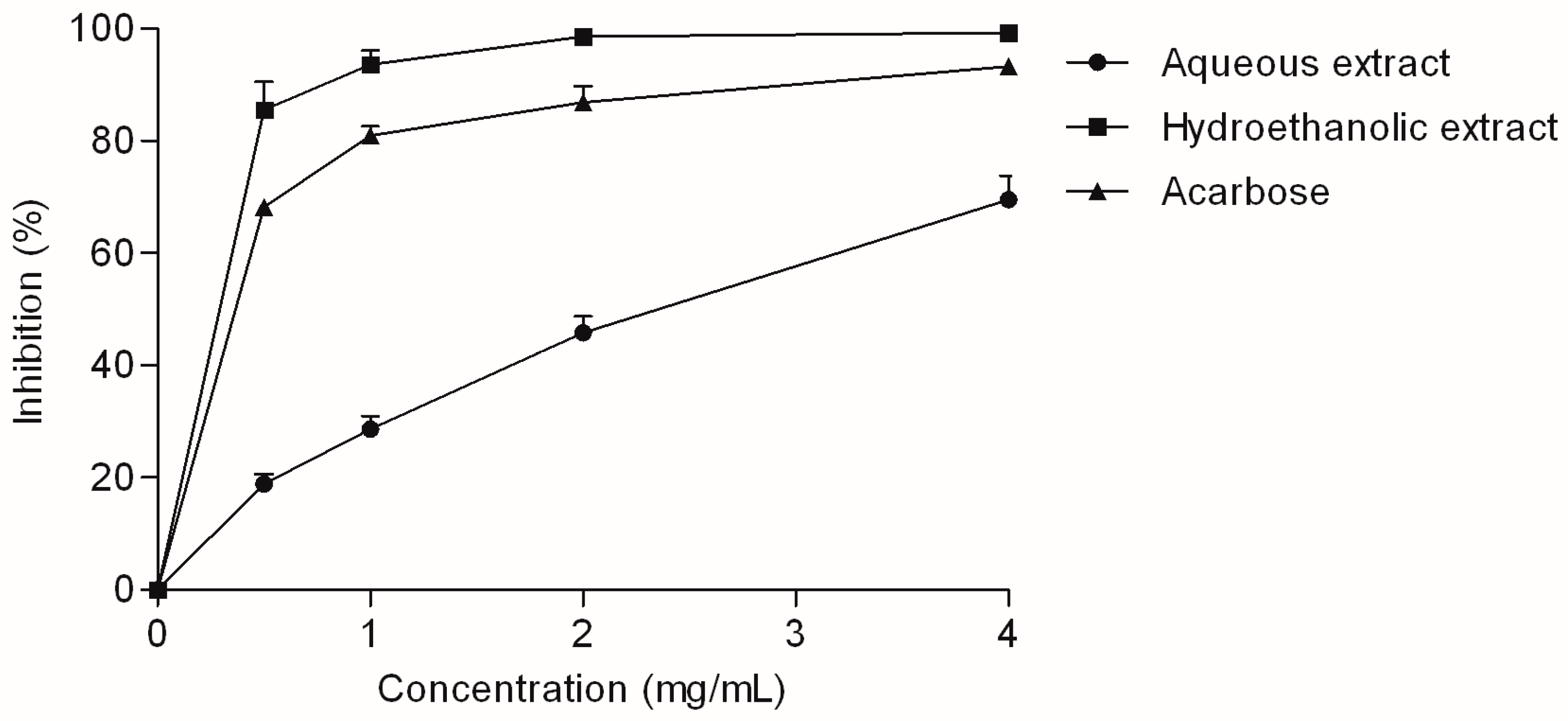
2.3. Enzyme-Inhibitory Activity of the Extracts
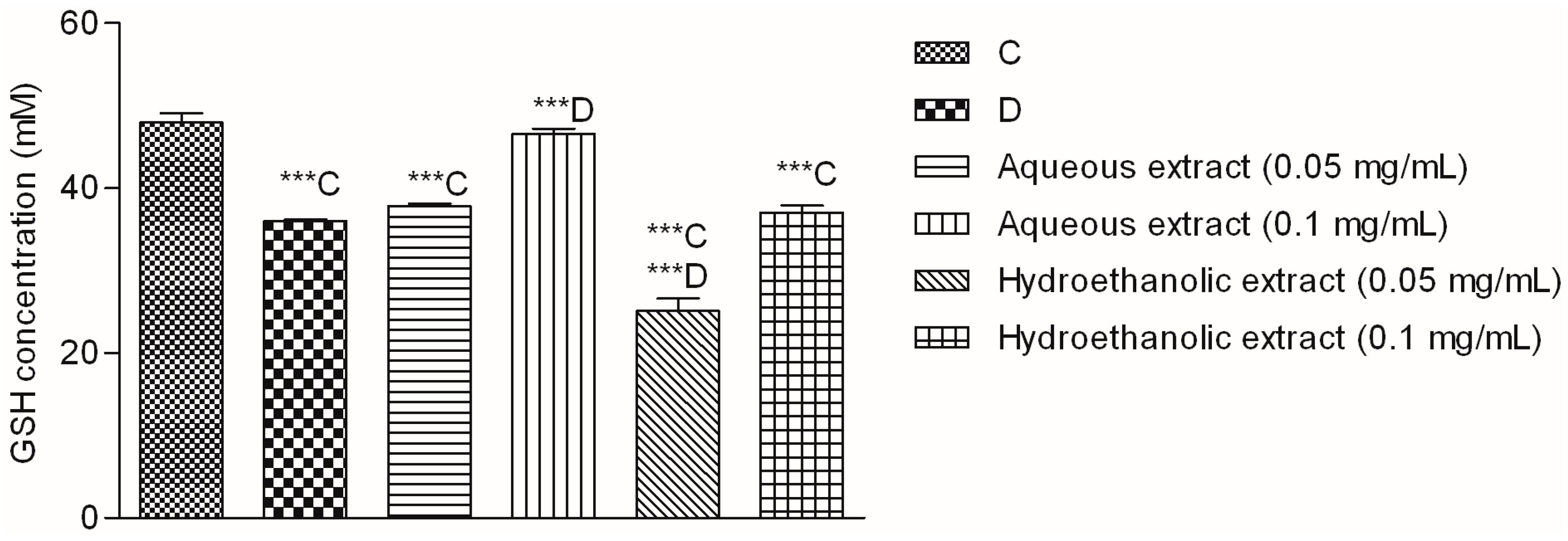
2.4. Effect of V. myrtillus Extracts on Glucose-Induced Oxidative Stress in Hep G2 Cells
3. Materials and Methods
3.1. Plant Materials and Chemicals
3.2. Determination of Metal Content in Plant Material
3.3. Preparation of the Extracts
3.4. Spectrophotometric Determination of Total Phenolic and Total Flavonoid Content
3.5. HPLC Analysis of Phenolic Constituents
3.6. Total Antioxidant Activity
3.7. Reducing Power (RP)
3.8. Ferric Reducing Antioxidant Power (FRAP)
3.9. β-Carotene-Linoleic Acid Assay
3.10. ABTS and DPPH Radical Scavenging Activity
3.11. Fe2+ Chelating Activity
3.12. Determination of α-Glucosidase Inhibiting Activity
3.13. Alpha-Amylase Inhibition Assay
3.14. Cell Culture and Treatment
3.15. Reduced Glutathione Content (GSH)
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kapoor, R.; Kakkar, P. Protective Role of Morin, a Flavonoid, against High Glucose Induced Oxidative Stress Mediated Apoptosis in Primary Rat Hepatocytes. PLoS ONE 2012, 7, e41663. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kaplowitz, N. Glutathione in liver diseases and hepatotoxicity. Mol. Asp. Med. 2009, 30, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.A.; Forman, H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Park, J.-H.; Jung, J.-H.; Yang, J.-Y.; Kim, H.-S. Olive leaf down-regulates the oxidative stress and immune dysregulation in streptozotocin-induced diabetic mice. Nutr. Res. 2013, 33, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Mol. Basel Switz. 2016, 21, E1374. [Google Scholar] [CrossRef] [PubMed]
- Dghaim, R.; Al Khatib, S.; Rasool, H.; Ali Khan, M. Determination of Heavy Metals Concentration in Traditional Herbs Commonly Consumed in the United Arab Emirates. J. Environ. Publ. Health 2015, 2015, e973878. [Google Scholar] [CrossRef]
- Krejpcio, Z.; Krol, E.; Sionkowski, S. Evaluation of Heavy Metals Contents in Spices and Herbs Available on the Polish Market. Pol. J. Environ. Stud. 2007, 16, 97–100. [Google Scholar]
- Huremović, J.; Badema, B.; Muhić-Šarac, T.; Selović, A.; Memić, M. Heavy Metal Contents in Spices from Markets in Sarajevo, Bosnia and Herzegovina (Sadržaj teških metala u začinskom bilju s tržišta u Sarajevu, Bosna i Hercegovina). Kem. Ind. 2014, 63, 77–81. [Google Scholar]
- Helmstädter, A.; Schuster, N. Vaccinium myrtillus as an antidiabetic medicinal plant—Research through the ages. Pharmacy 2010, 65, 315–321. [Google Scholar]
- Gašpar, K.; Zovko Končić, M. Traditional herbal products used for the management of diabetes in Croatia. In Proceedings of the International Conference on Natural Products Utilization: From Plants to Pharmacy Shelf, Bansko, Bulgaria, 3–6 November 2013. [Google Scholar]
- Ferrier, J.; Šačiragić, L.; Chen, E.C.H.; Trakić, S.; Saleem, A.; Alikadić, E.; Cuerrier, A.; Balick, M.J.; Arnason, J.T.; Redžić, S. Ways the Lukomir Highlanders of Bosnia and Herzegovina Treat Diabetes. In Ethnobotany and Biocultural Diversities in the Balkans; Pieroni, A., Quave, C.L., Eds.; Springer: New York, NY, USA, 2014; pp. 13–27. [Google Scholar]
- Kandziora-Ciupa, M.; Ciepał, R.; Nadgórska-Socha, A.; Barczyk, G. A comparative study of heavy metal accumulation and antioxidant responses in Vaccinium myrtillus L. leaves in polluted and non-polluted areas. Environ. Sci. Pollut. Res. Int. 2013, 20, 4920–4932. [Google Scholar] [CrossRef] [PubMed]
- Myrtilli folium. In Herbal Drugs and Phytopharmaceuticals, 3rd ed.; Wichtl, M. (Ed.) Medpharm Stuttgart: Boca Raton, FL, USA, 2004; pp. 407–410. [Google Scholar]
- Reimann, C.; Koller, F.; Frengstad, B.; Kashulina, G.; Niskavaara, H.; Englmaier, P. Comparison of the element composition in several plant species and their substrate from a 1,500,000-km2 area in Northern Europe. Sci. Total Environ. 2001, 278, 87–112. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2016, 196, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic distribution in liquid preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. PCA 2013, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.-P.; Yang, B. Characterization of metabolite profiles of leaves of bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef] [PubMed]
- Ponikierska, A.; Gugnacka-Fiedor, W.; Piwczynski, M.; Nicolaus, C.U. Morphological characteristics of Vaccinium × intermedium Ruthe. Dendrobiol. Pol. 2004, 51, 59–65. [Google Scholar]
- Moussa-Ayoub, T.E.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Identification and quantification of flavonol aglycons in cactus pear (Opuntia ficus indica) fruit using a commercial pectinase and cellulase preparation. Food Chem. 2011, 124, 1177–1184. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-Based Antioxidants and the In Vitro Methods Used for Their Assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Pokorny, J.; Yanishlieva, N.; Gordon, M.H. Antioxidants in Food: Practical Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Terrier, L.; Hay, A.-E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F.Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Mahjoub, S.; Pouramir, M.; Khadir, F. Hypoglycemic activity of Pyrus biossieriana Buhse leaf extract and arbutin: Inhibitory effects on alpha amylase and alpha glucosidase. Casp. J. Intern. Med. 2013, 4, 763–767. [Google Scholar]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative Evaluation of Quercetin, Isoquercetin and Rutin as Inhibitors of α-Glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Swaminathan, K.; Chatterjee, S.; Dey, A. Apoptosis in HepG2 cells exposed to high glucose. Toxicol. In Vitro 2010, 24, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Saso, L.; Firuzi, O. Pharmacological applications of antioxidants: Lights and shadows. Curr. Drug Targets 2014, 15, 1177–1199. [Google Scholar] [CrossRef]
- Lash, L. Mitochondrial Glutathione in Diabetic Nephropathy. J. Clin. Med. 2015, 4, 1428–1447. [Google Scholar] [CrossRef]
- Chobot, V.; Hadacek, F.; Kubicova, L. Effects of Selected Dietary Secondary Metabolites on Reactive Oxygen Species Production Caused by Iron (II) Autoxidation. Molecules 2014, 19, 20023–20033. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; An, X.-F.; Teng, S.-C.; Liu, J.-S.; Shang, W.-B.; Zhang, A.-H.; Yuan, Y.-G.; Yu, J.-Y. Pretreatment with the Total Flavone Glycosides of Flos Abelmoschus manihot and Hyperoside Prevents Glomerular Podocyte Apoptosis in Streptozotocin-Induced Diabetic Nephropathy. J. Med. Food 2012, 15, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, H.; Xu, Y.; Niu, Y.; An, X. Hyperoside reduces albuminuria in diabetic nephropathy at the early stage through ameliorating renal damage and podocyte injury. J. Nat. Med. 2016, 70, 740–748. [Google Scholar] [CrossRef]
- Verma, N.; Amresh, G.; Sahu, P.K.; Mishra, N.; Rao, C.V.; Singh, A.P. Pharmacological evaluation of hyperin for antihyperglycemic activity and effect on lipid profile in diabetic rats. Indian J. Exp. Biol. 2013, 51, 65–72. [Google Scholar] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evid. Based Complement. Altern. Med. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.M.; Lima, D.R.A. Coffee consumption, obesity and type 2 diabetes: A mini-review. Eur. J. Nutr. 2016, 55, 1345–1358. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Li, Z.-Y.; Zheng, Y.; Chen, Y.; Zhou, Z.-H.; Jin, J. The attenuation of chlorogenic acid on oxidative stress for renal injury in streptozotocin-induced diabetic nephropathy rats. Arch. Pharm. Res. 2016, 39, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Method Validation in Pharmaceutical Analysis: A Guide to Best Practice; Ermer, J. (Ed.) Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2013.
- Zovko Koncić, M.; Kremer, D.; Karlović, K.; Kosalec, I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. Toxicol. 2010, 48, 2176–2180. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Končić, M.Z.; Barbarić, M.; Perković, I.; Zorc, B. Antiradical, Chelating and Antioxidant Activities of Hydroxamic Acids and Hydroxyureas. Molecules 2011, 16, 6232–6242. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Swapna, M.; Ayesha, S.B.; Zehra, A.; Agawane, S.B.; Madhusudana, K. Identification of proglycemic and antihyperglycemic activity in antioxidant rich fraction of some common food grains. Int. Food Res. J. 2011, 18, 915–923. [Google Scholar]
- Apostolidis, E.; Kwon, Y.-I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Lee, K.J.; Woo, E.-R.; Choi, C.Y.; Shin, D.W.; Lee, D.G.; You, H.J.; Jeong, H.G. Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci. 2004, 74, 1051–1064. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Vaccinium myrtillus investigated in this work are available from the authors. |

| Before Hydrolysis | After Hydrolysis | |||
|---|---|---|---|---|
| Aqueous Extract | Hydroethanolic Extract | Aqueous Extract | Hydroethanolic Extract | |
| Arbutin (mg/g) | 10.60 | n.d. | n.d. | n.d. |
| Caffeic acid (mg/g) | n.d. | n.d. | n.d. | 6.58 |
| Chlorogenic acid (mg/g) | 20.82 | 11.21 | n.d. | n.d. |
| p-coumaric acid (mg/g) | 0.32 | 0.46 | n.d. | 11.90 |
| Protocatechuic acid (mg/g) | n.d. | n.d. | 176.45 | n.d. |
| Kaempferol (mg/g) | n.d. | n.d. | n.d. | 3.45 |
| Quercetin (mg/g) | n.d. | n.d. | 24.75 | 85.64 |
| Hyperoside (mg/g) | 32.16 | 44.43 | n.d. | n.d. |
| Extract | ANT | IC50 ABTS RSA | IC50 DPPH RSA | IC50 ChA | IC50 AG |
|---|---|---|---|---|---|
| % | mg/mL | µg/mL | µg/mL | mg/mL | |
| Aqueous extract | 93.4 ± 2.3 A | 251.1 ± 11.1 A | 59.0 ± 1.7 A | 135.2 ± 6.3 A | 2.53 ± 0.21 A |
| Hydroethanolic extract | 48.6 ± 13.4 B | 252.2 ± 21.2 A | 17.8 ± 0.1 B | 391.5±10.4 B | 0.29 ± 0.02 B |
| Standard | a 95.1 ± 2.4 A | b 41.7 ± 2.2 B | a 5.9 ± 0.1 C | c 4.0 ± 0.3 C | d 0.50 ± 0.01 B |
| Standard | Calibration Curve Equation | r2 | LOD (µg) | LOQ (µg) |
|---|---|---|---|---|
| Arbutin | y = 103.9x + 17.6 | 0.9999 | 0.005 | 0.016 |
| Caffeic acid | y = 15197.0x + 199.3 | 0.9997 | 0.035 | 0.105 |
| Chlorogenic acid | y = 2587.3x + 73.4 | 0.9996 | 0.036 | 0.110 |
| p-coumaric acid | y = 5735.6x + 89.3 | 0.9999 | 0.005 | 0.015 |
| Protocatechuic acid | y = 2415.8x + 17.6 | 0.9999 | 0.005 | 0.016 |
| Kaempferol | y = 2802.8x + 21.6 | 0.9998 | 0.026 | 0.078 |
| Quercetin | y = 2086.9x – 36.8 | 0.9998 | 0.027 | 0.083 |
| Hyperoside | y = 1426.2x + 15.4 | 0.9999 | 0.013 | 0.040 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves. Molecules 2017, 22, 703. https://doi.org/10.3390/molecules22050703
Bljajić K, Petlevski R, Vujić L, Čačić A, Šoštarić N, Jablan J, Saraiva de Carvalho I, Zovko Končić M. Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves. Molecules. 2017; 22(5):703. https://doi.org/10.3390/molecules22050703
Chicago/Turabian StyleBljajić, Kristina, Roberta Petlevski, Lovorka Vujić, Ana Čačić, Nina Šoštarić, Jasna Jablan, Isabel Saraiva de Carvalho, and Marijana Zovko Končić. 2017. "Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves" Molecules 22, no. 5: 703. https://doi.org/10.3390/molecules22050703
APA StyleBljajić, K., Petlevski, R., Vujić, L., Čačić, A., Šoštarić, N., Jablan, J., Saraiva de Carvalho, I., & Zovko Končić, M. (2017). Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves. Molecules, 22(5), 703. https://doi.org/10.3390/molecules22050703








