Immune-Stimulatory Effects of Althaea rosea Flower Extracts through the MAPK Signaling Pathway in RAW264.7 Cells
Abstract
:1. Introduction
2. Results
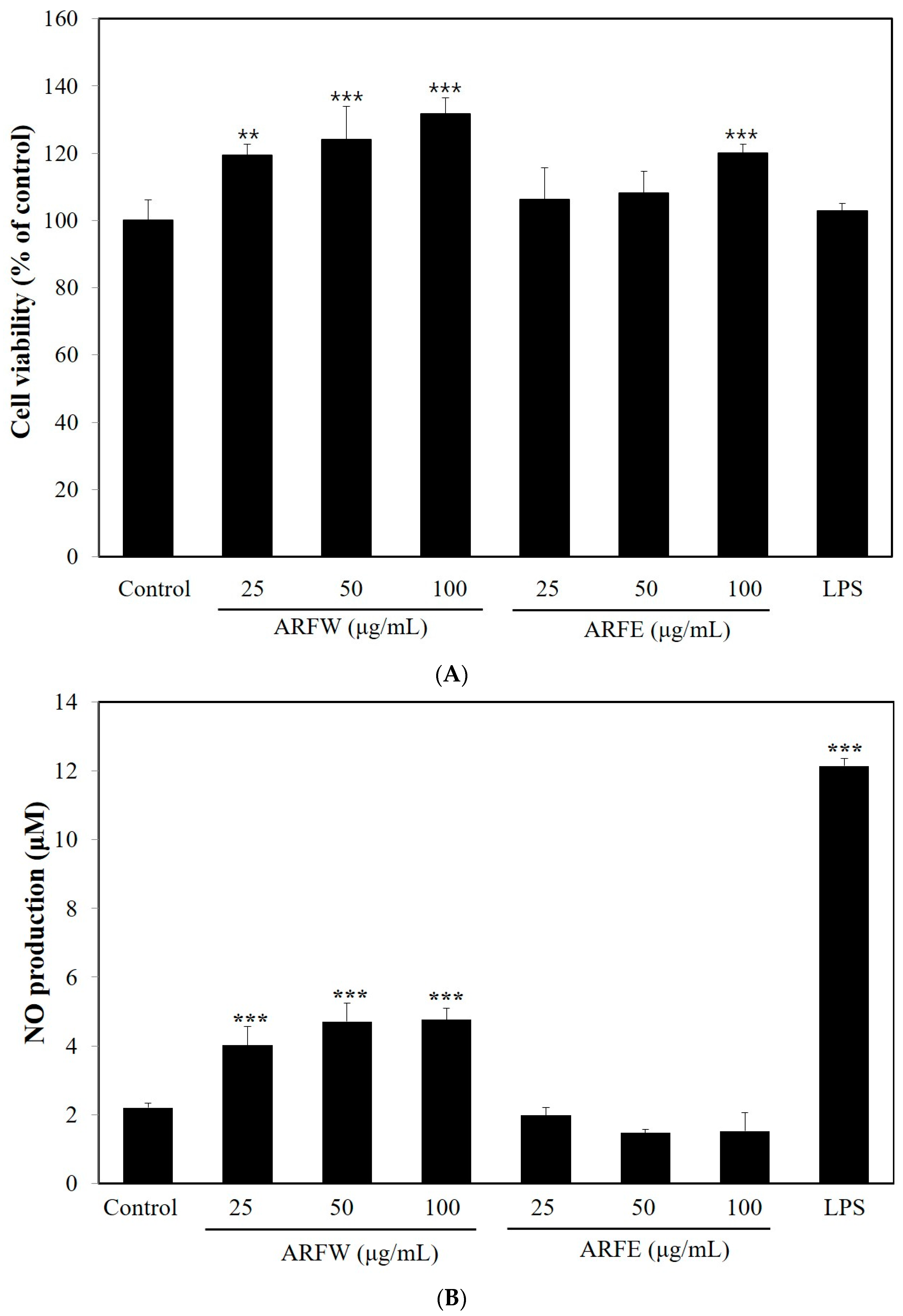
2.1. Effect of ARF Extracts on Cell Viability and NO Production in RAW264.7 Cells
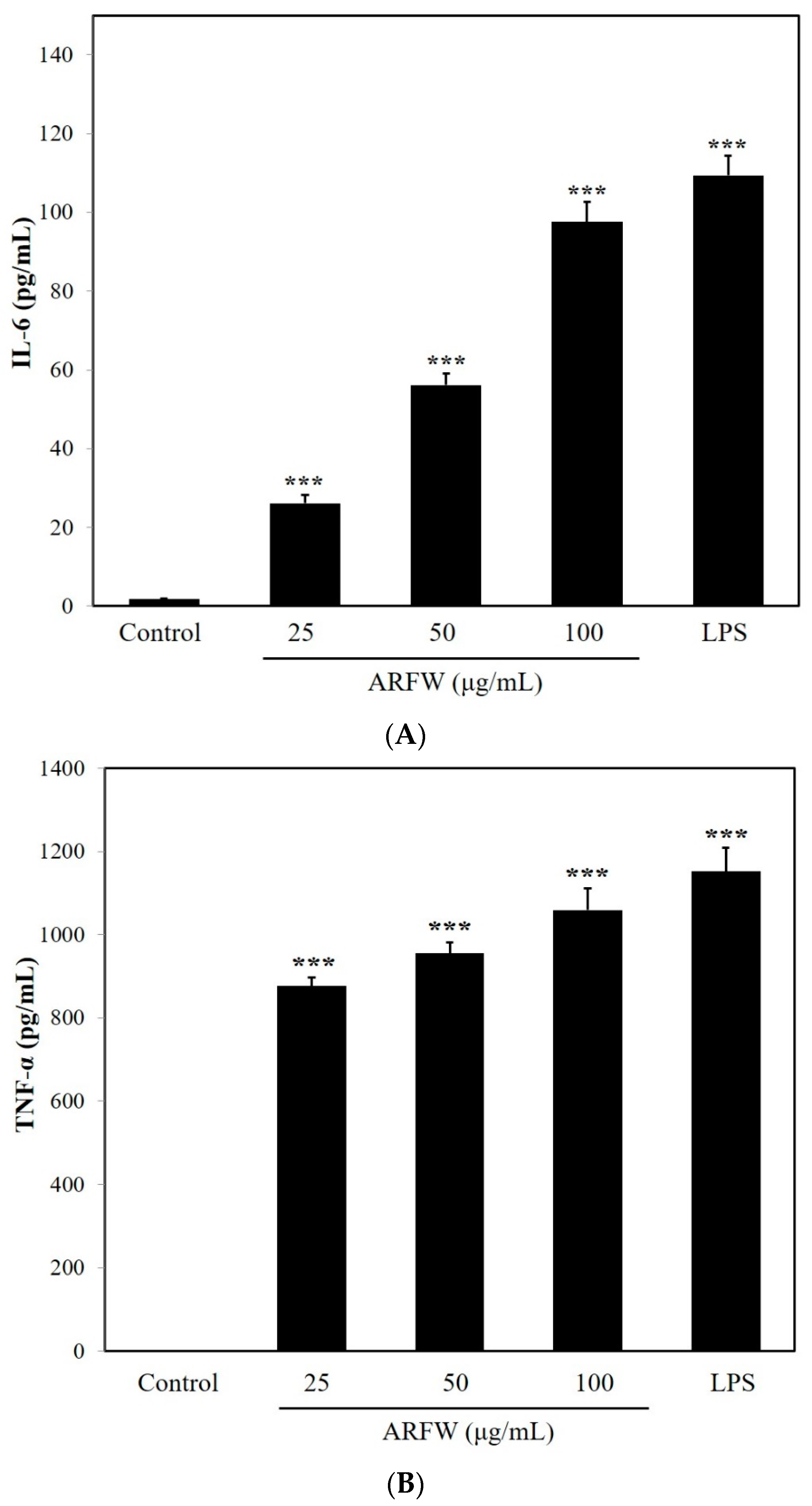
2.2. Effect of ARFW on the Expression of TNF-α and IL-6
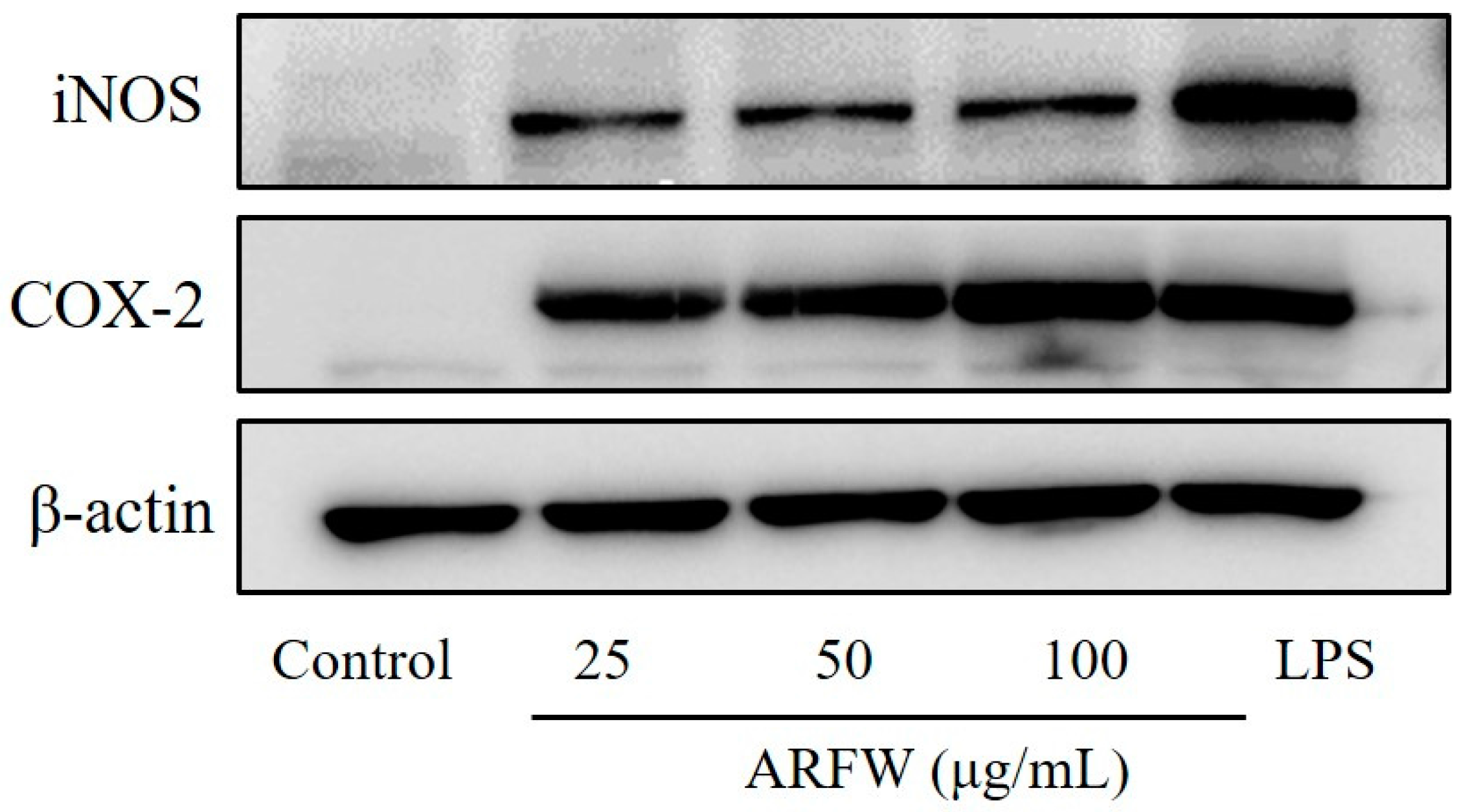
2.3. Effect of ARFW on the Expression of iNOS and COX-2
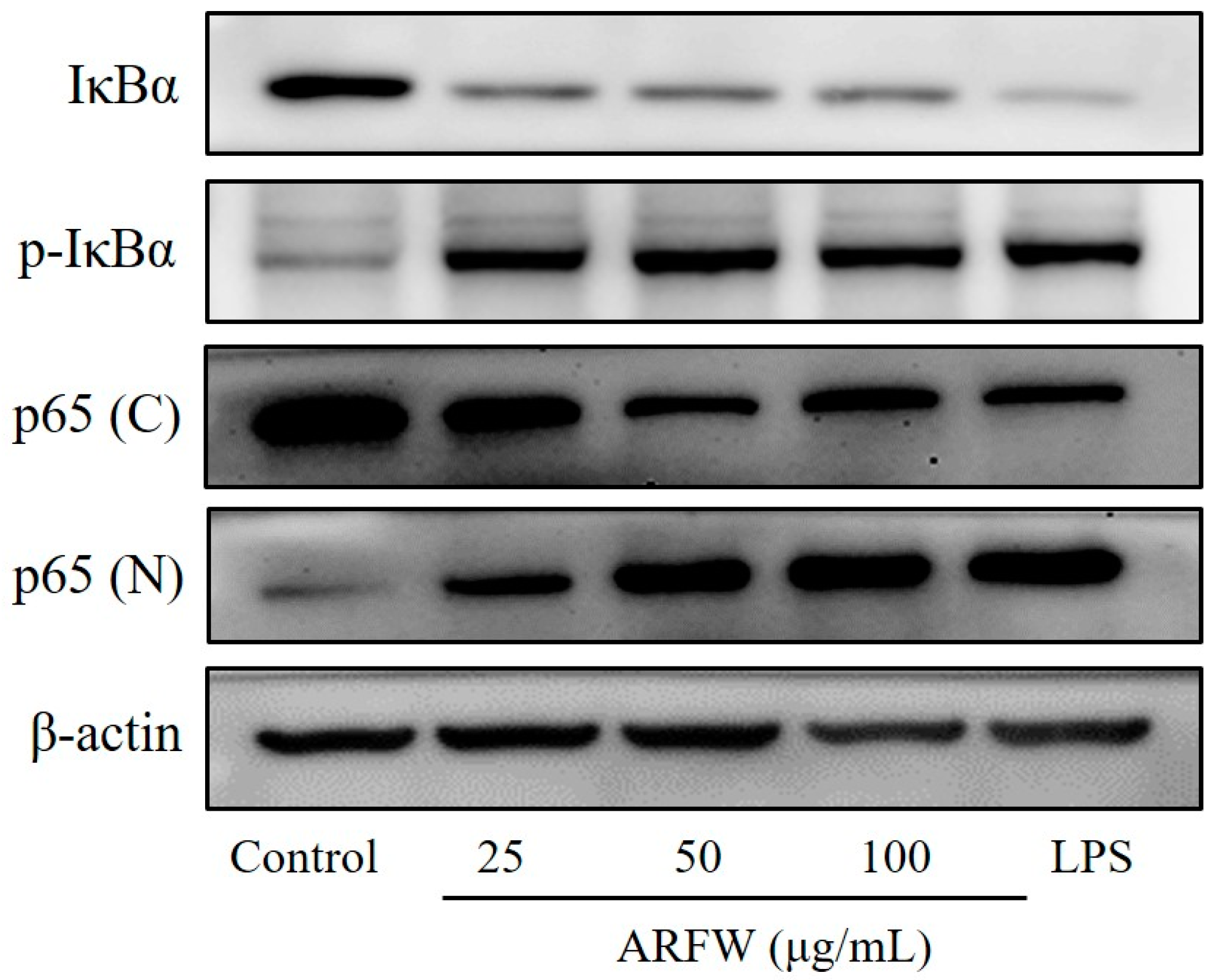
2.4. Effect of ARFW on IkBa Degradation and NF-kB Activation
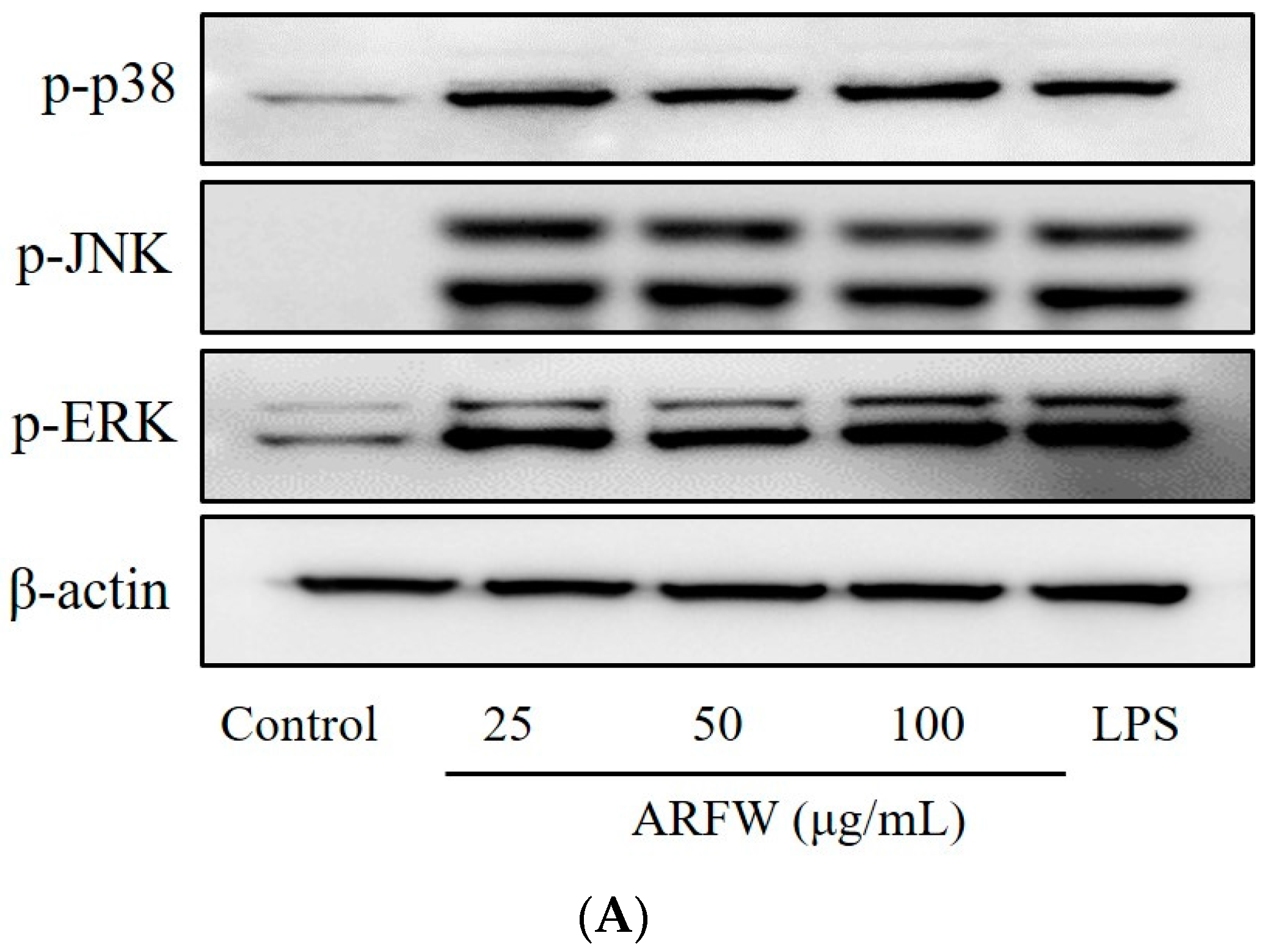
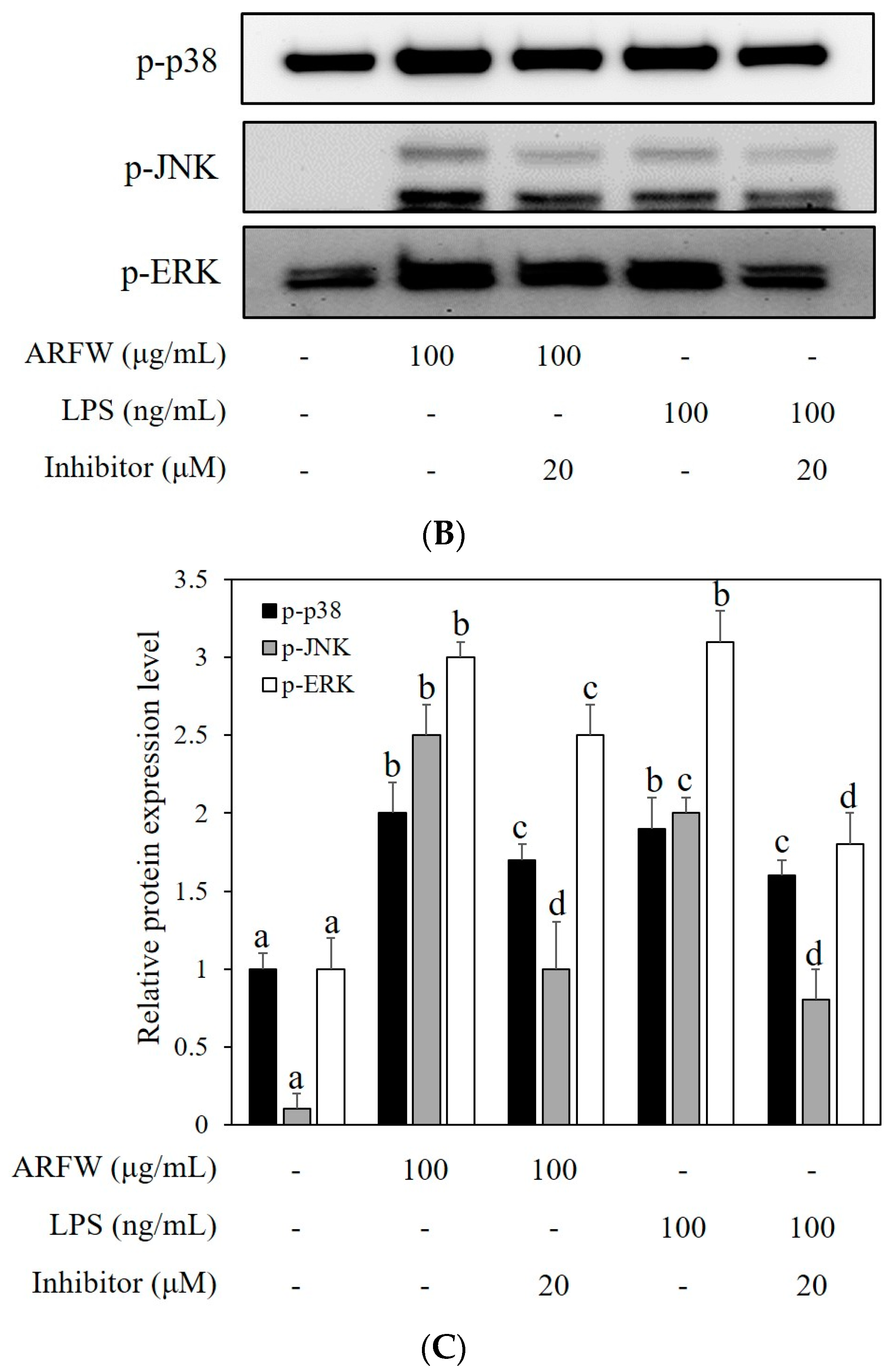
2.5. Effect of ARFW on MAPK Signalling Activation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of ARF Extracts
4.3. Cell Culture
4.4. Cell Viability
4.5. Measurement of Nitric Oxide (NO) Generation
4.6. Determination of Inflammatory Cytokine Production
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ali, E.A. The Pharmaceutical importance of Althaea officinalis and Althaea rosea: A review. Int. J. Pharm. Tech. Res. 2013, 5, 1378–1385. [Google Scholar]
- Zhang, Y.; Jin, L.; Chen, Q.; Wu, Z.; Dong, Y.; Han, L.; Wang, T. Hypoglycemic activity evaluation and chemical study on hollyhock flowers. Fitoterapia 2015, 102, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sadighara, P.; Gharibi, S.; Moghadam, J.A.; Jahed Khaniki, G.; Salari, S. The antioxidant and flavonoids contents of Althaea officinalis L. flowers based on their color. Avicenna J. Phytomed. 2012, 2, 113–117. [Google Scholar] [PubMed]
- Kwiatkowska, M.; Stępinski, D.; Poplonska, K.; Wojtczak, A.; Polit, J.T. ‘Elaioplasts’ identified as lipotubuloids in Althaea rosea, Funkia sieboldiana and Vanilla planifolia contain lipid bodies connected with microtubules. Acta Soc. Bot. Pol. 2011, 80, 211–219. [Google Scholar] [CrossRef]
- Liu, F.; Liu, W.; Tian, S. Artificial neural network optimization of Althaea rosea seeds polysaccharides and its antioxidant activity. Int. J. Biol. Macromol. 2014, 70, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Papiez, M.; Gancarczyk, M.; Bilinska, B. The compounds from the hollyhock extract (Althaea rosea Cav. Var. Nigra) affect the aromatization in rat testicular cells in vivo and in vitro. Folia Histochem. Cytobiol. 2002, 40, 353–359. [Google Scholar] [PubMed]
- Dudek, M.; Awska, I.M.; Szudlarek, M. Phenolic acids in the flowers of Althaea rosea var. nigra. Acta Pol. Pharm. 2006, 63, 207–211. [Google Scholar] [PubMed]
- Ammar, N.M.; El-Kashoury, E.S.A.; El-Kassem, L.T.; El-Hakeem, R.E. Evaluation of the phenolic content and antioxidant potential of Althaea rosea cultivated in Egypt. J. Arab. Soc. Med. Res. 2013, 8, 48–52. [Google Scholar] [CrossRef]
- Bruce, B. Tlr4: Central component of the sole mammalian LPS sensor. Curr. Op. Immunol. 2000, 12, 20–26. [Google Scholar]
- Ando, I.; Tsukumo, Y.; Wakabayashi, T.; Akashi, S.; Miyake, K.; Kataoka, T.; Nagai, K. Safflower polysaccharides activate the transcription factor NF-kappa B via Toll-like receptor 4 and induce cytokine production by macrophages. Int. Immunopharmacol. 2002, 2, 1155–1162. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Kim, D.K.; Ueno, M.; Okimura, T.; Yamaguchi, K.; Oda, T. Stimulatory effect of the sulfated polysaccharide ascophyllan on the respiratory burst in RAW264.7 macrophages. Int. J. Biol. Macromol. 2013, 52, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I.; Ernst, R.K.; Bader, M.K. LPS, TLR4 and infectious disease deversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Schepetkin, I.A.; Quinn, M.T. Immunomodulatory Activity of Acidic Polysaccharides Isolated from Tanacetum vulgare L. Int. Immunopharmacol. 2007, 7, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J. Immune stimulatory activity of BRP-4, an acidic polysaccharide from an edible plant, Basella rubra L. Asian Pac. J. Trop. Med. 2017, 7, 849–853. [Google Scholar] [CrossRef]
- Lim, T.S.; Na, K.; Choi, E.M.; Chung, J.Y.; Hwang, J.K. Immunomodulating activities of polysaccharides isolated from Panax ginseng. J. Med. Food 2004, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.M.; Zhou, H.Y.; Guo, L.Y.; Kim, J.A. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2008, 8, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, E.; Sekiyama, A.; Hori, M.; Hara, K.; Takahashi, N.; Konishi, M.; Sato, E.F.; Matsumoto, S.; Okamura, H.; Inoue, M. Mitochondrial density contributes to the immune response of macrophages to lipopolysaccharide via the MAPK pathway. FEBS Lett. 2011, 585, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Huanzhang, X.; Cheng, Y.; Zhang, X.; Zhang, X. Effects of taraxasterol on iNOS and COX-2 expression in LPS-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2014, 155, 753–757. [Google Scholar]
- Paul, P.T.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar]
- Lee, J.S.; Choi, J.W.; Sohng, J.K.; Pandey, R.; Park, Y.I. The immunostimulating activity of quercetin 3-O-xyloside in murine macrophages via activation of the ASK1/MAPK/NF-κB signaling pathway. Int. Immunopharmacol. 2016, 31, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Meili, N.; Christen, V.; Fent, K. Nodularin induces tumor necrosis factor-alpha and mitogen-activated protein kinases (MAPK) and leads to induction of endoplasmic reticulum stress. Toxicol. Appl. Pharm. 2016, 300, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.R.; Zhang, P.; Lee, J.C.; Hogan, E.L. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinasesregulate inducible nitric oxide synthase and tumor necrosis factor-alpha geneexpression in endotoxin-stimulated primary glial cultures. J. Neurosci. 1998, 18, 1633–1641. [Google Scholar] [PubMed]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immuneresponse. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Gallagher, E. From JNK to pay dirt: Jun kinases, their biochem-istry, physiology and clinical importance. IUBMB Life 2002, 57, 283–295. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Kim, E.-K.; Nawarathna, W.P.A.S.; Dong, X.; Shin, W.-B.; Park, J.-S.; Moon, S.-H.; Park, P.-J. Immune-Stimulatory Effects of Althaea rosea Flower Extracts through the MAPK Signaling Pathway in RAW264.7 Cells. Molecules 2017, 22, 679. https://doi.org/10.3390/molecules22050679
Kim Y-S, Kim E-K, Nawarathna WPAS, Dong X, Shin W-B, Park J-S, Moon S-H, Park P-J. Immune-Stimulatory Effects of Althaea rosea Flower Extracts through the MAPK Signaling Pathway in RAW264.7 Cells. Molecules. 2017; 22(5):679. https://doi.org/10.3390/molecules22050679
Chicago/Turabian StyleKim, Yon-Suk, Eun-Kyung Kim, Weligala Pahalagedara Amila Srilal Nawarathna, Xin Dong, Woen-Bin Shin, Jin-Su Park, Sang-Ho Moon, and Pyo-Jam Park. 2017. "Immune-Stimulatory Effects of Althaea rosea Flower Extracts through the MAPK Signaling Pathway in RAW264.7 Cells" Molecules 22, no. 5: 679. https://doi.org/10.3390/molecules22050679
APA StyleKim, Y.-S., Kim, E.-K., Nawarathna, W. P. A. S., Dong, X., Shin, W.-B., Park, J.-S., Moon, S.-H., & Park, P.-J. (2017). Immune-Stimulatory Effects of Althaea rosea Flower Extracts through the MAPK Signaling Pathway in RAW264.7 Cells. Molecules, 22(5), 679. https://doi.org/10.3390/molecules22050679





