Ameliorative Effects and Possible Molecular Mechanism of Action of Black Ginseng (Panax ginseng) on Acetaminophen-Mediated Liver Injury
Abstract
1. Introduction
2. Results
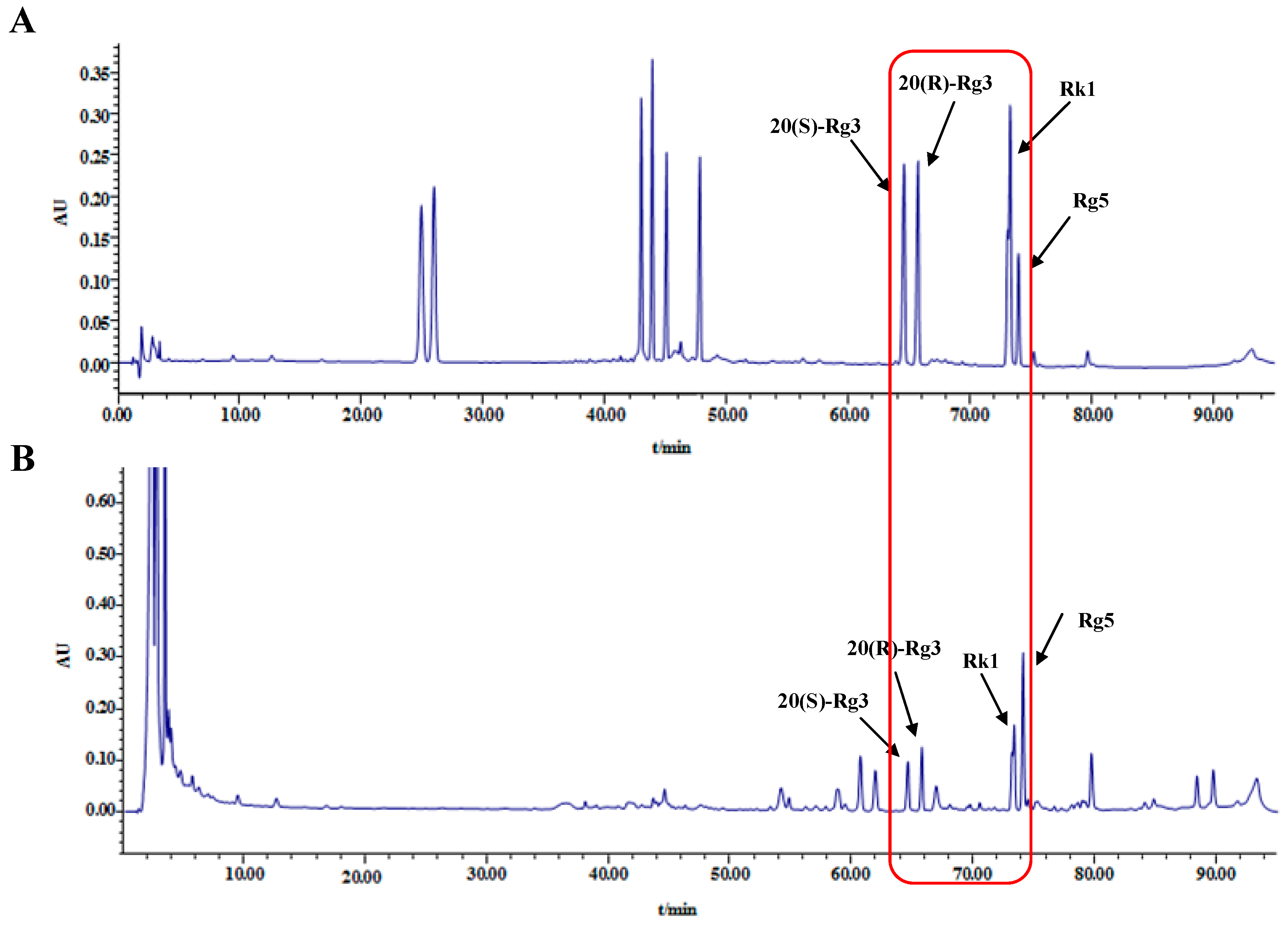
2.1. HPLC Analysis of Ginsenosides in Black Ginseng
2.2. Effect of Black Ginseng on Body Weight and Organ Indices
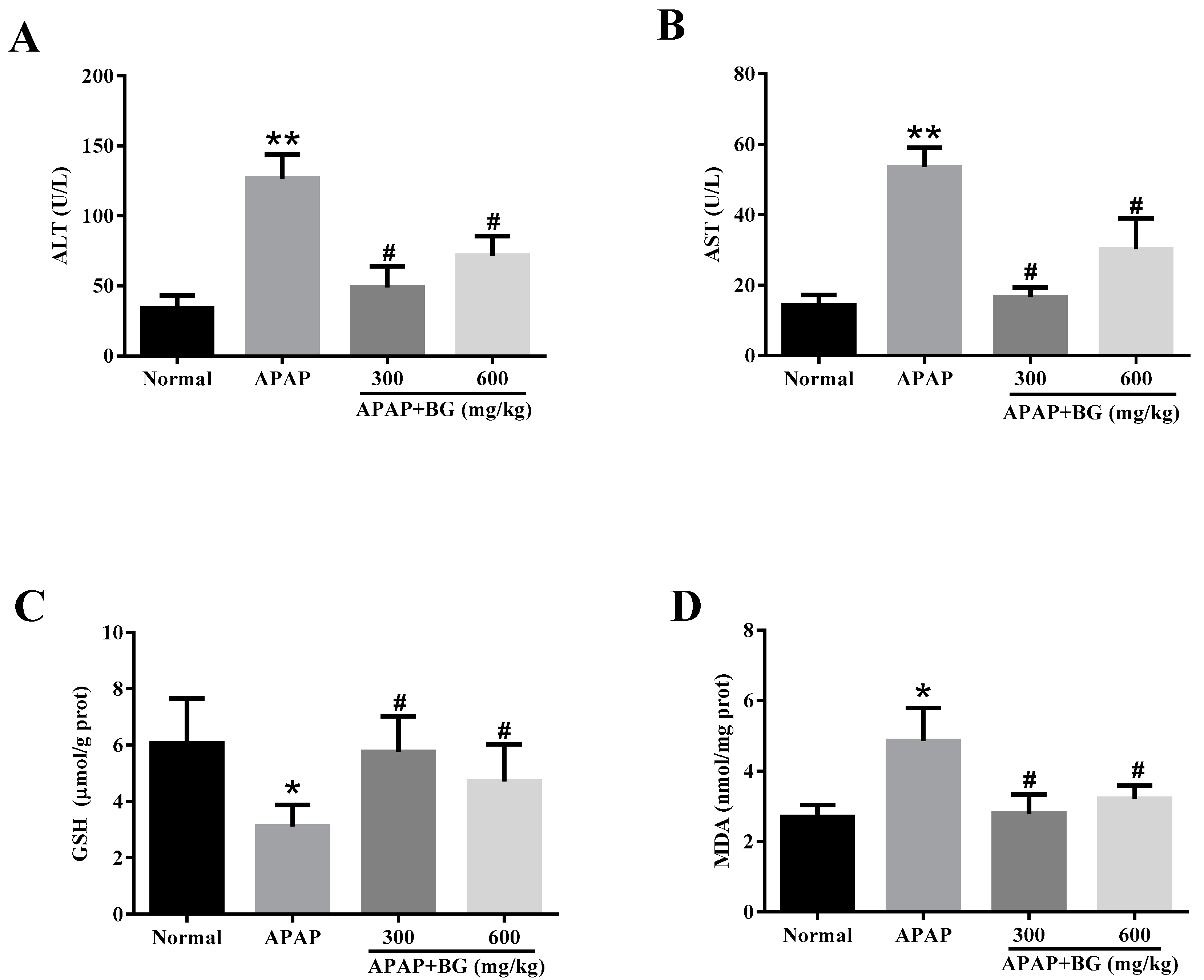
2.3. Effect of Black Ginseng on Serum Biochemical Markers
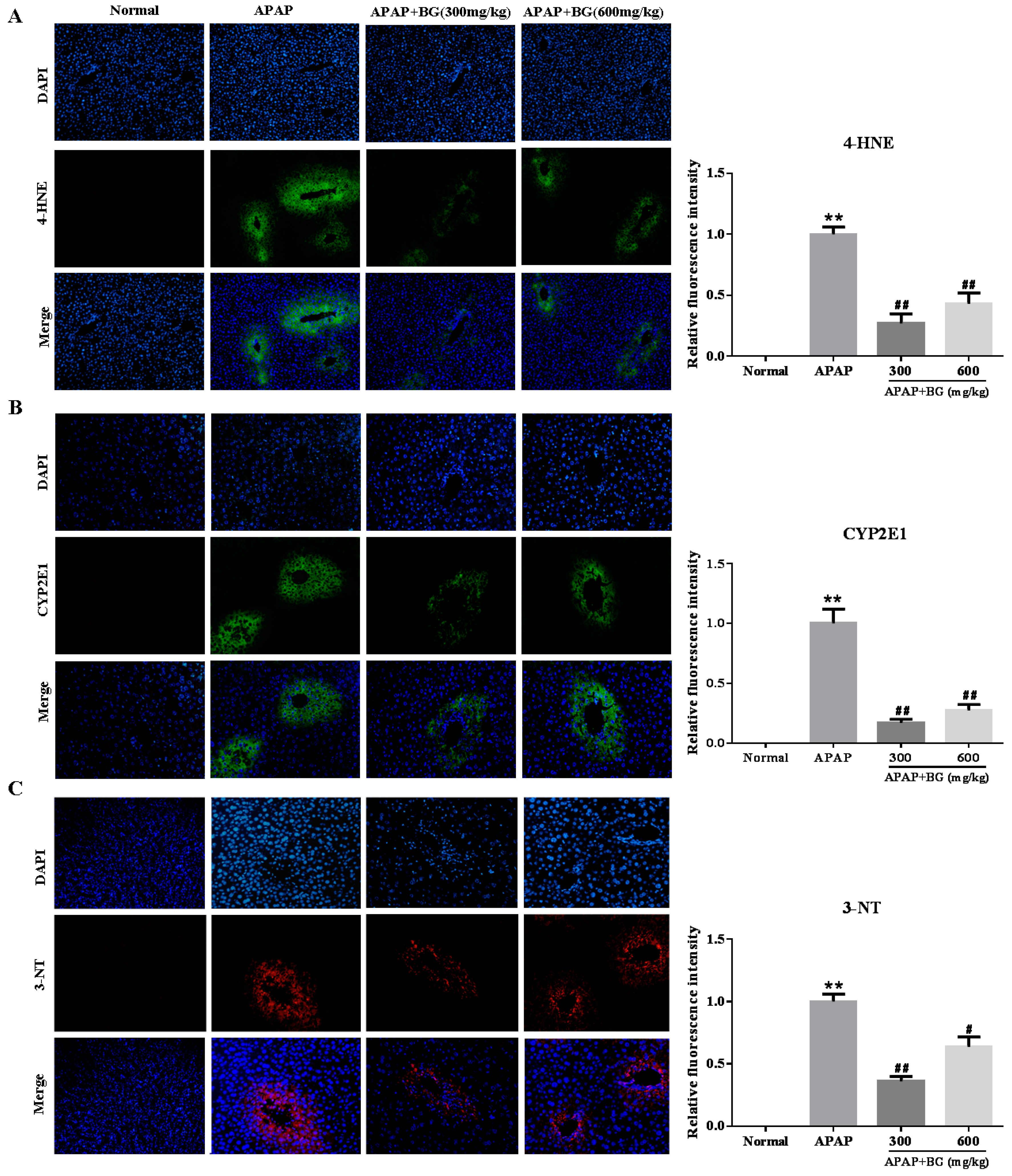
2.4. Effect of Black Ginseng on Attenuates APAP-Induced Oxidative Stress
2.5. Effect of Black Ginseng on Expression of 3-NT in Liver tissues
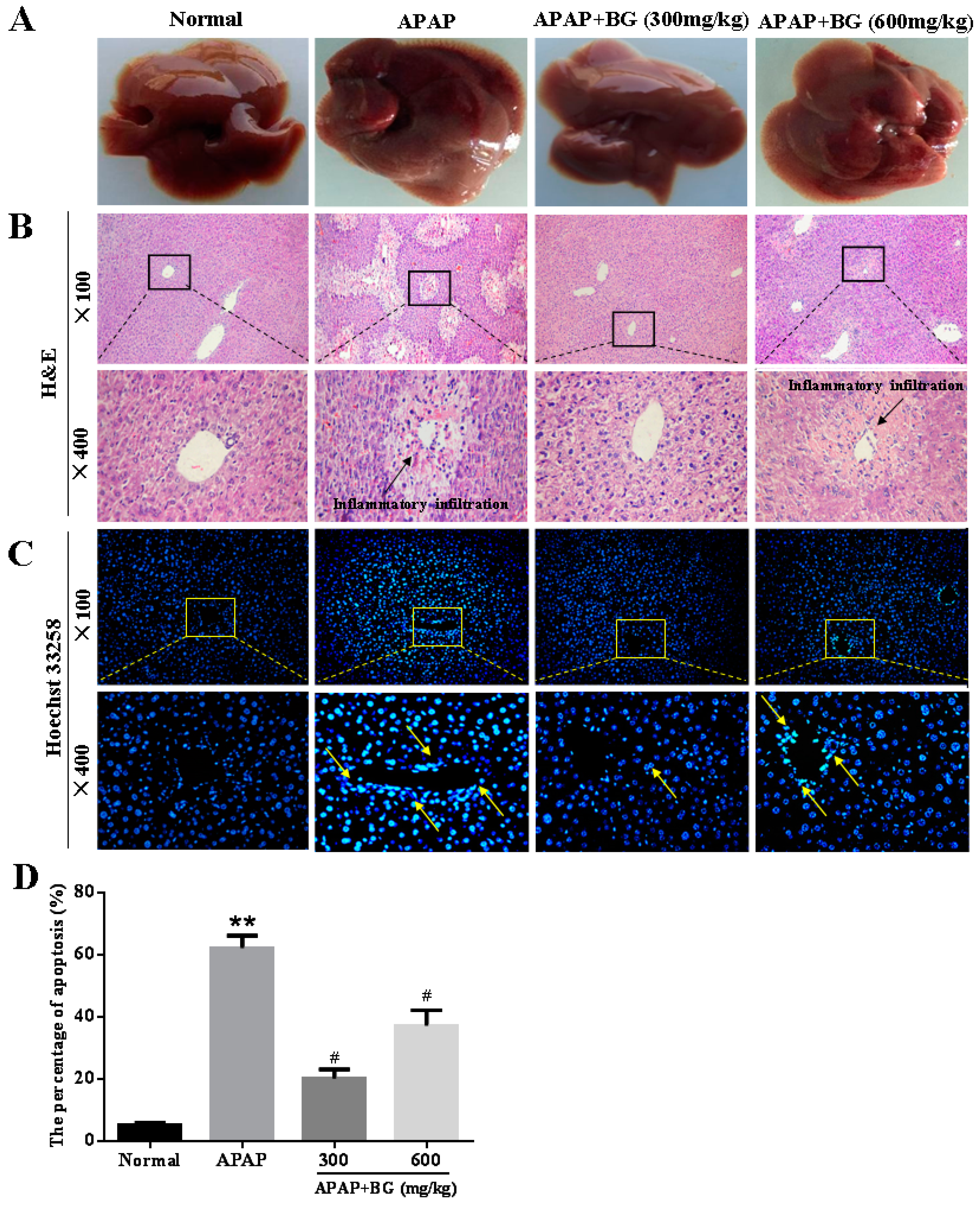
2.6. Effect of Black Ginseng on Liver Histopathology
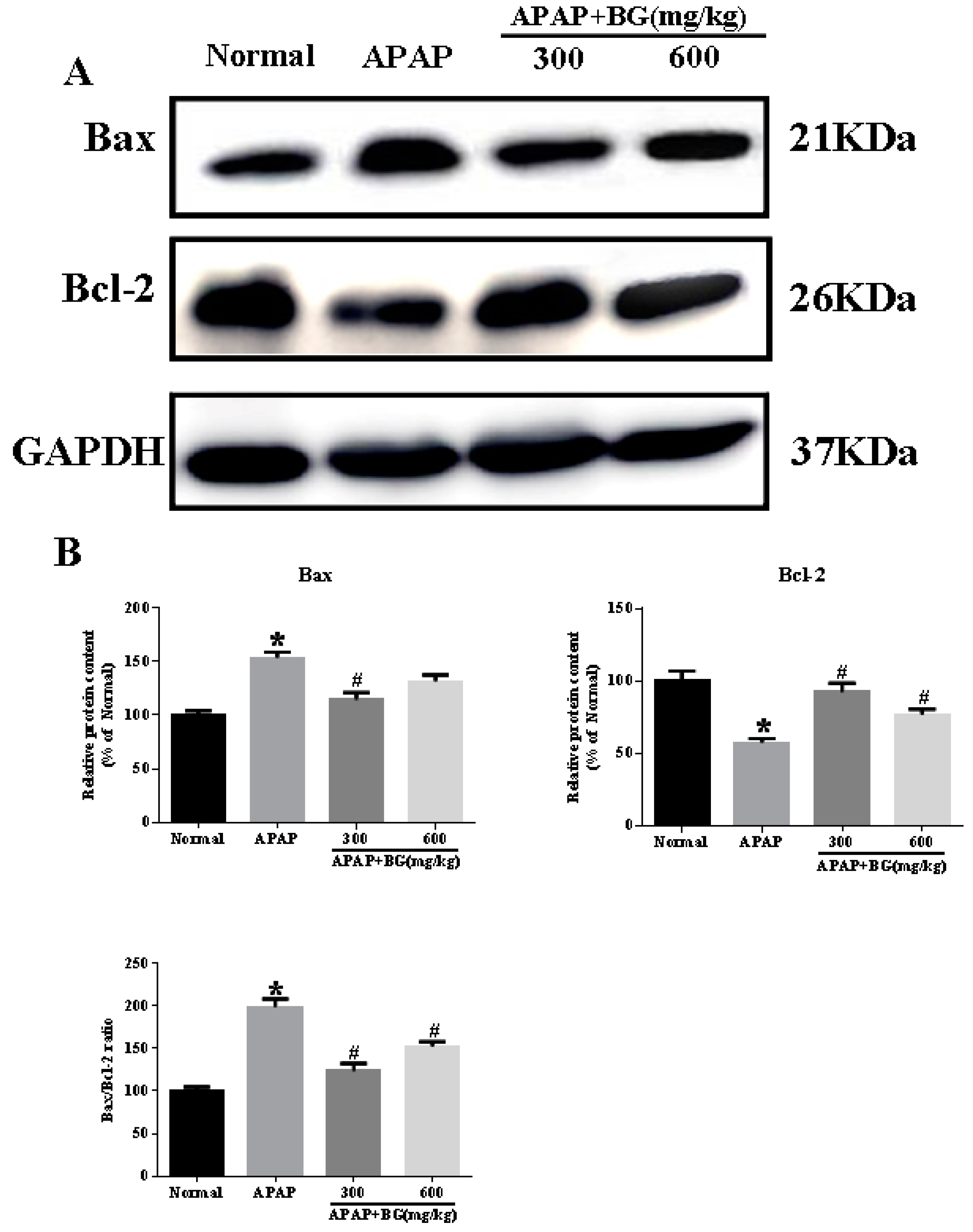
2.7. Effect of Black Ginseng on Hepatocyte Apoptosis in Mice
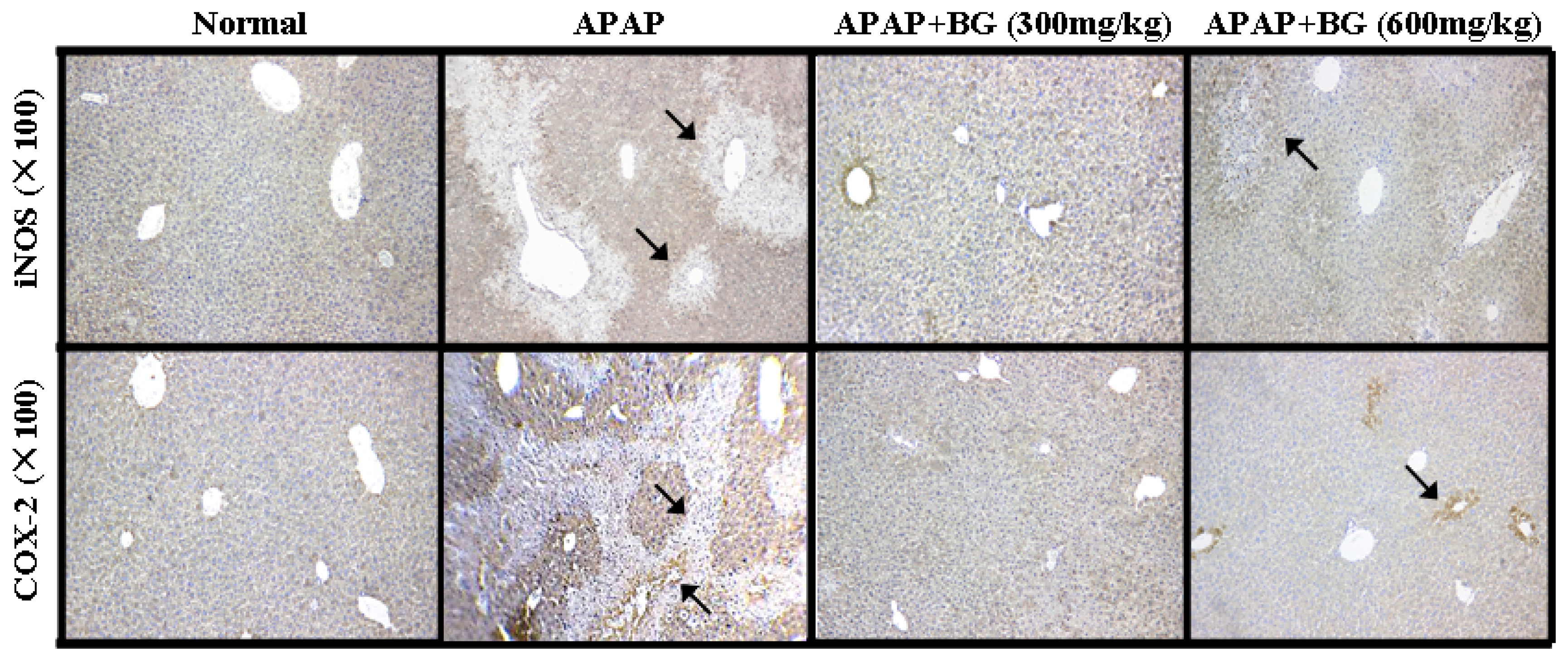
2.8. Effect of Black Ginseng on Expression of iNOS and COX-2 in Liver Tissues
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Identification and Analysis of Ginsenosides in Black Ginseng
4.4. Animals
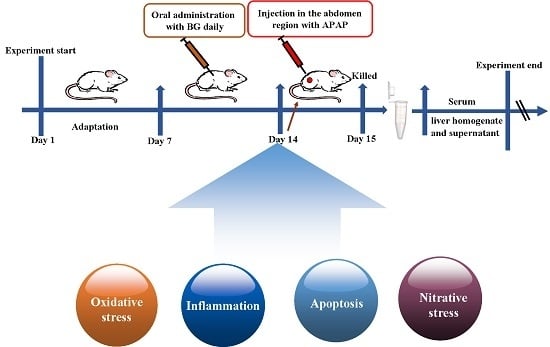
4.5. Experimental Groups and Treatment
4.6. Assay for Hepatic Function Biochemical Markers
4.7. Assay for GSH and MDA in Liver
4.8. Histopathological Examination
4.9. Hoechst 33258 Staining
4.10. Immunohistochemistry (IHC) and Immunofluorescence Analysis
4.11. Western Blotting Analysis
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BG | Black ginseng |
| APAP | Acetaminophen |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| 3-NT | 3-Nitrotyrosine |
| 4-HNE | 4-hydroxynonenal |
| CYP2E1 | Cytochrome P450 E1 |
References
- Zamora Nava, L.E.; Aguirre Valadez, J.; Chavez-Tapia, N.C.; Torre, A. Acute-on-chronic liver failure: A review. Ther. Clin. Risk Manag. 2014, 10, 295–303. [Google Scholar] [PubMed]
- Sun, H.; Chen, L.; Zhou, W.; Hu, L.; Li, L.; Tu, Q.; Chang, Y.; Liu, Q.; Sun, X.; Wu, M.; et al. The protective role of hydrogen-rich saline in experimental liver injury in mice. J. Hepatol. 2011, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Dargan, P.; Jones, A. Paracetamol: Balancing risk against benefit. QJM 2002, 95, 831–832. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, S.; Jiao, Y.; Lin, X.; Huang, J.; Chen, C.; Chen, Z.; Huang, R. Effects and mechanisms of rifampin on hepatotoxicity of acetaminophen in mice. Food Chem. Toxicol. 2012, 50, 3142–3149. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.M. Acetaminophen hepatotoxicity. Clin. Liver Dis. 2007, 11, 525–548. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010, 369–405. [Google Scholar]
- Prescott, L.F. Kinetics and metabolism of paracetamol and phenacetin. Br. J. Clin. Pharmacol. 1980, 10 (Suppl. 2), 291S–298S. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.A.; Cederbaum, A.I. Inhibition of cyp2e1 catalytic activity in vitro by s-adenosyl-l-methionine. Biochem. Pharmacol. 2005, 69, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. Iv. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973, 187, 211–217. [Google Scholar] [PubMed]
- Kon, K.; Kim, J.S.; Jaeschke, H.; Lemasters, J.J. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 2004, 40, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Reid, A.B.; McCullough, S.S.; James, L.P. Acetaminophen-induced hepatotoxicity: Role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab. Rev. 2004, 36, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.D. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990, 10, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Gum, S.I.; Cho, M.K. The amelioration of n-acetyl-p-benzoquinone imine toxicity by ginsenoside rg3: The role of nrf2-mediated detoxification and mrp1/mrp3 transports. Oxid. Med. Cell Longev. 2013, 2013, 957947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, F.; Wang, K.; Liu, G.; Yang, M.; Luan, Y.; Zhao, Z. Protective effect of allyl methyl disulfide on acetaminophen-induced hepatotoxicity in mice. Chem. Biol. Interact. 2016, 249, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Park, J.H. Trends in ginseng research in 2010. J. Ginseng Res. 2011, 35, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Gu, J.; Chen, L.; Hou, W.; Jin, Y.; Wang, Y. Bioconversion of ginsenoside rd to ginsenoside m1 by snailase hydrolysis and its enhancement effect on insulin secretion in vitro. Pharmazie 2015, 70, 340–346. [Google Scholar] [PubMed]
- Jin, Y.; Piao, J.; Lee, S.M. Evaluating the validity of the braden scale using longitudinal electronic medical records. Res. Nurs. Health 2015, 38, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, X.; Wang, M.; Li, B.; Liu, Z.; Liu, S. Chemical and antihyperglycemic activity changes of ginseng pectin induced by heat processing. Carbohydr. Polym. 2014, 114, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cha, H.Y.; Seo, J.J.; Hong, J.T.; Han, K.; Oh, K.W. Anxiolytic-like effects of ginseng in the elevated plus-maze model: Comparison of red ginseng and sun ginseng. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Yoo, K.M.; Lee, J.W.; Eom, S.H.; Hwang, I.K.; Lee, C.Y. Protective effect of steamed american ginseng (Panax quinquefolius L.) on v79-4 cells induced by oxidative stress. J. Ethnopharmacol. 2007, 111, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Igami, K.; Shimojo, Y.; Ito, H.; Miyazaki, T.; Kashiwada, Y. Hepatoprotective effect of fermented ginseng and its major constituent compound k in a rat model of paracetamol (acetaminophen)-induced liver injury. J. Pharm. Pharmacol. 2015, 67, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Joo, E.J.; Chun, J.; Ha, Y.W.; Lee, J.H.; Han, Y.; Kim, Y.S. Induction of apoptosis by ginsenoside rk1 in sk-mel-2-human melanoma. Arch. Pharm. Res. 2012, 35, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.S.; Gu, L.J.; Fang, Z.M.; Wang, C.Y.; Wang, Z.; Lee, M.R.; Li, Z.; Li, J.J.; Sung, C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from panax ginseng by hplc-elsd. J. Pharm. Biomed. Anal. 2009, 50, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.; Zhang, J.; Song, S.; Miao, R.; Liu, S.; Pang, Q.; Wu, Q.; Liu, C. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int. Immunopharmacol. 2015, 27, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Jodynis-Liebert, J.; Matlawska, I.; Bylka, W.; Murias, M. Protective effect of Aquilegia vulgaris (L.) on apap-induced oxidative stress in rats. J. Ethnopharmacol. 2005, 97, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; New, L.S.; Chan, E.C. Prevention of acetaminophen (apap)-induced hepatotoxicity by leflunomide via inhibition of apap biotransformation to n-acetyl-p-benzoquinone imine. Toxicol. Lett. 2008, 180, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, Y.J.; Jeon, J.N.; Wang, C.; Min, J.W.; Noh, H.Y.; Yang, D.C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.I.; Kim, D.S.; Lee, Y.H.; Park, J.D.; Lee, C.B.; Kim, S.I. Ginsenoside rh4, a genuine dammarane glycoside from korean red ginseng. Planta Med. 1996, 62, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Kim, H.Y.; Pyo, J.S.; Yokozawa, T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol. Pharm. Bull. 2006, 29, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Piao, L.Z.; Kwon, S.W.; Lee, Y.J.; Cho, S.Y.; Park, M.K.; Park, J.H. Cytotoxic dammarane glycosides from processed ginseng. Chem. Pharm. Bull. (Tokyo) 2002, 50, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, N.V. Toxicity of the ages generated from the maillard reaction: On the relationship of food-ages and biological-ages. Mol. Nutr. Food Res. 2006, 50, 1140–1149. [Google Scholar] [PubMed]
- Liu, L.C.; Wang, C.J.; Lee, C.C.; Su, S.C.; Chen, H.L.; Hsu, J.D.; Lee, H.J. Aqueous extract of Hibiscus sabdariffa L. Decelerates acetaminophen-induced acute liver damage by reducing cell death and oxidative stress in mouse experimental models. J. Sci. Food Agric. 2010, 90, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; McClain, C.J.; Chen, T. S-adenosylmethionine protects against acetaminophen-induced hepatotoxicity in mice. Pharmacology 2004, 71, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lin, C.C.; Liao, T.S.; Yin, M.C. Protective effect of s-allyl cysteine and s-propyl cysteine on acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2006, 44, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Dutta, A.; Chaudhuri, S.R.; Sharma, N.; Giri, A.K.; Chaudhuri, K. In vitro and in vivo reduction of sodium arsenite induced toxicity by aqueous garlic extract. Food Chem. Toxicol. 2008, 46, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.W.; Tsai, C.F.; Chen, W.K.; Lu, F.J. Protective effects of seabuckthorn (Hippophae rhamnoides L.) seed oil against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem. Toxicol. 2009, 47, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Yoo, J.S.; Lin, M.; Wang, E.J.; Yang, C.S. Protective effects of diallyl sulfide on acetaminophen-induced toxicities. Food Chem. Toxicol. 1996, 34, 963–969. [Google Scholar] [CrossRef]
- Hau, D.K.; Gambari, R.; Wong, R.S.; Yuen, M.C.; Cheng, G.Y.; Tong, C.S.; Zhu, G.Y.; Leung, A.K.; Lai, P.B.; Lau, F.Y.; et al. Phylla nthus urinaria extract attenuates acetaminophen induced hepatotoxicity: Involvement of cytochrome p450 cyp2e1. Phytomedicine 2009, 16, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Saito, C.; Lemasters, J.J.; Jaeschke, H. C-jun n-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010, 246, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Williams, C.D.; McGill, M.R.; Xie, Y.; Ramachandran, A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem. Toxicol. 2013, 55, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, J.; Liu, J.; Huang, Y.; Zhong, J.J.; Tang, W. Enhancement of il-2 and ifn-gamma expression and nk cells activity involved in the anti-tumor effect of ganoderic acid me in vivo. Int. Immunopharmacol. 2007, 7, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Luo, F.; Tang, X.; Li, K.; Hu, X.; Bai, J. Panaxatriol saponin ameliorated liver injury by acetaminophen via restoring thioredoxin-1 and pro-caspase-12. Liver Int. 2014, 34, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.S. Green-tea polyphenols downregulate cyclooxygenase and bcl-2 activity in acetaminophen-induced hepatotoxicity. Dig. Dis. Sci. 2008, 53, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.Y.; Park, J.H.; Kim, M.J.; Kim, H.O.; Hwang, J.K.; Lee, S.K.; Park, K.K. Xanthorrhizol inhibits 12-o-tetradecanoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase, cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-activated protein kinases and/or the nuclear factor-kappa b. Carcinogenesis 2007, 28, 1224–1231. [Google Scholar] [PubMed]
- Li, W.; Liu, Y.; Wang, Z.; Han, Y.; Tian, Y.H.; Zhang, G.S.; Sun, Y.S.; Wang, Y.P. Platycodin d isolated from the aerial parts of platycodon grandiflorum protects alcohol-induced liver injury in mice. Food Funct. 2015, 6, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Q.; Hu, J.N.; Han, X.Y.; Li, W.; Zhao, L.C. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, M.H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.S. Ginsenoside rg5 ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress, and apoptosis. Nutrients 2016, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, Q.; He, Y.F.; Liu, Y.; Yang, S.B.; Wang, Z.; Zhang, J.; Zhao, L.C. Anti-tumor effect of steamed codonopsis lanceolata in h22 tumor-bearing mice and its possible mechanism. Nutrients 2015, 7, 8294–8307. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, M.; Gu, J.; Meng, Z.J.; Zhao, L.C.; Zheng, Y.N.; Chen, L.; Yang, G.L. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound k on type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 2012, 83, 192–198. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 20(s)-Rg3, 20(r)-Rg3, Rk1, and Rg5 are available from the authors. |
| Groups | Dosage (mg/kg) | Body Weights (g) | Organ Indices (mg/g, ×100) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Liver | Spleen | Kidney | ||
| Normal | - | 28.92 ± 1.12 | 28.95 ± 2.13 | 5.71 ± 0.87 | 0.43 ± 0.06 | 1.65 ± 0.18 |
| APAP | - | 28.74 ± 1.36 | 27.02 ± 1.26 | 7.30 ± 0.78 ** | 0.53 ± 0.09 * | 1.54 ± 0.35 |
| APAP + BG | 300 | 28.76 ± 1.54 | 28.78 ± 1.62 | 4.95 ± 0.62 ## | 0.44 ± 0.03 # | 1.47 ± 0.12 |
| APAP + BG | 600 | 28.81 ± 1.79 | 28.53 ± 1.71 | 5.02 ± 0.35 ## | 0.33 ± 0.05 ## | 1.45 ± 0.13 |
| Groups | Dosage (mg/kg) | Necrocytosis Grade | Score | Ridit Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | 0 | 1 | 2 | 3 | 4 | ||||
| Normal | - | 8 | 8 | 0 | 0 | 0 | 0 | 0 | 0.27 |
| APAP | - | 8 | 0 | 2 | 1 | 4 | 1 | 20 | 0.81 ** |
| APAP + BG | 300 | 8 | 5 | 2 | 1 | 0 | 0 | 4 | 0.41 ## |
| APAP + BG | 600 | 8 | 4 | 1 | 2 | 1 | 0 | 8 | 0.51 ## |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.-N.; Liu, Z.; Wang, Z.; Li, X.-D.; Zhang, L.-X.; Li, W.; Wang, Y.-P. Ameliorative Effects and Possible Molecular Mechanism of Action of Black Ginseng (Panax ginseng) on Acetaminophen-Mediated Liver Injury. Molecules 2017, 22, 664. https://doi.org/10.3390/molecules22040664
Hu J-N, Liu Z, Wang Z, Li X-D, Zhang L-X, Li W, Wang Y-P. Ameliorative Effects and Possible Molecular Mechanism of Action of Black Ginseng (Panax ginseng) on Acetaminophen-Mediated Liver Injury. Molecules. 2017; 22(4):664. https://doi.org/10.3390/molecules22040664
Chicago/Turabian StyleHu, Jun-Nan, Zhi Liu, Zi Wang, Xin-Dian Li, Lian-Xue Zhang, Wei Li, and Ying-Ping Wang. 2017. "Ameliorative Effects and Possible Molecular Mechanism of Action of Black Ginseng (Panax ginseng) on Acetaminophen-Mediated Liver Injury" Molecules 22, no. 4: 664. https://doi.org/10.3390/molecules22040664
APA StyleHu, J.-N., Liu, Z., Wang, Z., Li, X.-D., Zhang, L.-X., Li, W., & Wang, Y.-P. (2017). Ameliorative Effects and Possible Molecular Mechanism of Action of Black Ginseng (Panax ginseng) on Acetaminophen-Mediated Liver Injury. Molecules, 22(4), 664. https://doi.org/10.3390/molecules22040664