Functional Assays in the Diagnosis of Heparin-Induced Thrombocytopenia: A Review
Abstract
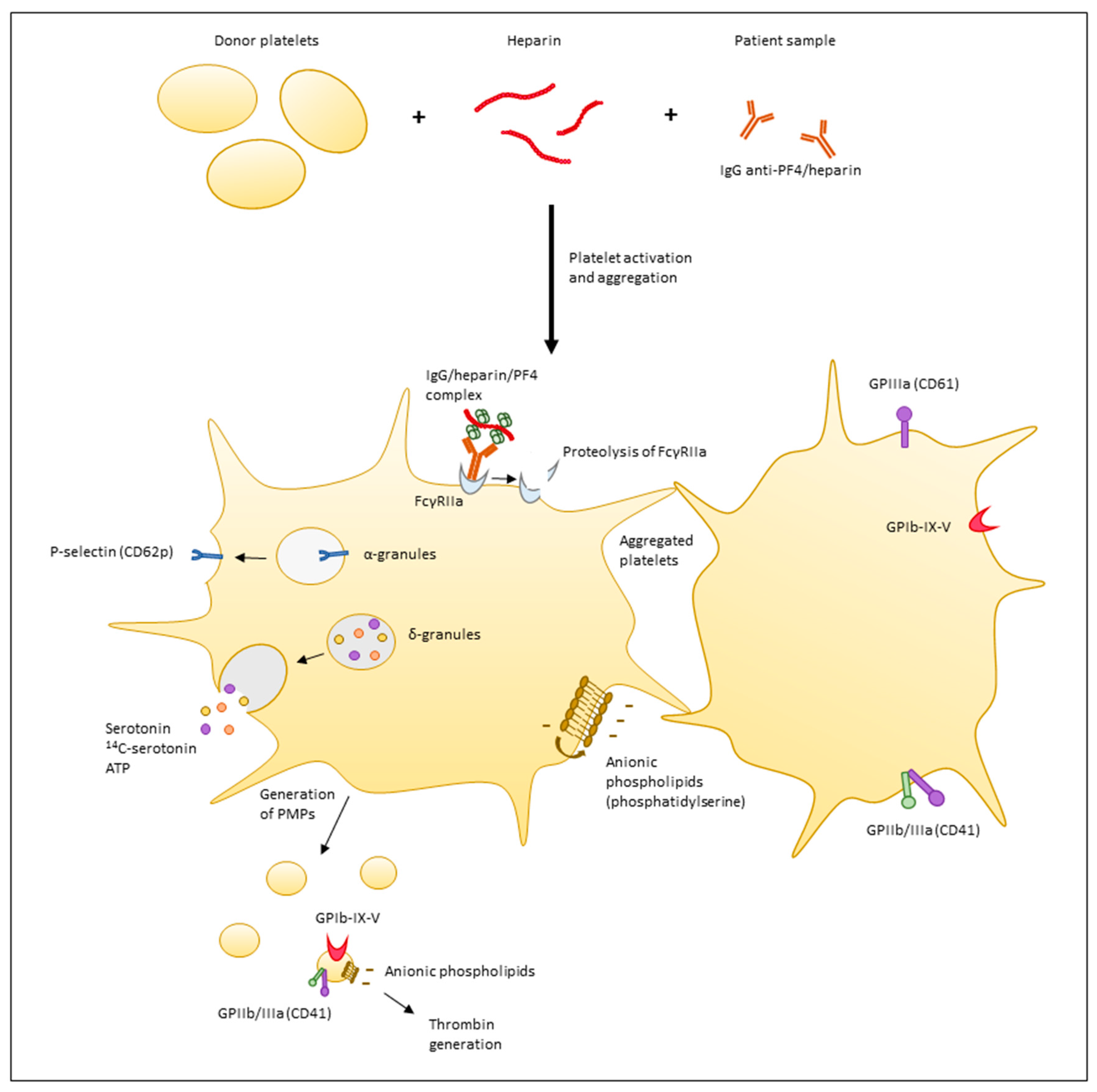
1. Introduction
2. Principles of Functional Assays
3. Activation Endpoints
3.1. Release of Dense Granules Content
3.1.1. Release of Serotonin
3.1.2. Release of Adenosine Triphosphate (ATP)
3.1.3. Platelet Aggregation
Visual Assessment
Optical Aggregometry
Impedance Aggregometry
3.1.4. Expression of Platelet Membrane Glycoproteins
3.1.5. Generation of Platelets Microparticles
3.1.6. Procoagulant Activity
3.1.7. FcγRIIa Proteolysis
3.1.8. Intracellular Luciferase Cell Activity
4. Platelets
4.1. Whole Blood, PRP or Washed Platelets
4.2. Platelet Donor Selection
5. Patient Sample
6. Heparin
7. Controls
7.1. Negative Controls
7.2. Heparin Dependency
7.3. Positive Controls
8. Other Variations
9. Results Expression
10. Results Interpretation
11. Threshold and Performances
12. Existing Functional Assays and Their Characteristics
13. Conclusions
Author Contributions
Conflicts of Interest
References
- Bakchoul, T. An update on heparin-induced thrombocytopenia: Diagnosis and management. Expert Opin. Drug Saf. 2016, 15, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A. Too many hits in HIT? Am. J. Hematol. 2007, 82, 1035–1036. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.K.; Sigouin, C.S.; Warkentin, T.E. What is the potential for overdiagnosis of heparin-induced thrombocytopenia? Am. J. Hematol. 2007, 82, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Elalamy, I.; Tardy-Poncet, B.; Mulot, A.; de Maistre, E.; Pouplard, C.; Nguyen, P.; Cleret, B.; Gruel, Y.; Lecompte, T.; Tardy, B.; et al. Risk factors for unfavorable clinical outcome in patients with documented heparin-induced thrombocytopenia. Thromb. Res. 2009, 124, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A. Heparin-induced thrombocytopenia. N. Engl. J. Med. 2015, 373, 1883–1884. [Google Scholar] [PubMed]
- Joseph, L.; Gomes, M.P.; Al Solaiman, F.; St John, J.; Ozaki, A.; Raju, M.; Dhariwal, M.; Kim, E.S. External validation of the HIT Expert Probability (HEP) score. Thromb. Haemost. 2015, 113, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Gimotty, P.A.; Crowther, M.A.; Warkentin, T.E. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood 2012, 120, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.K.; Juhl, D.; Warkentin, T.E.; Sigouin, C.S.; Eichler, P.; Greinacher, A. Evaluation of pretest clinical score (4 T′s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J. Thromb. Haemost. 2006, 4, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. Toward improved diagnosis of HIT. Blood 2015, 126, 563–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Linkins, L.A.; Bates, S.M.; Lee, A.Y.; Heddle, N.M.; Wang, G.; Warkentin, T.E. Combination of 4Ts score and PF4/H-PaGIA for diagnosis and management of heparin-induced thrombocytopenia: Prospective cohort study. Blood 2015, 126, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Nagler, M.; Fabbro, T.; Wuillemin, W.A. Prospective evaluation of the interobserver reliability of the 4Ts score in patients with suspected heparin-induced thrombocytopenia. J. Thromb. Haemost. 2012, 10, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. How I diagnose and manage HIT. ASH Educ. Program Book 2011, 1, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Nagler, M.; Bachmann, L.M.; Ten Cate, H.; Ten Cate-Hoek, A. Diagnostic value of immunoassays for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood 2015, 127, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Nagler, M.; Bakchoul, T. Clinical and laboratory tests for the diagnosis of heparin-induced thrombocytopenia. Thromb. Haemost. 2016, 116, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Ittermann, T.; Bagemuhl, J.; Althaus, K.; Furll, B.; Selleng, S.; Lubenow, N.; Schellong, S.; Sheppard, J.I.; Warkentin, T.E. Heparin-induced thrombocytopenia: Towards standardization of platelet factor 4/heparin antigen tests. J. Thromb. Haemost. 2010, 8, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Linkins, L.A.; Dans, A.L.; Moores, L.K.; Bona, R.; Davidson, B.L.; Schulman, S.; Crowther, M. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012, 141, e495S–e530S. [Google Scholar] [CrossRef] [PubMed]
- Farm, M.; Bakchoul, T.; Frisk, T.; Althaus, K.; Odenrick, A.; Norberg, E.M.; Berndtsson, M.; Antovic, J.P. Evaluation of a diagnostic algorithm for Heparin-Induced Thrombocytopenia. Thromb. Res. 2017, 152, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Bakchoul, T.; Zollner, H.; Greinacher, A. Current insights into the laboratory diagnosis of HIT. Int. J. Lab. Hematol. 2014, 36, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Michelson, A.D. Platelets, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2011; pp. 861–886. [Google Scholar]
- de Jong, W.H.; Wilkens, M.H.; De Vries, E.G.; Kema, I.P. Automated mass spectrometric analysis of urinary and plasma serotonin. Anal. Bioanal. Chem. 2010, 396, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; Anderson, G.M. The measurement of platelet-poor plasma serotonin: A systematic review of prior reports and recommendations for improved analysis. Clin. Chem. 2011, 57, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Arnold, D.M.; Nazi, I.; Kelton, J.G. The platelet serotonin-release assay. Am. J. Hematol. 2015, 90, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, D.; Carter, C.; Kelton, J.G. A diagnostic test for heparin-induced thrombocytopenia. Blood 1986, 67, 27–30. [Google Scholar] [PubMed]
- Harenberg, J.; Huhle, G.; Giese, C.; Wang, L.C.; Feuring, M.; Song, X.H.; Hoffmann, U. Determination of serotonin release from platelets by enzyme immunoassay in the diagnosis of heparin-induced thrombocytopenia. Br. J. Haematol. 2000, 109, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Odel, M.; Schmidt-Gayk, H.; Walch, S.; Budde, U.; Harenberg, J. Development of an HPLC method for the diagnosis of heparin-induced thrombocytopenia. Anastesiol. Intensivmed. Notfallmed. Schmerzther. 2002, 37 (Suppl. S1), S12. [Google Scholar] [CrossRef]
- Fouassier, M.; Bourgerette, E.; Libert, F.; Pouplard, C.; Marques-Verdier, A. Determination of serotonin release from platelets by HPLC and ELISA in the diagnosis of heparin-induced thrombocytopenia: Comparison with reference method by [C]-serotonin release assay. J. Thromb. Haemost. 2006, 4, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Sono-Koree, N.K.; Crist, R.A.; Frank, E.L.; Rodgers, G.M.; Smock, K.J. A high-performance liquid chromatography method for the serotonin release assay is equivalent to the radioactive method. Int. J. Lab. Hematol. 2016, 38, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Mirandola, P.; Tazzari, P.L.; Ricci, F.; Caimi, L.; Cacchioli, A.; Papa, S.; Conte, R.; Vitale, M. Flow cytometry detection of serotonin content and release in resting and activated platelets. Br. J. Haematol. 2003, 121, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W.; Etches, W.S.; Boshkov, L.K.; Gordon, P.A. Heparin-induced thrombocytopenia: An improved method of detection based on lumi-aggregometry. Br. J. Haematol. 1995, 91, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Teitel, J.M.; Gross, P.; Blake, P.; Garvey, M.B. A bioluminescent adenosine nucleotide release assay for the diagnosis of heparin-induced thrombocytopenia. Thromb. Haemost. 1996, 76, 479. [Google Scholar] [PubMed]
- Greinacher, A.; Michels, I.; Kiefel, V.; Mueller-Eckhardt, C. A rapid and sensitive test for diagnosing heparin-associated thrombocytopenia. Thromb. Haemost. 1991, 66, 734–736. [Google Scholar] [PubMed]
- Warkentin, T.E.; Moore, J.C. Laboratory evaluation of heparin-induced thrombocytopenia. In Quality in Laboratory Hemostasis and Thrombosis, 2nd ed.; Kitchen, S., Olson, J.D., Preston, F.E., Eds.; Wiley-Blackwell: Chichester, West Sussex, UK; Hoboken, NJ, USA, 2009. [Google Scholar]
- Chong, B.H.; Burgess, J.; Ismail, F. The clinical usefulness of the platelet aggregation test for the diagnosis of heparin-induced thrombocytopenia. Thromb. Haemost. 1993, 69, 344–350. [Google Scholar] [PubMed]
- Eichler, P.; Budde, U.; Haas, S.; Kroll, H.; Loreth, R.M.; Meyer, O.; Pachmann, U.; Potzsch, B.; Schabel, A.; Albrecht, D.; et al. First workshop for detection of heparin-induced antibodies: Validation of the heparin-induced platelet-activation test (HIPA) in comparison with a PF4/heparin ELISA. Thromb. Haemost. 1999, 81, 625–629. [Google Scholar] [PubMed]
- Greinacher, A.; Amiral, J.; Dummel, V.; Vissac, A.; Kiefel, V.; Mueller-Eckhardt, C. Laboratory diagnosis of heparin-associated thrombocytopenia and comparison of platelet aggregation test, heparin-induced platelet activation test, and platelet factor 4/heparin enzyme-linked immunosorbent assay. Transfusion 1994, 34, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.; Greinacher, A. Heparin-Induced Thrombocytopenia, 5th ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Fratantoni, J.C.; Pollet, R.; Gralnick, H.R. Heparin-induced thrombocytopenia: Confirmation of diagnosis with in vitro methods. Blood 1975, 45, 395–401. [Google Scholar] [PubMed]
- Prechel, M.; Jeske, W.P.; Walenga, J.M. Anticoagulants, Antiplatelets, and Thrombolytics. In Anticoagulants, Antiplatelets, and Thrombolytics; Mousa, S.A., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2004; Volume 93, pp. 83–94. [Google Scholar]
- Elalamy, I.; Galea, V.; Hatmi, M.; Gerotziafas, G.T. Heparin-induced multiple electrode aggregometry: A potential tool for improvement of heparin-induced thrombocytopenia diagnosis. J. Thromb. Haemost. 2009, 7, 1932–1934. [Google Scholar] [CrossRef] [PubMed]
- Minet, V.; Bailly, N.; Douxfils, J.; Osselaer, J.C.; Laloy, J.; Chatelain, C.; Elalamy, I.; Chatelain, B.; Dogne, J.M.; Mullier, F. Assessment of the performances of AcuStar HIT and the combination with heparin-induced multiple electrode aggregometry: A retrospective study. Thromb. Res. 2013, 132, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Morel-Kopp, M.C.; Mullier, F.; Gkalea, V.; Bakchoul, T.; Minet, V.; Elalamy, I.; Ward, C.M. Heparin-induced multi-electrode aggregometry method for heparin-induced thrombocytopenia testing: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 2548–2552. [Google Scholar] [CrossRef] [PubMed]
- Galea, V.; Khaterchi, A.; Robert, F.; Gerotziafas, G.; Hatmi, M.; Elalamy, I. Heparin-induced multiple electrode aggregometry is a promising and useful functional tool for heparin-induced thrombocytopenia diagnosis: Confirmation in a prospective study. Platelets 2013, 24, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Minet, V.; Baudar, J.; Bailly, N.; Douxfils, J.; Laloy, J.; Lessire, S.; Gourdin, M.; Devalet, B.; Chatelain, B.; Dogne, J.M.; et al. Rapid exclusion of the diagnosis of immune HIT by AcuStar HIT and heparin-induced multiple electrode aggregometry. Thromb. Res. 2014, 133, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Morel-Kopp, M.C.; Aboud, M.; Tan, C.W.; Kulathilake, C.; Ward, C. Whole blood impedance aggregometry detects heparin-induced thrombocytopenia antibodies. Thromb. Res. 2010, 125, e234–e239. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Mirandola, P.; Tazzari, P.L.; Talarico, E.; Caimi, L.; Martini, G.; Papa, S.; Conte, R.; Manzoli, F.A.; Vitale, M. New laboratory test in flow cytometry for the combined analysis of serologic and cellular parameters in the diagnosis of heparin-induced thrombocytopenia. Cytom. Part B Clin. Cytom. 2004, 58, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tomer, A.; Masalunga, C.; Abshire, T.C. Determination of heparin-induced thrombocytopenia: A rapid flow cytometric assay for direct demonstration of antibody-mediated platelet activation. Am. J. Hematol. 1999, 61, 53–61. [Google Scholar] [CrossRef]
- Vitale, M.; Tazzari, P.; Ricci, F.; Mazza, M.A.; Zauli, G.; Martini, G.; Caimi, L.; Manzoli, F.A.; Conte, R. Comparison between different laboratory tests for the detection and prevention of heparin-induced thrombocytopenia. Cytometry 2001, 46, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Tomer, A. A sensitive and specific functional flow cytometric assay for the diagnosis of heparin-induced thrombocytopenia. Br. J. Haematol. 1997, 98, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Mutsando, H.; Self, M.; Morel-Kopp, M.C.; Mollee, P. Using HitAlert flow cytometry to detect heparin-induced thrombocytopenia antibodies in a tertiary care hospital. Blood Coagul. Fibrinolysis 2013, 24, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Malicev, E.; Kozak, M.; Rozman, P. Evaluation of a flow cytometric assay for the confirmation of heparin-induced thrombocytopenia. Int. J. Lab. Hematol. 2016, 38, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kerenyi, A.; Debreceni, I.B.; Olah, Z.; Ilonczai, P.; Bereczky, Z.; Nagy, B., Jr.; Muszbek, L.; Kappelmayer, J. Evaluation of flow cytometric HIT assays in relation to an IgG-specific immunoassay and clinical outcome. Cytom. Part B Clin. Cytom. 2016. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Radu, C.M.; Duner, E.; Lombardi, A.M.; Spiezia, L.; Bendo, R.; Ferrari, S.; Simioni, P.; Fabris, F. Activated platelet-derived and leukocyte-derived circulating microparticles and the risk of thrombosis in heparin-induced thrombocytopenia: A role for PF4-bearing microparticles? Cytom. Part B Clin. Cytom. 2017. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Hayward, C.P.; Boshkov, L.K.; Santos, A.V.; Sheppard, J.A.; Bode, A.P.; Kelton, J.G. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: An explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood 1994, 84, 3691–3699. [Google Scholar] [PubMed]
- Hughes, M.; Hayward, C.P.; Warkentin, T.E.; Horsewood, P.; Chorneyko, K.A.; Kelton, J.G. Morphological analysis of microparticle generation in heparin-induced thrombocytopenia. Blood 2000, 96, 188–194. [Google Scholar] [PubMed]
- Mullier, F.; Bailly, N.; Cornet, Y.; Dubuc, E.; Robert, S.; Osselaer, J.C.; Chatelain, C.; Dogne, J.M.; Chatelain, B. Contribution of platelet microparticles generation assay to the diagnosis of type II heparin-induced thrombocytopenia. Thromb. Haemost. 2010, 103, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Mullier, F.; Minet, V.; Bailly, N.; Devalet, B.; Douxfils, J.; Chatelain, C.; Elalamy, I.; Dogne, J.M.; Chatelain, B. Platelet microparticle generation assay: A valuable test for immune heparin-induced thrombocytopenia diagnosis. Thromb. Res. 2014, 133, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Minet, V.; Bailly, N.; Dogne, J.M.; Mullier, F. Platelet microparticle generation assay for heparin-induced thrombocytopenia diagnosis: How should we express the results? Thromb. Res. 2015, 136, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Warkentin, T.E.; Denomme, G.A.; Hayward, C.P.; Kelton, J.G. A diagnostic test for heparin-induced thrombocytopenia: Detection of platelet microparticles using flow cytometry. Br. J. Haematol. 1996, 95, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Hemker, H.C.; Giesen, P.; Al Dieri, R.; Regnault, V.; de Smedt, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol. Haemost. Thromb. 2003, 33, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Hemker, H.C.; Giesen, P.; AlDieri, R.; Regnault, V.; de Smed, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. The calibrated automated thrombogram (CAT): A universal routine test for hyper- and hypocoagulability. Pathophysiol. Haemost. Thromb. 2002, 32, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Tardy-Poncet, B.; Piot, M.; Chapelle, C.; France, G.; Campos, L.; Garraud, O.; Decousus, H.; Mismetti, P.; Tardy, B. Thrombin generation and heparin-induced thrombocytopenia. J. Thromb. Haemost. 2009, 7, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; Karunakaran, D.; Arthur, J.F.; Mu, F.T.; Powell, M.S.; Baker, R.I.; Hogarth, P.M.; Kahn, M.L.; Andrews, R.K.; Berndt, M.C. Dual ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors: De-ITAM-izing FcgammaRIIa. Blood 2008, 111, 165–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gardiner, E.E.; Al-Tamimi, M.; Mu, F.T.; Karunakaran, D.; Thom, J.Y.; Moroi, M.; Andrews, R.K.; Berndt, M.C.; Baker, R.I. Compromised ITAM-based platelet receptor function in a patient with immune thrombocytopenic purpura. J. Thromb. Haemost. 2008, 6, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Nazi, I.; Arnold, D.M.; Smith, J.W.; Horsewood, P.; Moore, J.C.; Warkentin, T.E.; Crowther, M.A.; Kelton, J.G. FcgammaRIIa proteolysis as a diagnostic biomarker for heparin-induced thrombocytopenia. J. Thromb. Haemost. 2013, 11, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Rux, A.H.; Hinds, J.L.; Dela Cruz, M.; Yarovoi, S.V.; Brown, I.A.; Yang, W.; Konkle, B.A.; Arepally, G.M.; Watson, S.P.; et al. Novel diagnostic assays for heparin-induced thrombocytopenia. Blood 2013, 121, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.M.; Bain, B.J.; Bates, I.; Dacie, J.V.; Dacie, J.V. Dacie and Lewis Practical Haematology, 10th ed.; Churchill Livingstone: Philadelphia, PA, USA, 2006; pp. 475–478. [Google Scholar]
- Polgar, J.; Eichler, P.; Greinacher, A.; Clemetson, K.J. Adenosine diphosphate (ADP) and ADP receptor play a major role in platelet activation/aggregation induced by sera from heparin-induced thrombocytopenia patients. Blood 1998, 91, 549–554. [Google Scholar] [PubMed]
- Warkentin, T.E.; Greinacher, A.; Gruel, Y.; Aster, R.H.; Chong, B.H. Laboratory testing for heparin-induced thrombocytopenia: A conceptual framework and implications for diagnosis. J. Thromb. Haemost. 2011, 9, 2498–2500. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Winteroll, S. Laboratory diagnosis of heparin-induced thrombocytopenia and monitoring of alternative anticoagulants. Clin. Diagn. Lab. Immunol. 2003, 10, 731–740. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Favaloro, E.J.; Bernal-Hoyos, E.; Exner, T.; Koutts, J. Heparin-induced thrombocytopenia: Laboratory investigation and confirmation of diagnosis. Pathology 1992, 24, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Walenga, J.M.; Jeske, W.P.; Fasanella, A.R.; Wood, J.J.; Ahmad, S.; Bakhos, M. Laboratory diagnosis of heparin-induced thrombocytopenia. Clin. Appl. Thromb. Hemost. 1999, 5, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Pouplard, C.; Amiral, J.; Borg, J.Y.; Laporte-Simitsidis, S.; Delahousse, B.; Gruel, Y. Decision analysis for use of platelet aggregation test, carbon 14-serotonin release assay, and heparin-platelet factor 4 enzyme-linked immunosorbent assay for diagnosis of heparin-induced thrombocytopenia. Am. J. Clin. Pathol. 1999, 111, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Morel-Kopp, M.C.; Aboud, M.; Tan, C.W.; Kulathilake, C.; Ward, C. Heparin-induced thrombocytopenia: Evaluation of IgG and IgGAM ELISA assays. Int. J. Lab. Hematol. 2011, 33, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kappa, J.R.; Fisher, C.A.; Berkowitz, H.D.; Cottrell, E.D.; Addonizio, V.P. Heparin-induced platelet activation in sixteen surgical patients: Diagnosis and management. J. Vasc. Surg. 1987, 5, 101–109. [Google Scholar] [CrossRef]
- Chong, B.H.; Pilgrim, R.L.; Cooley, M.A.; Chesterman, C.N. Increased expression of platelet IgG Fc receptors in immune heparin-induced thrombocytopenia. Blood 1993, 81, 988–993. [Google Scholar] [PubMed]
- Chong, B.H.; Murray, B.; Berndt, M.C.; Dunlop, L.C.; Brighton, T.; Chesterman, C.N. Plasma P-selectin is increased in thrombotic consumptive platelet disorders. Blood 1994, 83, 1535–1541. [Google Scholar] [PubMed]
- Griffiths, E.; Dzik, W.H. Assays for heparin-induced thrombocytopenia. Transfus. Med. 1997, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arman, M.; Krauel, K. Human platelet IgG Fc receptor FcgammaRIIA in immunity and thrombosis. J. Thromb. Haemost. 2015, 13, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, P.A.; van de Winkel, J.G.; Gosselin, E.J.; Capel, P.J. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32). J. Exp. Med. 1990, 172, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.R.; Stuart, S.G.; Kimberly, R.P.; Ory, P.A.; Goldstein, I.M. A single amino acid distinguishes the high-responder from the low-responder form of Fc receptor II on human monocytes. Eur. J. Immunol. 1991, 21, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, Y.; Kunicki, T.J.; Zipf, T.F.; Ford, S.B.; Aster, R.H. Response of human platelets to activating monoclonal antibodies: Importance of Fc gamma RII (CD32) phenotype and level of expression. Blood 1992, 80, 2261–2268. [Google Scholar] [PubMed]
- Burgess, J.K.; Lindeman, R.; Chesterman, C.N.; Chong, B.H. Single amino acid mutation of Fc gamma receptor is associated with the development of heparin-induced thrombocytopenia. Br. J. Haematol. 1995, 91, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.T.; Isenhart, C.E.; Osborne, J.M.; Ahmed, A.; Anderson, C.L. On the role of platelet Fc gamma RIIa phenotype in heparin-induced thrombocytopenia. Thromb. Haemost. 1995, 74, 1564–1572. [Google Scholar] [PubMed]
- Denomme, G.A.; Warkentin, T.E.; Horsewood, P.; Sheppard, J.A.; Warner, M.N.; Kelton, J.G. Activation of platelets by sera containing IgG1 heparin-dependent antibodies: An explanation for the predominance of the Fc gammaRIIa “low responder” (his131) gene in patients with heparin-induced thrombocytopenia. J. Lab. Clin. Med. 1997, 130, 278–284. [Google Scholar] [CrossRef]
- Norris, C.F.; Pricop, L.; Millard, S.S.; Taylor, S.M.; Surrey, S.; Schwartz, E.; Salmon, J.E.; McKenzie, S.E. A naturally occurring mutation in Fc gamma RIIA: A Q to K127 change confers unique IgG binding properties to the R131 allelic form of the receptor. Blood 1998, 91, 656–662. [Google Scholar] [PubMed]
- Bachelot-Loza, C.; Saffroy, R.; Lasne, D.; Chatellier, G.; Aiach, M.; Rendu, F. Importance of the FcgammaRIIa-Arg/His-131 polymorphism in heparin-induced thrombocytopenia diagnosis. Thromb. Haemost. 1998, 79, 523–528. [Google Scholar] [PubMed]
- Francis, J.L. A critical evaluation of assays for detecting antibodies to the heparin-PF4 complex. Semin. Thromb. Hemost. 2004, 30, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Price, E.A.; Hayward, C.P.M.; Moffat, K.A.; Moore, J.C.; Warkentin, T.E.; Zehnder, J.L. Laboratory testing for heparin-induced thrombocytopenia is inconsistent in North America: A survey of North American specialized coagulation laboratories. Thromb. Haemost. 2007, 98, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Morel-Kopp, M.C.; Tan, C.W.; Brighton, T.A.; McRae, S.; Baker, R.; Tran, H.; Mollee, P.; Kershaw, G.; Joseph, J.; Ward, C.; et al. Validation of whole blood impedance aggregometry as a new diagnostic tool for HIT: Results of a large Australian study. Thromb. Haemost. 2012, 107, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Rollin, J.; Pouplard, C.; Gratacap, M.P.; Leroux, D.; May, M.A.; Aupart, M.; Gouilleux-Gruart, V.; Payrastre, B.; Gruel, Y. Polymorphisms of protein tyrosine phosphatase CD148 influence FcgammaRIIA-dependent platelet activation and the risk of heparin-induced thrombocytopenia. Blood 2012, 120, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Rollin, J.; Pouplard, C.; Sung, H.C.; Leroux, D.; Saada, A.; Gouilleux-Gruart, V.; Thibault, G.; Gruel, Y. Increased risk of thrombosis in FcgammaRIIA 131RR patients with HIT due to defective control of platelet activation by plasma IgG2. Blood 2015, 125, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Cerletti, C.; Harrison, P.; Hayward, C.P.; Kenny, D.; Nugent, D.; Nurden, P.; Rao, A.K.; Schmaier, A.H.; Watson, S.P.; et al. Recommendations for the standardization of light transmission aggregometry: A consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J. Thromb. Haemost. 2013, 11, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Legnani, C.; Cini, M.; Pili, C.; Boggian, O.; Frascaro, M.; Palareti, G. Evaluation of a new automated panel of assays for the detection of anti-PF4/heparin antibodies in patients suspected of having heparin-induced thrombocytopenia. Thromb. Haemost. 2010, 104, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; Arnold, D.M.; Warkentin, T.E.; Warkentin, A.E.; Kelton, J.G. An algorithm for resolving ‘indeterminate’ test results in the platelet serotonin release assay for investigation of heparin-induced thrombocytopenia. J. Thromb. Haemost. 2008, 6, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Poley, S.; Mempel, W. Laboratory diagnosis of heparin-induced thrombocytopenia: Advantages of a functional flow cytometric test in comparison to the heparin-induced platelet-activation test. Eur. J. Haematol. 2001, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Ward, C.M.; Morel-Kopp, M.C. Evaluating heparin-induced thrombocytopenia: The old and the new. Semin. Thromb. Hemost. 2012, 38, 135–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greinacher, A.; Gopinadhan, M.; Gunther, J.U.; Omer-Adam, M.A.; Strobel, U.; Warkentin, T.E.; Papastavrou, G.; Weitschies, W.; Helm, C.A. Close approximation of two platelet factor 4 tetramers by charge neutralization forms the antigens recognized by HIT antibodies. Arterioscler. Thrombo. Vasc. Biol. 2006, 26, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Krauel, K.; Hackbarth, C.; Furll, B.; Greinacher, A. Heparin-induced thrombocytopenia: In vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood 2012, 119, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Levine, M.N.; Hirsh, J.; Horsewood, P.; Roberts, R.S.; Gent, M.; Kelton, J.G. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N. Engl. J. Med. 1995, 332, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Michels, I.; Mueller-Eckhardt, C. Heparin-associated thrombocytopenia: The antibody is not heparin specific. Thromb. Haemost. 1992, 67, 545–549. [Google Scholar] [PubMed]
- Kikta, M.J.; Keller, M.P.; Humphrey, P.W.; Silver, D. Can low molecular weight heparins and heparinoids be safely given to patients with heparin-induced thrombocytopenia syndrome? Surgery 1993, 114, 705–710. [Google Scholar] [PubMed]
- Vun, C.M.; Evans, S.; Chong, B.H. Cross-reactivity study of low molecular weight heparins and heparinoid in heparin-induced thrombocytopenia. Thromb. Res. 1996, 81, 525–532. [Google Scholar] [CrossRef]
- Makhoul, R.G.; Greenberg, C.S.; McCann, R.L. Heparin-associated thrombocytopenia and thrombosis: A serious clinical problem and potential solution. J. Vasc. Surg. 1986, 4, 522–528. [Google Scholar] [CrossRef][Green Version]
- Chong, B.H.; Ismail, F.; Cade, J.; Gallus, A.S.; Gordon, S.; Chesterman, C.N. Heparin-induced thrombocytopenia: Studies with a new low molecular weight heparinoid, Org 10172. Blood 1989, 73, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Feigl, M.; Mueller-Eckhardt, C. Crossreactivity studies between sera of patients with heparin associated thrombocytopenia and a new low molecular weight heparin, reviparin. Thromb. Haemost. 1994, 72, 644–645. [Google Scholar] [PubMed]
- Greinacher, A.; Alban, S.; Omer-Adam, M.A.; Weitschies, W.; Warkentin, T.E. Heparin-induced thrombocytopenia: A stoichiometry-based model to explain the differing immunogenicities of unfractionated heparin, low-molecular-weight heparin, and fondaparinux in different clinical settings. Thromb. Res. 2008, 122, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Rauova, L.; Poncz, M.; McKenzie, S.E.; Reilly, M.P.; Arepally, G.; Weisel, J.W.; Nagaswami, C.; Cines, D.B.; Sachais, B.S. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood 2005, 105, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Potzsch, B.; Amiral, J.; Dummel, V.; Eichner, A.; Mueller-Eckhardt, C. Heparin-associated thrombocytopenia: Isolation of the antibody and characterization of a multimolecular PF4-heparin complex as the major antigen. Thromb. Haemost. 1994, 71, 247–251. [Google Scholar] [PubMed]
- White, M.M.; Siders, L.; Jennings, L.K.; White, F.L. The effect of residual heparin on the interpretation of heparin-induced platelet aggregation in the diagnosis of heparin-associated thrombocytopenia. Thromb. Haemost. 1992, 68, 88. [Google Scholar] [PubMed]
- Potzsch, B.; Keller, M.; Madlener, K.; Muller-Berghaus, G. The use of heparinase improves the specificity of crossreactivity testing in heparin-induced thrombocytopenia. Thromb. Haemost. 1996, 76, 1121. [Google Scholar] [PubMed]
- Socher, I.; Kroll, H.; Jorks, S.; Santoso, S.; Sachs, U.J. Heparin-independent activation of platelets by heparin-induced thrombocytopenia antibodies: A common occurrence. J. Thromb. Haemost. 2008, 6, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Arepally, G.; Reynolds, C.; Tomaski, A.; Amiral, J.; Jawad, A.; Poncz, M.; Cines, D.B. Comparison of PF4/heparin ELISA assay with the 14C-serotonin release assay in the diagnosis of heparin-induced thrombocytopenia. Am. J. Clin. Pathol. 1995, 104, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Prechel, M.M.; McDonald, M.K.; Jeske, W.P.; Messmore, H.L.; Walenga, J.M. Activation of platelets by heparin-induced thrombocytopenia antibodies in the serotonin release assay is not dependent on the presence of heparin. J. Thromb. Haemost. 2005, 3, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Horlait, G.; Minet, V.; Mullier, F.; Michaux, I. Persistent heparin-induced thrombocytopenia: Danaparoid cross-reactivity or delayed-onset heparin-induced thrombocytopenia? A case report. Blood Coagul. Fibrinolysis 2017, 28, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Tardy, B.; Presles, E.; Akrour, M.; de Maistre, E.; Lecompte, T.; Tardy-Poncet, B. Experts’ opinion or the serotonin release assay as a gold standard for the diagnosis of heparin-induced thrombocytopenia (HIT)? J. Thromb. Haemost. 2011, 9, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Sheppard, J.A.; Moore, J.C.; Cook, R.J.; Kelton, J.G. Studies of the immune response in heparin-induced thrombocytopenia. Blood 2009, 113, 4963–4969. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Sheppard, J.I.; Moore, J.C.; Kelton, J.G. The use of well-characterized sera for the assessment of new diagnostic enzyme-immunoassays for the diagnosis of heparin-induced thrombocytopenia. J. Thromb. Haemost. 2010, 8, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Hayward, C.P.; Smith, C.A.; Kelly, P.M.; Kelton, J.G. Determinants of donor platelet variability when testing for heparin-induced thrombocytopenia. J. Lab. Clin. Med. 1992, 120, 371–379. [Google Scholar] [PubMed]
- Arepally, G.M.; Kamei, S.; Park, K.S.; Kamei, K.; Li, Z.Q.; Liu, W.; Siegel, D.L.; Kisiel, W.; Cines, D.B.; Poncz, M. Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood 2000, 95, 1533–1540. [Google Scholar] [PubMed]
- Warkentin, T.E.; Sheppard, J.I. Generation of platelet-derived microparticles and procoagulant activity by heparin-induced thrombocytopenia IgG/serum and other IgG platelet agonists: A comparison with standard platelet agonists. Platelets 1999, 10, 319–326. [Google Scholar] [PubMed]
- Warkentin, T.E.; Sheppard, J.A.; Moore, J.C.; Moore, K.M.; Sigouin, C.S.; Kelton, J.G. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: How much class do we need? J. Lab. Clin. Med. 2005, 146, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Pauzner, R.; Greinacher, A.; Selleng, K.; Althaus, K.; Shenkman, B.; Seligsohn, U. False-positive tests for heparin-induced thrombocytopenia in patients with antiphospholipid syndrome and systemic lupus erythematosus. J. Thromb. Haemost. 2009, 7, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Isenhart, C.E.; Brandt, J.T. Platelet aggregation studies for the diagnosis of heparin-induced thrombocytopenia. Am. J. Clin. Pathol. 1993, 99, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Garritsen, H.S.; Probst-Kepper, M.; Legath, N.; Eberl, W.; Samaniego, S.; Woudenberg, J.; Schuitemaker, J.H.; Kroll, H.; Gurney, D.A.; Moore, G.W.; et al. High sensitivity and specificity of a new functional flow cytometry assay for clinically significant heparin-induced thrombocytopenia antibodies. Int. J. Lab. Hematol. 2014, 36, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. Clinical picture of heparin-induced thrombocytopenia (HIT) and its differentiation from non-HIT thrombocytopenia. Thromb. Haemost. 2016, 116, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Basciano, P.A.; Knopman, J.; Bernstein, R.A. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood 2014, 123, 3651–3654. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Makris, M.; Jay, R.M.; Kelton, J.G. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am. J. Med. 2008, 121, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Kopolovic, I.; Warkentin, T.E. Progressive thrombocytopenia after cardiac surgery in a 67-year-old man. Can. Med. Assoc. J. 2014, 186, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Tvito, A.; Bakchoul, T.; Rowe, J.M.; Greinacher, A.; Ganzel, C. Severe and persistent heparin-induced thrombocytopenia despite fondaparinux treatment. Am. J. Hematol. 2015, 90, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, K.A.; Al-Nasser, S.M.; Bates, S.M.; Patel, A.; Warkentin, T.E.; Arnold, D.M. Delayed-onset HIT caused by low-molecular-weight heparin manifesting during fondaparinux prophylaxis. Am. J. Hematol. 2008, 83, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Pruthi, R.K.; Daniels, P.R.; Nambudiri, G.S.; Warkentin, T.E. Heparin-induced thrombocytopenia (HIT) during postoperative warfarin thromboprophylaxis: A second example of postorthopedic surgery ‘spontaneous’ HIT. J. Thromb. Haemost. 2009, 7, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Jones, C.G.; Bougie, D.W.; Curtis, B.R.; McFarland, J.G.; Wang, D.; Aster, R.H. Heparin-independent, PF4-dependent binding of HIT antibodies to platelets: Implications for HIT pathogenesis. Blood 2015, 125, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Kelton, J.G. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann. Intern. Med. 2001, 135, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.; Attisha, W.K.; Drexler, A.; Francis, J.L. Delayed-onset heparin-induced thrombocytopenia. Ann. Intern. Med. 2002, 136, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Bernstein, R.A. Delayed-onset heparin-induced thrombocytopenia and cerebral thrombosis after a single administration of unfractionated heparin. N. Engl. J. Med. 2003, 348, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.; Carlson, K.B.; DeSancho, M.T. A patient with ‘spontaneous’ heparin-induced thrombocytopenia and thrombosis after undergoing knee replacement. Blood Coagul. Fibrinolysis 2011, 22, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A. Me or not me? The danger of spontaneity. Blood 2014, 123, 3536–3538. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. Fondaparinux: Does it cause HIT? Can it treat HIT? Expert Rev. Hematol. 2010, 3, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Michels, I.; Liebenhoff, U.; Presek, P.; Mueller-Eckhardt, C. Heparin-associated thrombocytopenia: Immune complexes are attached to the platelet membrane by the negative charge of highly sulphated oligosaccharides. Br. J. Haematol. 1993, 84, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Duffau, P.; Seneschal, J.; Nicco, C.; Richez, C.; Lazaro, E.; Douchet, I.; Bordes, C.; Viallard, J.F.; Goulvestre, C.; Pellegrin, J.L.; et al. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2010, 2, 47ra63. [Google Scholar] [CrossRef] [PubMed]
- Bakchoul, T.; Giptner, A.; Bein, G.; Santoso, S.; Sachs, U.J. Performance characteristics of two commercially available IgG-specific immunoassays in the assessment of heparin-induced thrombocytopenia (HIT). Thromb. Res. 2011, 127, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. Heparin-induced thrombocytopenia in the ICU: A transatlantic perspective. Chest J. 2012, 142, 815–816. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. Heparin-induced thrombocytopenia: Pathogenesis and management. Br. J. Haematol. 2003, 121, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Tcherniantchouk, O.; Ley, E.J.; Salim, A.; Mirocha, J.; Martin-Stone, S.; Stolpner, D.; Margulies, D.R. Overdiagnosis of heparin-induced thrombocytopenia in surgical ICU patients. J. Am. Coll. Surg. 2011, 213, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Demma, L.J.; Winkler, A.M.; Levy, J.H. A diagnosis of heparin-induced thrombocytopenia with combined clinical and laboratory methods in cardiothoracic surgical intensive care unit patients. Anesthesia Analg. 2011, 113, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.A.; Cook, D.J.; Albert, M.; Williamson, D.; Meade, M.; Granton, J.; Skrobik, Y.; Langevin, S.; Mehta, S.; Hebert, P.; et al. The 4Ts scoring system for heparin-induced thrombocytopenia in medical-surgical intensive care unit patients. J. Crit. Care 2010, 25, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, N.M.; Hegazy, M.A.; Hassan, E.A.; Ramadan, Y.K.; Nasr, A.S. Egyptian experience of reliability of 4T's score in diagnosis of heparin induced thrombocytopenia syndrome. Blood Coagul. Fibrinolysis 2011, 22, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Arepally, G.; Crowther, M.A.; Rice, L.; Datko, F.; Hook, K.; Propert, K.J.; Kuter, D.J.; Ortel, T.L.; Konkle, B.A.; et al. The HIT Expert Probability (HEP) Score: A novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. J. Thromb. Haemost. 2010, 8, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. Platelet microparticle generation assay for detection of HIT antibodies: Advance, retreat, or too soon to tell? Thromb. Res. 2014, 133, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Sachs, U.J.; von Hesberg, J.; Santoso, S.; Bein, G.; Bakchoul, T. Evaluation of a new nanoparticle-based lateral-flow immunoassay for the exclusion of heparin-induced thrombocytopenia (HIT). Thromb. Haemost. 2011, 106, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A. Clinical and laboratory diagnosis of heparin-induced thrombocytopenia: An integrated approach. Semin. Thromb. Hemost. 2014, 40, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Cines, D.B. How I treat heparin-induced thrombocytopenia. Blood 2012, 119, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Kaywin, P.; Bina, M.; Tomaski, A.; Schreiber, A.D. Heparin associated thrombocytopenia. N. Engl. J. Med. 1980, 303, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Braun, S.; Morath, T.; Mehilli, J.; Vogt, W.; Schomig, A.; Kastrati, A.; von Beckerath, N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 2009, 53, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Caton, S.; O‘Brien, E.; ′annelay, A.J.; Cook, R.G. Assessing the clinical and cost impact of on-demand immunoassay testing for the diagnosis of heparin induced thrombocytopenia. Thromb. Res. 2016, 140, 155–162. [Google Scholar] [CrossRef] [PubMed]
| Pattern | Absence of Added Heparin | Low Concentration(s) of Heparin | High Concentration of Heparin | Monoclonal Ab IV.3 | Potential Causes |
|---|---|---|---|---|---|
| 1 | Neg | Neg | Neg | Neg | No HIT HIT and low platelet reactivity Technical problem |
| 2 | Neg | Pos | Neg | Neg | HIT |
| 3 | Pos | Pos | Neg | Neg | HIT with residual heparin Syndromes of autoimmune HIT: Delayed-onset HIT Persisting HIT Spontaneous HIT syndrome Fondaparinux-associated HIT |
| 4 | Pos | Pos | Neg | Pos | Residual thrombin Very strong HIT |
| 5 | Pos | Pos | Pos | Neg | Heat-aggregated IgG High-titer HLA class I alloantibodies Systemic lupus erythematosus Other platelet-activating factor |
| 6 | Pos | Pos | Pos | Pos | Very strong HIT |
| Assay | Technique/Technology | Endpoint | Platelets Used | Advantages | Limitations |
|---|---|---|---|---|---|
| 14C-SRA | β-counter | 14C-radiolabeled serotonin release from dense granules of activated platelets | Washed platelets (PRP) | High sensitivity High specificity | Time-consuming High technical expertise Radioactivity and specific license Expensive equipment Limited availability |
| EIA-SRA | ELISA | Serotonin release from dense granules of activated platelets | Washed platelets | Endogenous serotonin No radioactive serotonin preloading No special equipment needed Quantitative determination of serotonin | Time-consuming |
| HPLC-SRA | HPLC | Serotonin release from dense granules of activated platelets | Washed platelets | Endogenous serotonin No radioactive serotonin preloading Rapid Quantitative determination of serotonin | High technical expertise Expensive equipment Not widely available |
| FCA-intraplatelet serotonin | Flow cytometer | Loss of intraplatelet content of serotonin from activated platelets | PRP | Rapid Reproducible | High technical expertise Expensive equipment Not widely available |
| HIPA | Visual observation | Visual assessment of platelet aggregation | Washed platelets | High sensitivity High specificity No special equipment needed Repeated evaluation of platelet activation over time Moderate level of expertise required Moderate time consumption | Subjective visual assessment Possible interference with visual interpretation |
| PAT | Aggregometer | Change of light transmittance caused by platelet aggregation | PRP | Largely available equipment in laboratory Easy-to-perform Objective assessment of platelet aggregation Record over time | Low sensitivity Moderate specificity |
| HIMEA | Multiple electrode platelet aggregometry | Changes in impedance caused by platelet aggregation on electrodes | Whole blood | Easy-to-perform Semi-automated No platelet handling and preparation Moderate level of expertise required Rapid Largely available equipment in laboratory | Compatible blood group donor |
| ATP release assay | Lumiaggregometer/Standard scintillation counter | Detection of ATP release from activated platelets | Washed platelets PRP | Easy-to-perform Rapid | Not widely available |
| FCA-membrane GP | Flow cytometer | Expression of platelet activation markers (anionic phospholipids or P-selectin) in platelet population (CD61 or CD41) | PRP | Rapid Cost-effective | Expensive equipment High technical expertise Not widely available |
| PMPGA | Flow cytometer | Generation of PMPs | Washed platelets Whole blood PRP | Rapid Cost-effective | Expensive equipment High technical expertise Not widely available |
| TGA | Fluorometer | Generation of thrombin | PRP | ||
| FcγRIIa proteolysis assay | Western blot/densitometer | Proteolysis of FcγRIIa | Washed platelets | Specific for FcγRIIa-mediated platelet activation | Not widely available |
| DT40-luciferase | Luminometer | Luciferase activity induced by cell activation | Platelet substitutes: chicken B lymphocytes | No need of donor platelets Cell line stored at −80 °C and retrieved as neededEasy-to-perform | Not widely available |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minet, V.; Dogné, J.-M.; Mullier, F. Functional Assays in the Diagnosis of Heparin-Induced Thrombocytopenia: A Review. Molecules 2017, 22, 617. https://doi.org/10.3390/molecules22040617
Minet V, Dogné J-M, Mullier F. Functional Assays in the Diagnosis of Heparin-Induced Thrombocytopenia: A Review. Molecules. 2017; 22(4):617. https://doi.org/10.3390/molecules22040617
Chicago/Turabian StyleMinet, Valentine, Jean-Michel Dogné, and François Mullier. 2017. "Functional Assays in the Diagnosis of Heparin-Induced Thrombocytopenia: A Review" Molecules 22, no. 4: 617. https://doi.org/10.3390/molecules22040617
APA StyleMinet, V., Dogné, J.-M., & Mullier, F. (2017). Functional Assays in the Diagnosis of Heparin-Induced Thrombocytopenia: A Review. Molecules, 22(4), 617. https://doi.org/10.3390/molecules22040617





