Chemical and Biological Properties of S-1-Propenyl-ʟ-Cysteine in Aged Garlic Extract
Abstract
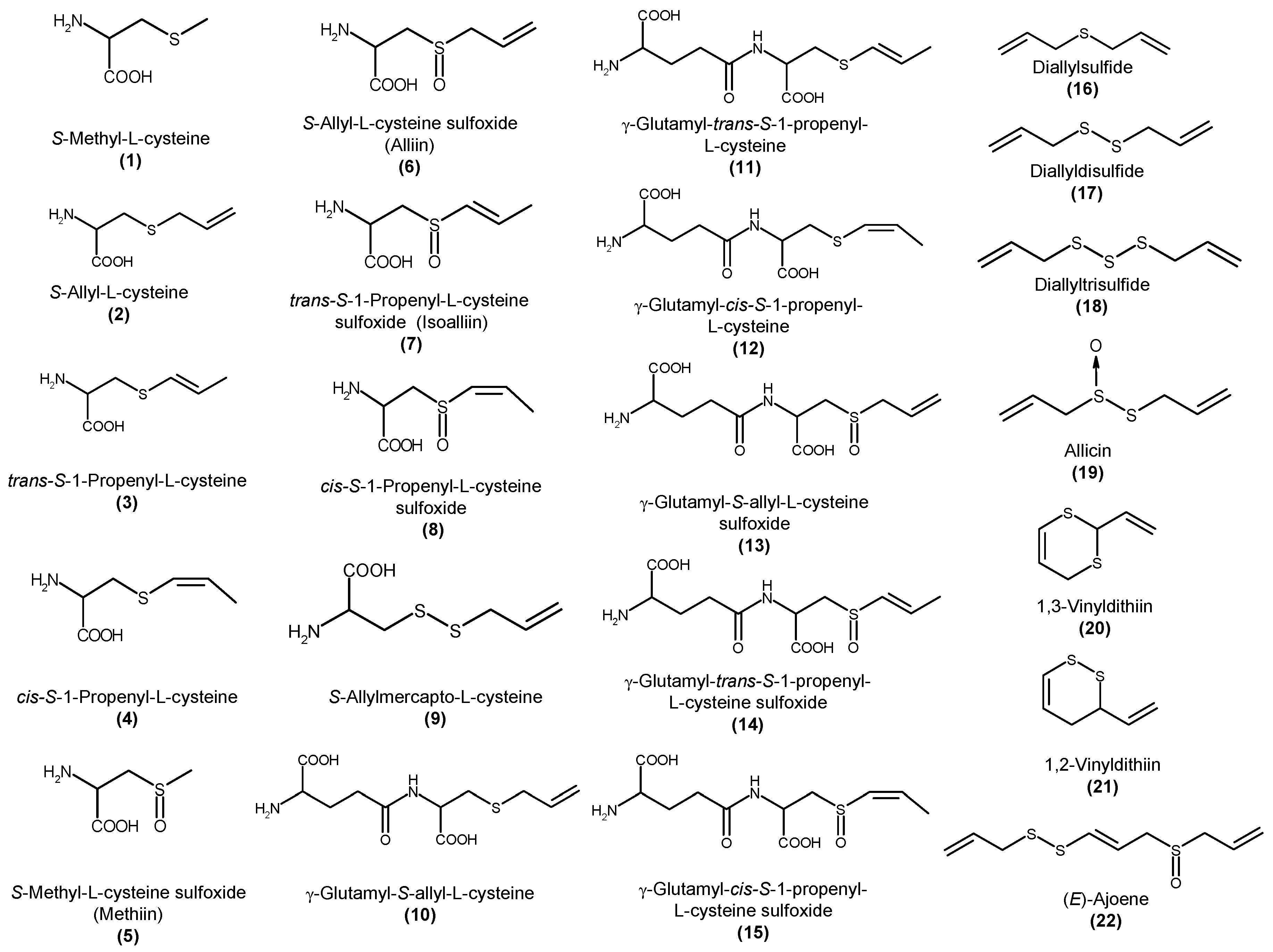
:1. Introduction
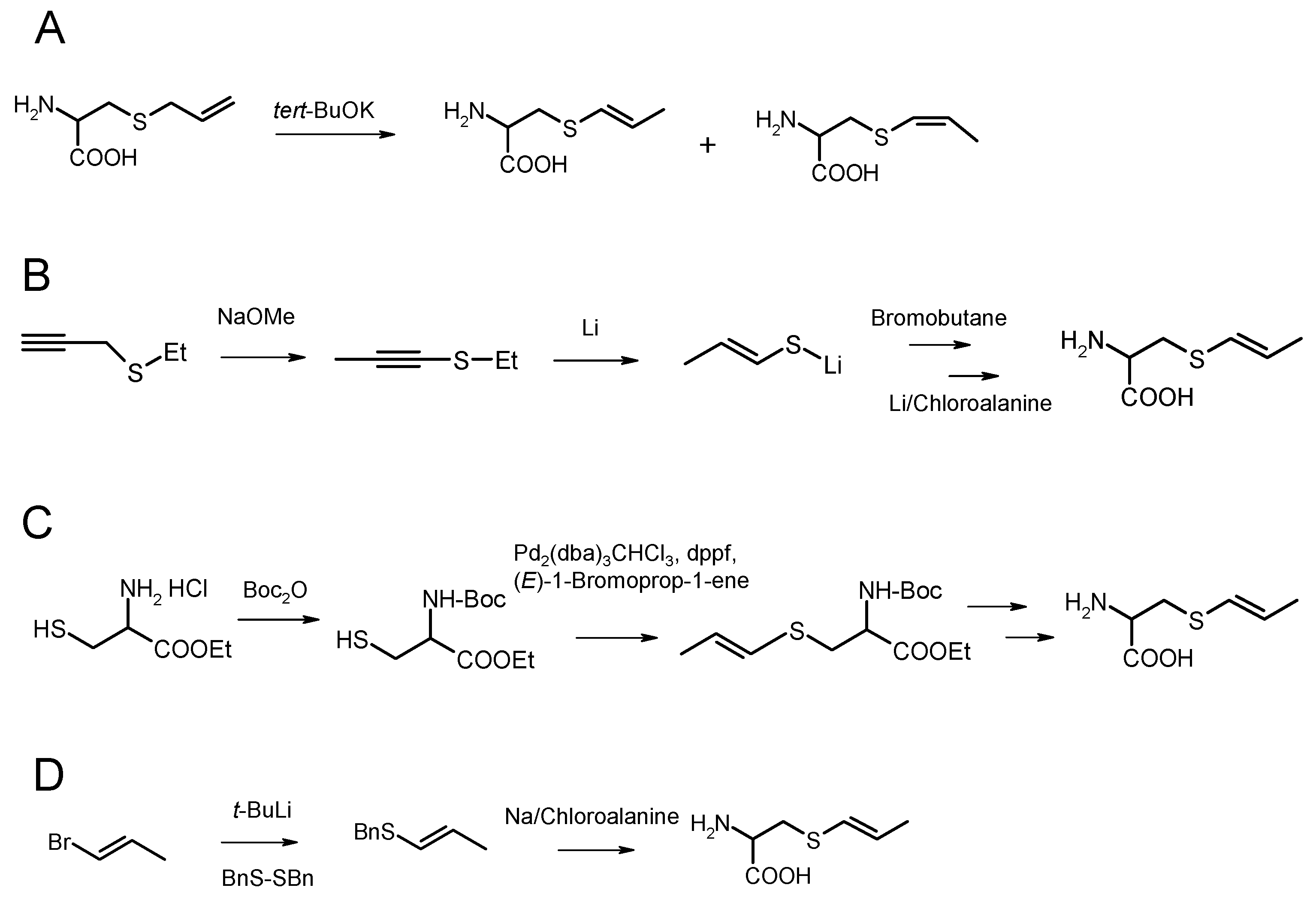
2. Organic Synthesis of trans-S-1-propenyl-l-cysteine
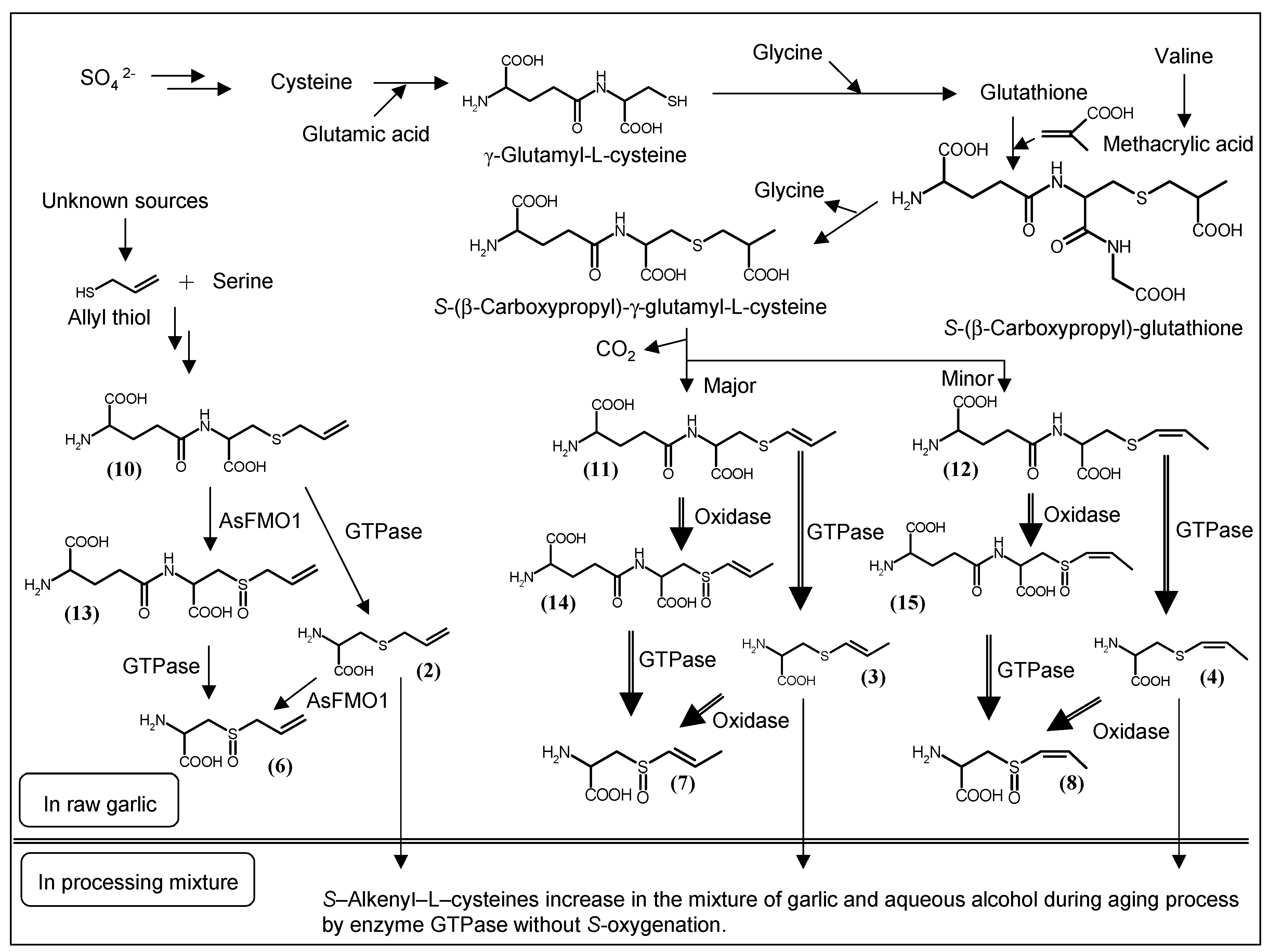
3. Biosynthesis of S-1-propenyl-l-cysteine in Allium Plants
4. Analysis of Sulfur-Containing Compounds in Garlic and Its Preparations
5. Change of S-1-propenyl-l-cysteine Content during Processing
6. Biological Properties of S-1-propenyl-l-cysteine
6.1. Immunomodulation
6.2. Antihypertension
6.3. Pharmacokinetics and Safety
7. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Science and Therapeutic Application of Allium Sativum L and Related Species; Koch, H.P.; Lawson, L.D. (Eds.) Williams & Wilkins: Baltimore, MD, USA, 1996. [Google Scholar]
- Chauhan, N.B. Multiplicity of garlic health effects and Alzheimer’s disease. J. Nutr. Health Aging 2005, 9, 421–432. [Google Scholar] [PubMed]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. The “aged garlic extract”: (AGE) and one of its active ingredients S-allyl-l-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer’s disease (AD). Curr. Med. Chem. 2011, 18, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, R. Historical perspective on the use of garlic. J. Nutr. 2001, 131 (Suppl. 3), 951S–954S. [Google Scholar] [PubMed]
- Garlic and Other Aliums, The Lore and the Science; Block, E. (Ed.) The Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.D.; Wang, Z.J. Pre-hepatic fate of the organosulfur compounds derived from garlic (Allim sativum). Planta Med. 1993, 59, 688A–689A. [Google Scholar] [CrossRef]
- Freeman, F.; Kodera, Y. Garlic chemistry: Stability of S-(2-propyl) 2-propen-1-sulfinothioate (allicin) in blood, solvents, and stimulated physiological fluids. J. Agric. Food Chem. 1995, 43, 2332–2338. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131 (Suppl. 3), 955S–962S. [Google Scholar] [PubMed]
- Colin-Gonzalez, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chanez-Cardenas, M.E.; Santamaria, A.; Maldonado, P.D. The Antioxidant Mechanisms Underlying the Aged Garlic Extract and S-Allylcysteine-Induced Protection. Oxid. Med. Cell Longev. 2012, 2012, 907162. [Google Scholar] [CrossRef] [PubMed]
- Nagae, S.; Ushijima, M.; Hatono, S.; Imai, J.; Kasuga, S.; Matsuura, H.; Itakura, Y.; Higashi, Y. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994, 60, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Kodera, Y.; Suzuki, A.; Imada, O.; Kasuga, S.; Sumioka, I.; Kanezawa, A.; Taru, N.; Fujikawa, M.; Nagae, S.; Masamoto, K.; et al. Physical, chemical, and biological properties of S-allylcysteine, an amino acid derived from garlic. J. Agric. Food. Chem. 2002, 50, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Sugii, M.; Suzuki, T.; Nagasawa, S. Isolation of (–)S-propenyl-l-cysteiene from garlic. Chem. Abstr. 1963, 59, 6509. [Google Scholar]
- Carson, J.F.; Boggs, L.E. The synthesis and base-catalyzed cyslization of (+)– and (–)-cis-S-(1-propenyl)-l-cysteine sulfoxides. J. Org. Chem. 1966, 31, 2862–2864. [Google Scholar] [CrossRef]
- Nishimura, H.; Mizuguchi, A.; Mizutani, J. Stereoselective synthesis of S-(trans-prop-1-enyl)-cysteine sulphoxide. Tet. Lett. 1975, 37, 3201–3202. [Google Scholar] [CrossRef]
- Namyslo, J.C.; Stanitzek, C. A palladium–catalyzed synthesis of isoalliin, the main cysteine sulfoxide in onion (Allim cepa). Synthesis 2006, 20, 3367–3369. [Google Scholar] [CrossRef]
- Lee, A.; Kim, J.N.; Choung, D.H.; Lee, H.K. Facile synthesis of trans-S-1-propenyl-L-cysteine sulfoxide (Isoalliin) in onions (Allium cepa). Bull. Korean Chem. Soc. 2011, 32, 319–320. [Google Scholar] [CrossRef]
- Kodera, Y.; Matsutomo, T.; Itoh, K. The evidence for the production mechanism of cis-S-1-propenylcysteine in aged garlic extract based on a model reaction approach using its isomers and deuterated solvents. Planta Med. Lett. 2015, 2, e69–e72. [Google Scholar] [CrossRef]
- Matsutomo, T.; Kodera, Y. Development of an analytical method for sulfur compounds in aged garlic extract with the use of a postcolumn high performance liquid chromatography method with sulfur-specific detection. J. Nutr. 2016, 146 (Suppl. 2), 450S–455S. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Yamaguchi, T.; Natsutomo, T.; Amano, H.; Morihara, N.; Kodera, Y. S-1-Propenylcysteine promotes the differentiation of B cell into IgA-producing cells by the induction of Erk1/2-dependent Xbp1 expression in Peyer’s patches. Nutrition 2016, 32, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Matsutomo, T.; Ushijima, M.; Kodera, Y.; Nakamoto, M.; Takashima, M.; Morihara, N.; Tamura, K. Metabolomic study on the antihypertensive effect of S-1-propenylcysteine in spontaneously hypertensive rats using liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry. J. Chromatogr. B 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Harauma, A.; Moriguchi, T. Aged garlic extract improves blood pressure in spontaneous hypertensive rats more safely than raw garlic. J. Nutr. 2006, 136 (Suppl. 3), 769S–773S. [Google Scholar] [PubMed]
- Sridharan, K.; Sivaramakrishnan, G. Interaction of Citrus Juices with Cyclosporine: Systematic Review and Meta-Analysis. Eur. J. Drug. Metab. Pharmacokinet. 2016, 41, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Fifer, E.K.; Gardner, Z. Phytochemicals modulators of human drug metabolism: Drug interactions with fruits, vegetables and botanical dietary supplements. In Encyclopedia of Drug Metabolism and Interactions; Wienkers, L., Ed.; John Wiley & Son, Inc.: Hoboken, NJ, USA, 15 May 2012. [Google Scholar]
- Tapaninen, T.; Neuvonen, P.J.; Niemi, M. Grapefruit juice greatly reduces the plasma concentrations of the OATP2B1 and CYP3A4 substrate aliskiren. Clin. Pharmacol. Ther. 2010, 88, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Vaes, L.P.; Chyka, PA. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: Nature of the evidence. Ann. Pharmacother. 2000, 34, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Macan, H.; Uykimpang, R.; Alconcel, M.; Takasu, J.; Razon, R.; Amagase, H.; Niihara, Y. Aged garlic extract may be safe for patients on warfarin therapy. J. Nutr. 2006, 136 (Suppl. 3), 793S–795S. [Google Scholar] [PubMed]
- Amano, H.; Kazamori, D.; Itoh, K. Pharmacokinetics and N-acetylation metabolism of S-methyl-l-cysteine and trans-S-1-propenyl-l-cysteine in rats and dogs. Xenobiotica 2016, 46, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Kazamori, D.; Itoh, K. Evaluation of the effects of S-allyl-l-cysteine, S-methyl-l-cysteine, trans-S-1-propenyl-l-cysteine, and their N-acetylated and S-oxidized metabolites on human CYP activities. Biol. Pharm. Bull. 2016, 39, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Block, E. The organosulfur chemistry of the genus Allium—Implication for the organic chemistry of sulfur. Angew. Chem. Int. Ed. Engl. 1992, 31, 113–1178. [Google Scholar] [CrossRef]
- Lawson, L.D.; Wang, Z.Y.; Hughes, B.G. γ-Glutamyl-S-alklycysteines in garlic and other Allium spp.: Precursor of age-dependent trans-1-propenyl thiosulfinates. J. Nat. Prod. 1991, 54, 436–444. [Google Scholar] [CrossRef]
- Masters, P.M.; Friedman, M. Racemization of amino acids in alkali-treated food proteins. J. Agric. Food Chem. 1979, 27, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Liardon, R.; Hurrell, R.F. Amino acid racemization in heated and alkali-treated proteins. J. Agric. Food Chem. 1983, 31, 432–437. [Google Scholar] [CrossRef]
- Smith, G.G.; Sivakua, T. Mechanism of the racemization of amino acids. Kinetics of racemization of arylglycines. J. Org. Chem. 1983, 48, 627–634. [Google Scholar] [CrossRef]
- Turnbull, A.; Galpin, I.J.; Collin, H.A. Comparison of the onion plant (Allium cepa) and onion tissue culture. III. Feeding of 14C lS1PC has two forms of isomersabeled precursor of the flavor precursor compounds. New Phytol. 1980, 85, 485–487. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Show, M.L. γ-Glutamyl peptides in the biosynthesis of S-alk(en)yl-l-cysteine sulphoxides (flavor precursors) in Allium. Phytochemistry 1989, 28, 455–460. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Onuma, M.; Mizuno, S.; Sugino, Y.; Nakabayashi, R.; Imai, S.; Tsuneyoshi, T.; Sumi, S.; Saito, K. Identification of a flavine-containing S-oxygenating monooxygenase involved in alliin biosynthesis in garlic. Plant J. 2015, 83, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Yabe, A.; Sugino, Y.; Murakami, S.; Sai-Ngam, N.; Sumi, S.; Tsuneyoshi, T.; Saito, K. Garlic γ-glutamyl transpeptidases that catalyze deglutamylation of biosynthetic intermediate of alliin. Front. Plant Sci. 2015, 5, 758. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.J.; Sood, G.R. Investigations of the biosynthesis of trans-(+)-S-1-propenyl-l-cysteine sulfoxide in onions (Allium cepa). J. Am. Chem. Soc. 1989, 111, 4514–4515. [Google Scholar] [CrossRef]
- Parry, R.J.; Lii, F.L. Investigations of the biosynthesis of trans-(+)-S-1-propenyl-l-cysteine sulfoxide. Elucidation of the stereochemistry of the oxidative decarboxylation process. J. Am. Chem. Soc. 1991, 113, 4704–4706. [Google Scholar] [CrossRef]
- Schlaich, N.L. Flavin-containing monooxygenase in plants: Looking beyond detox. Trends Plant Sci. 2007, 12, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.F.; Wong, F.P. The volatile flavor components of onion. J. Agric. Food Chem. 1961, 9, 140–143. [Google Scholar] [CrossRef]
- Brondnitz, M.H.; Pollck, C.L.; Vallon, P.P. Flavor components of onion oil. J. Agric. Food Chem. 1969, 17, 760–763. [Google Scholar] [CrossRef]
- Cai, X.L.; Uden, P.C.; Block, E.; Zhang, X.; Quimby, B.D.; Sullivan, J.J. Allium chemistry: Identification of natural abundance organoselenium volatiles from garlic, elephant garlic, onion, and Chinese chive using headspace gas chromatography with atomic emission detection. J. Agric. Food Chem. 1994, 42, 2081–2084. [Google Scholar] [CrossRef]
- Itakura, Y.; Ichikawa, M.; Mori, Y.; Okino, R.; Udayama, M.; Morita, T. How to distinguish garlic from the other Allium vegetables. J. Nutr. 2001, 131, 963S–967S. [Google Scholar] [PubMed]
- Brodnitz, M.H.; Pascale, J.V.; Derslice, L.V. Flavor components of garlic extract. J. Agric. Food Chem. 1971, 19, 273–275. [Google Scholar] [CrossRef]
- Block, E. Flavor artifacts. J. Agric. Food Chem. 1993, 41, 692. [Google Scholar] [CrossRef]
- Block, E.; Calvey, E.M. Facts and artifacts in Allium chemistry Sulfur Compounds in Foods. In ACS Symposium Series 564; Mussinan, C.L., Keelan, M.E., Eds.; American Chemical Society: Washington, DC, USA, 1994; Chapter 6; pp. 63–79. [Google Scholar]
- Lawson, L.D.; Wang, Z.J.; Hughes, B.G. Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfides in commercial garlic products. Planta Med. 1991, 57, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Tokunaga, T.; Okuno, T. Quantitative determination of eleven flavor precursor (S-alke(en)yl cysteine derivatives) in the garlic with an HPLC method. Nippon Shoyaku Kagaku Kaishi 2005, 52, 160–166. [Google Scholar] [CrossRef]
- Thomas, D.J.; Parkin, K.L. Quantification of alk(en)yl-l-cysteine sulfoxides and related amino acids in Alliums by high-performance liquid chromatography. J. Agric. Food Chem. 1994, 42, 1632–1638. [Google Scholar] [CrossRef]
- Ichikawa, M.; Ide, N.; Yoshida, J.; Yamaguchi, H.; Ono, K. Determination of seven organosulfur compounds in garlic by high-performance liquid chromatography. J. Agric Food Chem. 2006, 54, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Kubec, R.; Dadakova, E. Chromatographic methods for determination of S-substituted cysteine—A comparative study. J. Chromatogr. A 2009, 1216, 6957–6963. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.H.; Wu, C.M.; Rosen, R.T.; Hartman, T.G.; Ho, C.T. Volatile compounds generated from thermal degradation of alliin and deoxyalliin in an aqueous solution. J. Agric. Food Chem. 1994, 42, 146–153. [Google Scholar] [CrossRef]
- Kubec, R.; Svobodova, M.; Velisek, J. Gas chromatographic determination of S-alk(en)ylcysteine sulfoxides. J. Chromatogr. A 1999, 862, 85–94. [Google Scholar] [CrossRef]
- Kubec, R.; Dadakova, E. Quantitative determination of S-alk(en)ylcysteine-S-oxides by micellar electrokinetic capillary chromatography. J. Chromatogr. A 2008, 1212, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Awwad, H.K.; Adelstein, S.J. A quantitative method for the determination of the specific radioactivity of sulfur-containing amino acids separated by paper chromatography. Anal. Biochem. 1966, 16, 433–437. [Google Scholar] [CrossRef]
- Fowler, B.; Robins, A.J. Methods for the quantitative analysis of sulphur–containing compounds in physiological fluids. J. Chromatogr. 1972, 72, 105–111. [Google Scholar] [CrossRef]
- Kawano, S.; Yasui, Y.; Hayashi, M.; Shono, T.; Chone, Y.; Hirai-(Emoto), S.; Aoki, M. Post-column derivatization using hexaiodoplatinate for determination of sulfur-containing amino acids by high performance liquid chromatography. Shimadzu Hyoron 1997, 54, 3–8. [Google Scholar]
- Dirsch, V.M.; Kiemer, A.K.; Wagner, H.; Vollmar, A.M. Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis 1998, 139, 333–339. [Google Scholar] [CrossRef]
- Kang, N.S.; Moon, E.Y.; Cho, C.G.; Pyo, S. Immunomodulating effect of garlic component, allicin, on murine peritoneal macrophages. Nutr. Res. 2001, 21, 617–626. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, X.; Wang, Q.; Jiang, Y.; Shang, H.; Cui, L.; Cao, Y. Allicin enhances host pro–inflammatory immune responses and protects against acute murine malaria infection. Malar. J. 2012, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.P.; Chen, Y.H. Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition 2005, 21, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.P.; Hung, S.Y.; Chen, Y.H. Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. J. Agric. Food Chem. 2005, 53, 2530–2534. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Chen, H.W.; Wang, R.Y.; Lei, Y.P.; Sheen, L.Y.; Lii, C.K. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-kappaB activation in RAW264.7 macrophages. J. Agric. Food Chem. 2006, 54, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabian, S.; Ortuno-sahagun, D.; Vazquez-Carrera, M.; Lopez-Roa, R.I. Alliin, a garlic (Allium sativum) compound, prevents LPS-induced inflammation in 3T3-L1 adipocytes. Mediat. Inflamm. 2013, 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.; Bergman, M.; Bessler, H.; Punsky, I.; Djaldetti, M. Effect of a garlic derivative (Alliin) on peripheral bloodcell immune responses. Int. J. Immunopharm. 1999, 21, 589–597. [Google Scholar] [CrossRef]
- Kyo, E.; Uda, N.; Kasuga, S.; Itakura, Y. Immunomodulatory effects of aged garlic extract. J. Nutr. 2001, 131 (Suppl. 3), 1075S–1079S. [Google Scholar] [PubMed]
- Ishikawa, H.; Saeki, T.; Otani, T.; Suzuki, T.; Shimozuma, K.; Nishino, H.; Fukuda, S.; Morimoto, K. Aged garlic extract prevents a decline of NK number and activity in patients with advanced cancer. J. Nutr. 2006, 136 (Suppl. 3), 816S–820S. [Google Scholar] [PubMed]
- Fallah-Rostami, F.; Tabari, M.A.; Esfandiari, B.; Aghajanzadeh, H.; Behzadi, M.T. Immunomodulatory activity of aged garlic extract against implanted fibrosarcoma tumor in mice. N. Am. J. Med. Sci. 2013, 5, 207–212. [Google Scholar] [PubMed]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanilka, J.M.; Percival, S.S. Supplementation with aged garlic extract improves both NK and γδ–T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind, placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, S.; Honjo, T. Intestinal IgA synthesis: Regulation of front-line body defences. Nat. Rev. Immunol. 2003, 3, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Geuking, M.B.; McCoy, K.D. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012, 33, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Suzuki, K.; Kinoshita, K.; Fagarasan, S. Dynamic interactions between bacteria and immune cells leading to intestinal IgA synthesis. Semin. Immunol. 2008, 20, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.B.; Hayakawa, Y.; Zerafa, N.; Sheehan, K.C.; Scott, B.; Schreiber, R.D.; Hertzog, P.; Smyth, M.J. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 2007, 178, 7540–7549. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Miah, S.M.; Wilson, Z.S.; Erick, T.K.; Banh, C.; Brossay, L. Role of type I interferon receptor signaling on NK cell development and functions. PLoS ONE 2014, 9, e111302. [Google Scholar] [CrossRef] [PubMed]
- Madera, S.; Rapp, M.; Firth, M.A.; Beilke, J.N.; Lanier, L.L.; Sun, J.C. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J. Exp. Med. 2016, 213, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Iwakoshi, N.N.; Pypaert, M.; Glimcher, L.H. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 2007, 204, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Chen, X.; Lee, A.H.; Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010, 11, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mujaj, S.; Gandhi, M.; Vari, F.; Nourse, J. Modulation of the unfolded protein response via XBP1 splicing: A novel mechanism that regulates Natural Killer cell effector function (172.10). J. Immunol. 2012, 188 (Suppl. 1), 172.10. [Google Scholar]
- Zheng, X.; Wang, Y.; Wei, H.; Sun, R.; Tian, Z. LFA-1 and CD2 synergize for the erk1/2 activation in the natural killer (NK) cell immunological synapse. J. Biol. Chem. 2009, 284, 21280–21287. [Google Scholar] [CrossRef] [PubMed]
- Global Health Observatory (GHO) Data: Raised Blood Pressure. Available online: http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/ (accessed on 21 December 2016).
- Sim, J.J.; Bhandari, S.K.; Shi, J.; Reynolds, K.; Calhoun, D.A.; Kalantar-Zadeh, K.; Jacobsen, S.J. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015, 88, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Ordunez, P.; DiPette, D.; Escobar, M.C.; Hassell, T.; Wyss, F.; Hennis, A.; Asma, S.; Angell, S. Improved blood pressure control to reduce cardiovascular disease morbidity and mortality: The standardized hypertension treatment and prevention project. J. Clin. Hypertens. 2016, 18, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Ried, K. Garlic Lowers Blood Pressure in Hypertensive Individuals, Regulates Serum Cholesterol, and Stimulates Immunity: An Updated Meta-analysis and Review. J. Nutr. 2016, 146 (Suppl. 2), 389S–396S. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract reduces blood pressure in hypertensives: A dose-response trial. Eur. J. Clin. Nutr. 2013, 67, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: A randomised controlled trial. Maturitas 2010, 67, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Tsuchikura, S.; Iida, H. Age-related changes in blood pressure, hematological values, concentrations of serum biochemical constituents and weights of organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp. Anim. 2004, 53, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Muller, D.; Pentz, R.; Kress, G.; Siegers, C.-P. Bioavailability of sulphur-containing ingredients of garlic in the rat. Planta Med. 1990, 56, 692–693. [Google Scholar] [CrossRef]
- Lachmann, G.; Lorenz, D.; Radeck, W.; Steiper, M. The pharmacokinetics of the S35 labeled labeled garlic constituents alliin, allicin and vinyldithiine. Arzneimittelforschung 1994, 44, 734–743. [Google Scholar] [PubMed]
- Cruz, C.; Correa-Rotter, R.; Sánchez-González, D.J.; Hernández-Pando, R.; Maldonado, P.D.; Martínez-Martínez, C.M.; Medina-Campos, O.N.; Tapia, E.; Aguilar, D.; Chirino, Y.I.; et al. Renoprotective and antihypertensive effects of S-allylcysteine in 5/6 nephrectomized rats. Am. J. Physiol. Renal Physiol. 2007, 293, F1691–F1698. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; Atkinson, W.; George, P.M.; Chambers, S.T. Sex differences in the control of plasma concentrations and urinary excretion of glycine betaine in patients attending a lipid disorders clinic. Clin. Biochem. 2007, 40, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Ogawa, Y.; Tobise, K.; Kikuchi, K. Mechanism of endothelium-dependent vasorelaxation evoked by lysophosphatidylcholine. Hypertens. Res. 1998, 21, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pushpendran, C.K.; Devasagayam, T.P.; Chintalwar, G.J.; Banerji, A.; Eapen, J. The metabolic fate of (35S)-diallyl disulphide in mice. Experientia 1980, 36, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Pushpendran, C.K.; Eapen, J. Diallyl disulphide induced changes in microsomal enzymes of suckling rats. Indian J. Exp. Biol. 1982, 20, 430–432. [Google Scholar] [PubMed]
- Wang, W.; Tang, J.; Peng, A. The isolation, identification, and bioactivities of selenoproteine in selsenium-rich garlic. Shengwu Huaxue Zazhi 1989, 5, 229–234. [Google Scholar]
- Matsutomo, T.; Ichikawa, M.; Kodera, Y. Pharmoacokinetics of water soluble organosulfur compounds from garlic. J. Clin. Biochem. Nutr. 2008, 45 (Suppl. 1), 100–104. [Google Scholar]
- Amano, H.; Kazamori, D.; Itoh, K.; Kodera, Y. Metabolism, excretion, and pharmacokinetics of S-allyl-l-cysteine in rats and dogs. Drug Metab. Dispos. 2015, 43, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Fifer, K.E.; Gardner, Z. Pharmacokinetic herb–drug interactions (Part 2): Drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta Med. 2012, 78, 1490–1514. [Google Scholar] [CrossRef] [PubMed]




| Metabolite b | Control vs. WKY c | S1PC vs. Control c |
|---|---|---|
| Betaine | ↑↑ d | ↓ |
| Tryptophan | ↓↓ | ↑↑ |
| LysoPC(16:0) | ↓↓ | ↑↑ |
| LysoPC(18:3) | ↓↓ | ↑↑ |
| LysoPC(20:4) | ↓↓ | ↑ |
| 3-(Acetyloxy)-2-hydroxypropyl icosanoate | ↑ | ↓↓ |
| Caproic acid | ↑↑ | ↓ |
| Animal | Compound | Cmax (mg/L) | T1/2 (h) | AUC (mg·h/L) | BA (%) |
|---|---|---|---|---|---|
| Rat | SMC b | 1.3 ± 0.33 | 2.6 ± 0.75 | 6.0 ± 1.7 | 95.8 |
| SAC c | 3.9 ± 0.64 | 1.2 ± 0.19 | 5.1 ± 0.58 | 91.9 | |
| S1PC c | 4.2 ± 0.92 | 0.56 ± 0.072 | 3.2 ± 0.34 | 88.0 | |
| Dog | SMC b | 2.6 ± 0.62 | 8.0 ± 1.1 | 41 ± 5.3 | 95.5 |
| SAC b | 2.1 ± 0.14 | 12 ± 1.2 | 39 ± 3.5 | 92.0 | |
| S1PC b | 2.0 ± 0.22 | 5.6 ± 0.58 | 19 ± 2.4 | 100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodera, Y.; Ushijima, M.; Amano, H.; Suzuki, J.-i.; Matsutomo, T. Chemical and Biological Properties of S-1-Propenyl-ʟ-Cysteine in Aged Garlic Extract. Molecules 2017, 22, 570. https://doi.org/10.3390/molecules22040570
Kodera Y, Ushijima M, Amano H, Suzuki J-i, Matsutomo T. Chemical and Biological Properties of S-1-Propenyl-ʟ-Cysteine in Aged Garlic Extract. Molecules. 2017; 22(4):570. https://doi.org/10.3390/molecules22040570
Chicago/Turabian StyleKodera, Yukihioro, Mitsuyasu Ushijima, Hirotaka Amano, Jun-ichiro Suzuki, and Toshiaki Matsutomo. 2017. "Chemical and Biological Properties of S-1-Propenyl-ʟ-Cysteine in Aged Garlic Extract" Molecules 22, no. 4: 570. https://doi.org/10.3390/molecules22040570
APA StyleKodera, Y., Ushijima, M., Amano, H., Suzuki, J.-i., & Matsutomo, T. (2017). Chemical and Biological Properties of S-1-Propenyl-ʟ-Cysteine in Aged Garlic Extract. Molecules, 22(4), 570. https://doi.org/10.3390/molecules22040570





