Chemical Constituents of Smilax china L. Stems and Their Inhibitory Activities against Glycation, Aldose Reductase, α-Glucosidase, and Lipase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Identification of Isolated Compounds from SCS
2.2. Bioactivity Assay
2.2.1. Inhibition of AGEs Formation and AR Activity
Inhibition of AGEs Formation and AR Activity by Four Smilax Samples
Inhibition of AGEs Formation and AR Activity by SCS Extract and Fractions
Inhibition of AGEs Formation and AR Activity by EAF/NBF-Isolated Compounds
Inhibition of AGEs Formation and AR Activity by SCS Extracts from Six Different Regions of Korea
Quantitative HPLC Analysis of Four Bioactive Compounds from SCS Extracts
2.2.2. α-Glucosidase and Lipase Inhibition Assays
Inhibitory Effect of SCS Extract and Its Fractions on α-Glucosidase and Lipase Activities
Inhibitory Effect of EAF/NBF-Isolated Compounds against α-Glucosidase and Lipase Activities
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction, Fractionation, and Isolation (Scheme 1)
3.4. Component Identification
3.4.1. NMR
3.4.2. UHPLC-ESI/LTQ-Orbitrap-HRMS
3.5. Structure Elucidation
3.6. Biological Evaluation
3.6.1. AGEs Formation Inhibition Assay
3.6.2. AR assay
3.6.3. α-Glucosidase Inhibition Assay
3.6.4. Lipase Inhibition Assay
3.7. Quantitative Analysis by HPLC
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas 2015. Available online: http://www.diabetesatlas.org/ (accessed on 10 December 2016).
- Nathan, D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Yamagishi, S.; Matsui, T.; Nakamura, K.; Imaizumi, T. Role of advanced glycation end products (ages) in thrombogenic abnormalities in diabetes. Curr. Neurovasc. Res. 2006, 3, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.D.; Clements, R.S., Jr.; Winegrad, A.I. Effects of elevated glucose concentrations on the metabolism of the aortic wall. J. Clin. Investig. 1972, 51, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.J.; Korwar, A.M.; Mary, S.; Bhonsle, H.S.; Giri, A.P. Glycated proteome: From reaction to intervention. Proteom. Clin. Appl. 2013, 7, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Lee, I.S.; Jung, S.H.; Lee, Y.M.; Lee, Y.R.; Kim, J.H.; Sun, H.; Kim, J.S. Caffeoylated phenylpropanoid glycosides from brandisia hancei inhibit advanced glycation end product formation and aldose reductase in vitro and vessel dilation in larval zebrafish in vivo. Planta Med. 2013, 79, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Srivastava, S.K. Aldose reductase: Congenial and injurious profiles of an enigmatic enzyme. Biochem. Med. Metab. Biol. 1992, 48, 91–121. [Google Scholar] [CrossRef]
- Kawanishi, K.; Ueda, H.; Moriyasu, M. Aldose reductase inhibitors from the nature. Curr. Med. Chem. 2003, 10, 1353–1374. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Choi, S.H.; Moon, H.E.; Park, J.J.; Jung, H.A.; Woo, M.H.; Woo, H.C.; Choi, J.S. The inhibitory activities of the edible green alga capsosiphon fulvescens on rat lens aldose reductase and advanced glycation end products formation. Eur. J. Nutr. 2014, 53, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.-P.; Song, C.-Q.; Yuan, P.; Mao, R.-G. A-glucosidase and α-amylase inhibitory activity of common constituents from traditional chinese medicine used for diabetes mellitus. Chin. J. Nat. Med. 2010, 8, 349–352. [Google Scholar] [CrossRef]
- Seghrouchni, I.; Drai, J.; Bannier, E.; Rivière, J.; Calmard, P.; Garcia, I.; Orgiazzi, J.; Revol, A. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. Clin. Chim. Acta 2002, 321, 89–96. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Hiroyuki, F.; Tomohide, Y.; Kazunori, O. Efficacy and safety of touchi extract, an alpha-glucosidase inhibitor derived from fermented soybeans, in non-insulin-dependent diabetic mellitus. J. Nutr. Biochem. 2001, 12, 351–356. [Google Scholar] [CrossRef]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two alpha-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Ramirez, G.; Zamilpa, A.; Zavala, M.; Perez, J.; Morales, D.; Tortoriello, J. Chrysoeriol and other polyphenols from tecoma stans with lipase inhibitory activity. J. Ethnopharmacol. 2016, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Kim, Y.J.; Jung, S.-H.; Kim, J.-H.; Kim, J.S. Flavonoids from litsea japonica inhibit ages formation and rat lense aldose reductase in vitro and vessel dilation in zebrafish. Planta Med. 2017, 83, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Nisar, M.; Ebad, F.; Nadeem, S.; Saeed, M.; Khan, H.; Samiullah; Khuda, F.; Karim, N.; Ahmad, Z. Anti-inflammatory activities of sieboldogenin from Smilax china linn.: Experimental and computational studies. J. Ethnopharmacol. 2009, 121, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-K.; Lee, J.-H.; Kim, H.-S.; Lee, C.-K.; Lee, S.-C. Antioxidant and antimicrobial activities of Smilax china L. Leaf extracts. Food Sci. Biotechnol. 2012, 21, 1723–1727. [Google Scholar] [CrossRef]
- Chen, L.; Yin, H.; Lan, Z.; Ma, S.; Zhang, C.; Yang, Z.; Li, P.; Lin, B. Anti-hyperuricemic and nephroprotective effects of Smilax china L. J. Ethnopharmacol. 2011, 135, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Lu, Z.-Q.; Chen, G.-T.; Zhang, J.-Q.; Wang, W.; Yang, M.; Guo, D.-A. Phenylpropanoid-substituted catechins and epicatechins from Smilax china. Helv. Chim. Acta 2007, 90, 1751–1757. [Google Scholar] [CrossRef]
- Li, Y.L.; Gan, G.P.; Zhang, H.Z.; Wu, H.Z.; Li, C.L.; Huang, Y.P.; Liu, Y.W.; Liu, J.W. A flavonoid glycoside isolated from Smilax china L. Rhizome in vitro anticancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.S.; Gao, Z.H.; Yang, X.L. Anti-inflammatory and anti-nociceptive activities of Smilax china L. Aqueous extract. J. Ethnopharmacol. 2006, 103, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.L. A study of the anti-inflammatory components of Smilax china. Herald Med. 2005, 24, 670–672. [Google Scholar]
- Zhao, B.T.; Le, D.D.; Nguyen, P.H.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Shin, H.M.; Rhee, H.I.; Woo, M.H. Ptp1b, α-glucosidase, and dpp-iv inhibitory effects for chromene derivatives from the leaves of Smilax china L. Chem. Biol. Interact. 2016, 253, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Lee, Y.K.; Kim, S.D.; Cheng, J.; Yang, S.H.; Suh, J.W. Xanthine and aldehyde oxidase inhibitory activities, and antihyperuricemic effects of fermented Smilax china L. Leaf extracts and fractions. J. Appl. Biol. Chem. 2014, 57, 53–59. [Google Scholar] [CrossRef]
- Shao, B.; Guo, H.Z.; Cui, Y.J.; Liu, A.H.; Yu, H.L.; Guo, H.; Xu, M.; Guo, D.A. Simultaneous determination of six major stilbenes and flavonoids in Smilax china by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2007, 44, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.Y.; Park, J.R.; Kim, J.H. Electron donating abilities, nitrite scavenging effects and antimicrobial activities of Smilax china leaf. J. Korean Soc. Food Sci. Nutr. 2004, 33, 621–625. [Google Scholar]
- Kim, H.Y.; Oh, J.H. Screening of Korean forest plants for rat lens aldose reductase inhibition. Biosci. Biotechnol. Biochem. 1999, 63, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Whang, W.K. Pharmaceutical Compositions Comprising an Extract from Smilax china Linne for Preventing or Treating Obesity, Hyperlipidemia or Fatty Liver. Google Patents 10-1225486, 17 January 2013. [Google Scholar]
- Lee, J.Y.; Lee, S.Y. Cosmetic Composition Containing Smilax china L. Stem Extracts. KR2014126205A, 30 October 2014. [Google Scholar]
- Mazumder, P.M.; Rathinavelusamy, P.; Sasmal, D. Role of antioxidants in phytomedicine with special reference to antidiabetic herbs. Asian Pac. J. Trop. Dis. 2012, 2, S969–S979. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.S.; Kim, H.P.; Lee, J.-H.; Kang, S.S. Phenolic constituents from the flower buds of lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar] [CrossRef]
- Nakatani, N.; Kayano, S.I.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food. Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, H.K.; Linthorst, H.J.M.; Hollander, J.G.; Lefeber, A.W.M.; Erkelens, C.; Nuzillard, J.M.; Verpoorte, R. Nmr metabolomics to revisit the tobacco mosaic virus infection in nicotiana tabacum leaves. J. Nat. Prod. 2006, 69, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Bajko, E.; Kalinowska, M.; Borowski, P.; Siergiejczyk, L.; Lewandowski, W. 5-o-caffeoylquinic acid: A spectroscopic study and biological screening for antimicrobial activity. LWT Food Sci. Technol. 2016, 65, 471–479. [Google Scholar] [CrossRef]
- Jung, H.A.; Islam, M.D.N.; Kwon, Y.S.; Jin, S.E.; Son, Y.K.; Park, J.J.; Sohn, H.S.; Choi, J.S. Extraction and identification of three major aldose reductase inhibitors from artemisia montana. Food Chem. Toxicol. 2011, 49, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Luan, G. Separation and purification of five flavone glucosides and one lignan from caragana korshinskii kom. By the combination of hsccc and semi-preparative rplc. Chromatographia 2016, 79, 823–831. [Google Scholar] [CrossRef]
- Pauli, G.F. Higher order and substituent chemical shift effects in the proton nmr of glycosides. J. Nat. Prod. 2000, 63, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Özden, S.; Dürüst, N.; Toki, K.; Saito, N.; Honda, T. Acylated kaempferol glycosides from the flowers of delphinium formosum. Phytochemistry 1998, 49, 241–245. [Google Scholar] [CrossRef]
- Fukunaga, T.; Nishiya, K.; Kajikawa, I.; Watanabe, Y.; Suzuki, N.; Takeya, K.; Itokawa, H. Chemical studies on the constituents of hyphear tanakae hosokawa from different host trees. Chem. Pharm. Bull. (Tokyo) 1988, 36, 1180–1184. [Google Scholar] [CrossRef]
- Sarg, T.M.; Hafez, S.S.; Dora, G.A.; Mohamed, S.A. Phytochemical and biological studies of Cynara cardunculus L. Egypt J. Biomed. Sci. 2004, 15, 339–354. [Google Scholar]
- Commodari, F.; Khiat, A.; Ibrahimi, S.; Brizius, A.R.; Kalkstein, N. Comparison of the phytoestrogen trans-resveratrol (3,4′,5-trihydroxystilbene) structures from X-ray diffraction and solution nmr. Magn. Reson. Chem. 2005, 43, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Khaligh, P.; Salehi, P.; Farimani, M.M.; Ali-Asgari, S.; Esmaeili, M.A.; Nejad Ebrahimi, S. Bioactive compounds from smilax excelsa l. J. Iranian Chem. Soc. 2016, 13, 1055–1059. [Google Scholar] [CrossRef]
- Nakano, K.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. Feruloyl sucrose derivatives from heloniopsis orientalis. Chem. Pharm. Bull. 1986, 34, 5005–5010. [Google Scholar]
- Panda, P.; Appalashetti, M.; Natarajan, M.; Mary, C.-P.; Venkatraman, S.S.; Judeh, Z.M.A. Synthesis and antiproliferative activity of helonioside a, 3′,4′,6′-tri-o-feruloylsucrose, lapathoside c and their analogs. Eur. J. Med. Chem. 2012, 58, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Gao, W.; Zhang, Y.; Wang, Y. A new phenylpropanoid glycosides from Paris polyphylla var. Yunnanensis. Fitoterapia 2008, 79, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Zhu, N.; Lin-Shiau, S.-Y.; Lin, J.-K.; Ho, C.-T. Antioxidant activity of flavanols and flavonoid glycosides in oolong tea. ACS Symp. Ser. 2002, 803, 292–303. [Google Scholar]
- Kuo, Y.-H.; Hsu, Y.-W.; Liaw, C.-C.; Lee, J.K.; Huang, H.-C.; Kuo, L.-M.Y. Cytotoxic phenylpropanoid glycosides from the stems of Smilax china. J. Nat. Prod. 2005, 68, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- CrascÌ, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs. metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2016, in press. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem. Pharm. Bull. 2002, 50, 788–795. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Song, S.; Zou, Z.; Feng, M.; Wang, D.; Wang, Y.; Li, X.; Ye, X. The hypoglycemic and synergistic effect of loganin, morroniside, and ursolic acid isolated from the fruits of cornus officinalis. Phytother. Res. 2016, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Camp, J.V. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Yamashita, H.; Hiemori, M.; Inagaki, E.; Kimoto, M.; Okamoto, M.; Tsuji, H.; Memon, A.N.; Mohammadi, A.; Natori, Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of nerium indicum. J. Nutr. Sci. Vitaminol. (Tokyo) 2007, 53, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Karamac, M.; Amarowicz, R. Inhibition of pancreatic lipase by phenolic acids-examination in vitro. Z. Naturforsch. C 1996, 51, 903–905. [Google Scholar] [PubMed]
- Chang, Y.X.; Ge, A.H.; Jiang, Y.; Teye Azietaku, J.; Li, J.; Gao, X.M. A bioactivity-based method for screening, identification of lipase inhibitors, and clarifying the effects of processing time on lipase inhibitory activity of polygonum multiflorum. Evid. Based Complement. Altern. Med. 2016, 2016, 5965067. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Asker, M.; Amin, R.; Eissa, R. Anti-obesity effect of resveratrol in rats on high fat diet through regulation of gene expression of visceral white adipose tissue. Int. J. Pharm. Pharm. Sci. 2016, 8, 378–384. [Google Scholar]
- Zhang, C.; Luo, J.; Yu, B.; Chen, J.; Chen, D. Effects of resveratrol on lipid metabolism in muscle and adipose tissues: A reevaluation in a pig model. J. Funct. Foods 2015, 14, 590–595. [Google Scholar] [CrossRef]
- Nakai, M.; Fukui, Y.; Asami, S.; Toyoda-Ono, Y.; Iwashita, T.; Shibata, H.; Mitsunaga, T.; Hashimoto, F.; Kiso, Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J. Agric. Food. Chem. 2005, 53, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Aradate, T.; Sasaki, C.; Kojima, H.; Ohara, M.; Hasegawa, J.; Ubukata, M. Screening system for the maillard reaction inhibitor from natural product extracts. J. Health Sci. 2002, 48, 520–526. [Google Scholar] [CrossRef]
- Sato, S.; Kador, P.F. Inhibition of aldehyde reductase by aldose reductase inhibitors. Biochem. Pharmacol. 1990, 40, 1033–1042. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-D’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
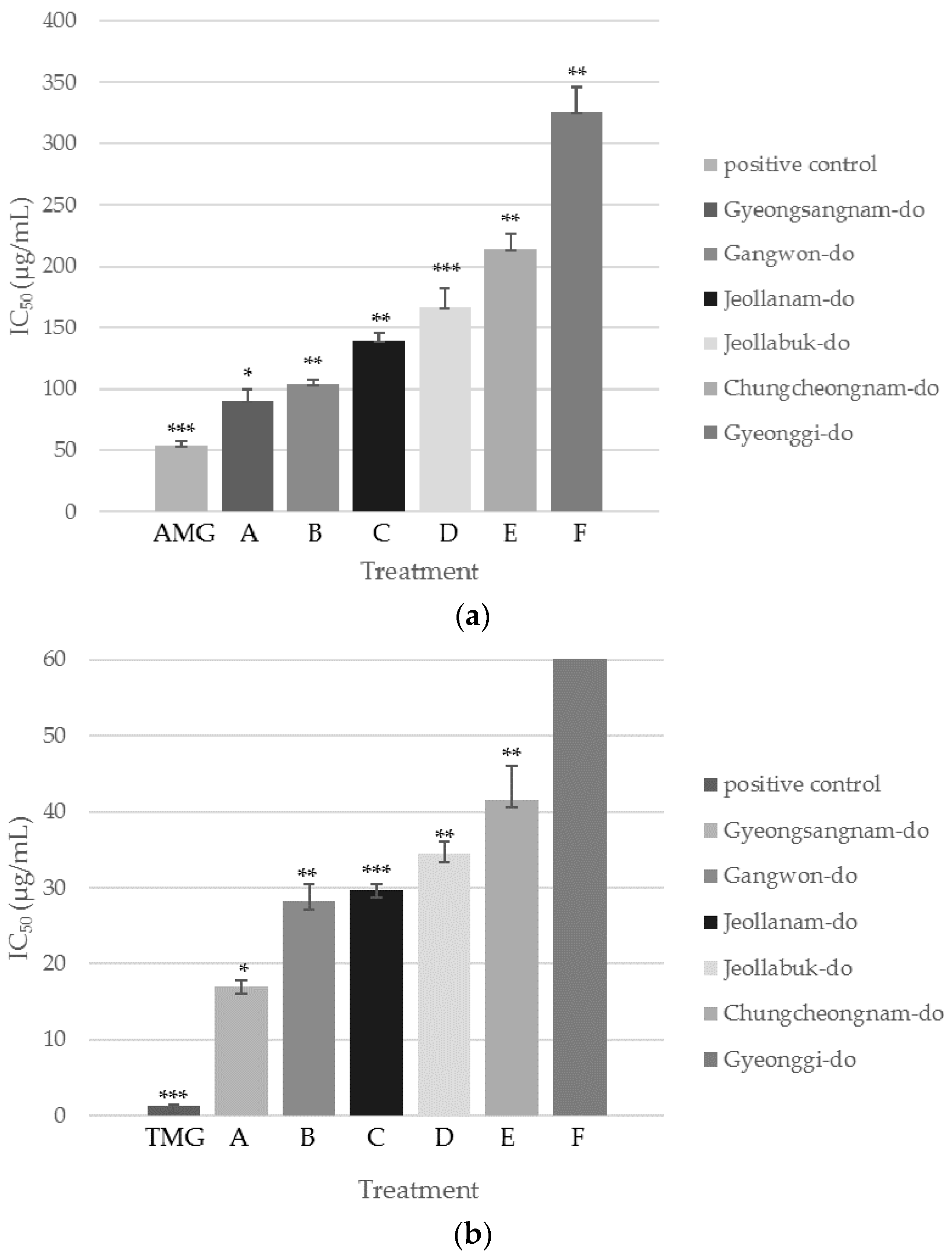
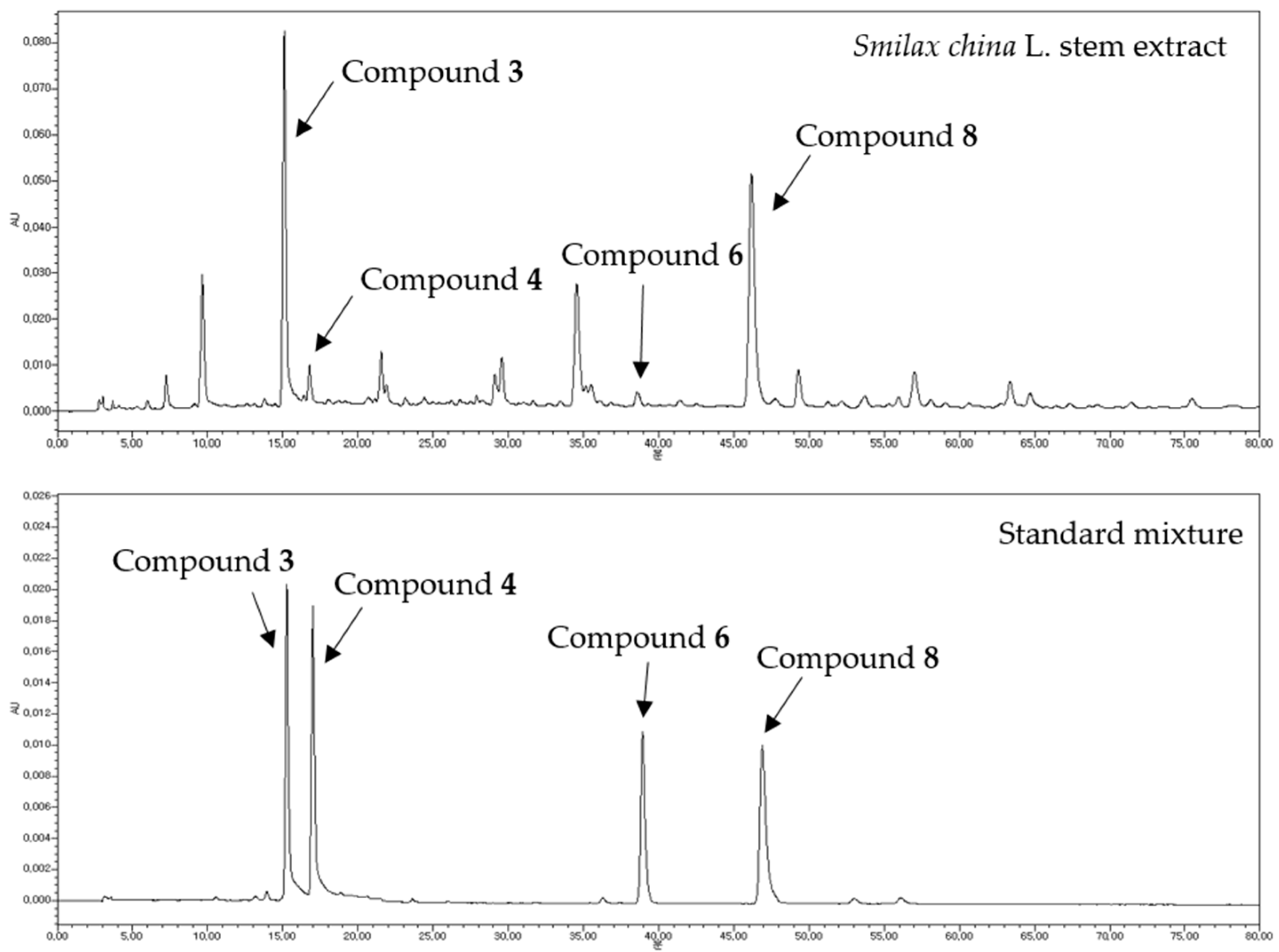
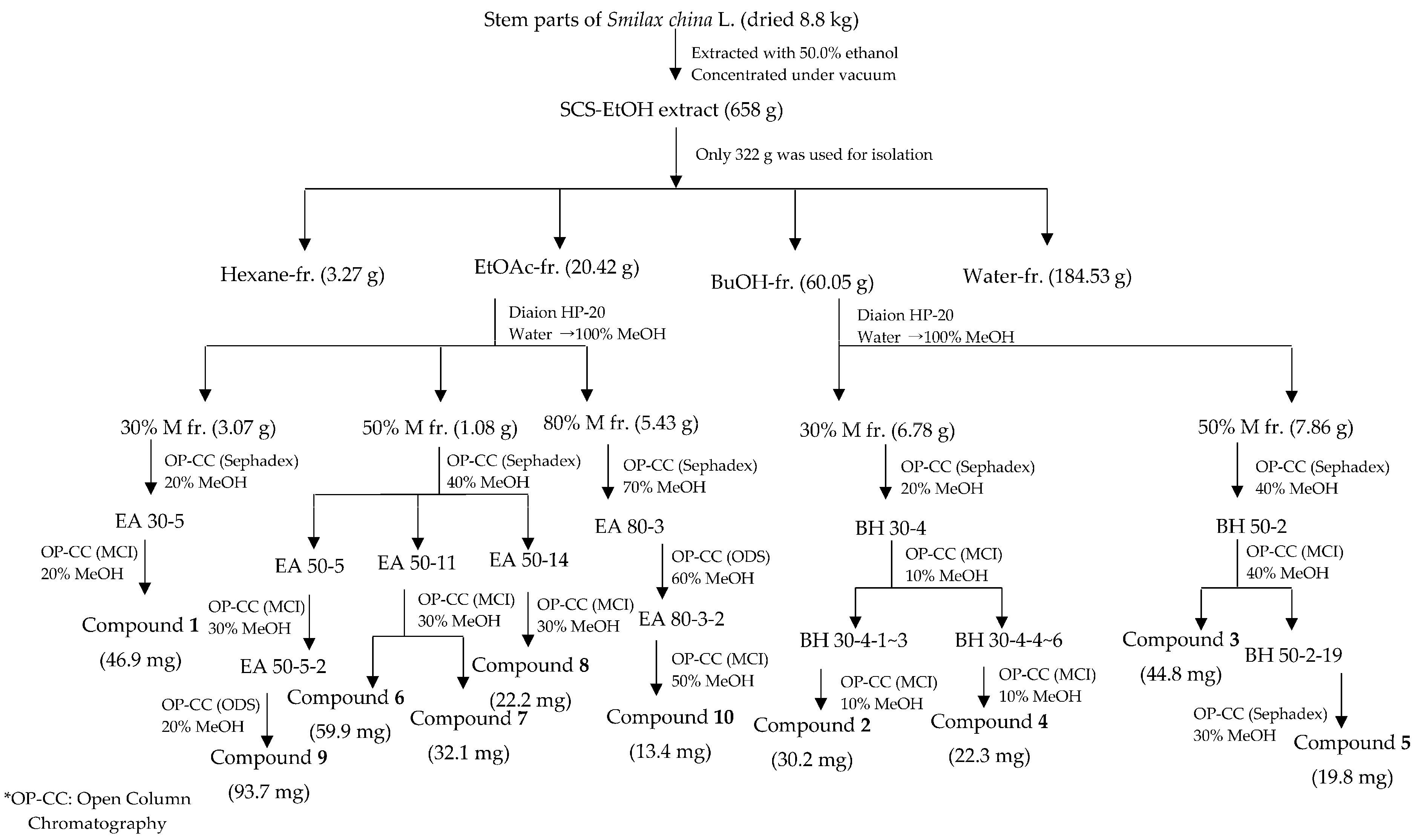

| Test Materials | AGEs | Aldose Reductase |
|---|---|---|
| IC50 (μg/mL) | IC50 (μg/mL) | |
| Smilax china L. | 145 ± 8 ** | 37.0 ± 4.6 ** |
| Smilax sieboldii | 366 ± 29 *** | 80.8 ± 6.3 ** |
| Smilax riparia var. ussuriensis | 413 ± 17 *** | 41.6 ± 1.8 ** |
| Smilax ferox Wall. ex Kunth | 186 ± 11 ** | 37.0 ± 1.7 ** |
| AMG a | 54.8 ± 2.1 *** | - |
| TMG b | - | 1.30 ± 0.10 *** |
| Test Materials | AGEs | Aldose Reductase | α-Glucosidase | Lipase |
|---|---|---|---|---|
| IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | IC50 (mg/mL) | |
| SCS extract | 145 ± 8 ** | 37.0 ± 4.6 ** | 51.7 ± 2.1 ** | 2.75 ± 0.22 ** |
| Hexane fraction | ND | 42.1 ± 1.1 ** | 41.6 ± 4.5 ** | 0.10 ± 0.02 * |
| EAF | 40.5 ± 4.7 * | 10.4 ± 0.3 ** | 9.48 ± 0.37 *** | 0.14 ± 0.01 ** |
| NBF | 140 ± 18 * | 39.0 ± 3.6 *** | 33.8 ± 2.4 | 0.44 ± 0.03 ** |
| Water fraction | 410 ± 16 *** | ND | 56.7 ± 1.1 * | 4.12 ± 0.25 ** |
| AMG a | 54.8 ± 2.1 *** | - | - | - |
| TMG b | - | 1.30 ± 0.10 *** | - | - |
| Acarbose c | - | - | 63.5 ± 1.9 *** | - |
| Orlistat d | - | - | - | 0.001 * |
| Test Materials | AGEs | Aldose Reductase | α-Glucosidase | Lipase |
|---|---|---|---|---|
| IC50 (µM) | IC50 (µM) | IC50 (µM) | IC50 (µM) | |
| Protocatechuic acid (1) | 418 ± 10 * | ND | 176 ± 7 | 22.9 ± 0.8 *** |
| 5-O-Caffeoylquinic acid (2) | 215 ± 18 *** | 87.5 ± 1.6 *** | 389 ± 14 | 474 ± 35 ** |
| 3-O-Caffeoylquinic acid (3) | 80.5 ± 5.5 ** | 0.60 ± 0.03 ** | 608 ± 5 *** | >1000 |
| 4-O-Caffeoylquinic acid (4) | 74.1 ± 3.1 ** | 20.1 ± 0.1 *** | 736 ± 15 | >1000 |
| Kaempferol 3-O-β-d-glucopyranosyl-7-O-α-l-rhamnopyranoside (5) | 193 ± 9 ** | 9.38 ± 1.46 | 609 ± 32 *** | >1000 |
| Quercitrin (6) | 58.0 ± 2.5 ** | 0.56 ± 0.14 ** | 135 ± 13 * | 96.7 ± 7.0 ** |
| Afzelin (7) | 75.4 ± 3.8 ** | 87.9 ± 2.1 *** | 31.7 ± 1.6 ** | 572 ± 102 * |
| trans-Resveratrol (8) | 148 ± 14 *** | 60.5 ± 16.8 * | 5.97 ± 0.20 ** | 61.2 ± 11.7 * |
| Helonioside A (9) | 227 ± 16 ** | 34.5 ± 0.1 *** | 154 ± 4 | >1000 |
| Isoscutellarein-8-O-rhamnoside (10) | 440 ± 17 * | 17.0 ± 3.6 | 45.1 ± 2.6 ** | 101 ± 18 * |
| AMG a | 515 ± 33 *** | - | - | - |
| TMG b | - | 6.97 ± 0.56 *** | - | - |
| Acarbose c | - | - | 172 ± 17 ** | - |
| Orlistat d | - | - | - | 1.40 ± 0.12 * |
| Sample No. | Contents (µg/ext. g) | |||
|---|---|---|---|---|
| Compound 3 | Compound 4 | Compound 6 | Compound 8 | |
| A | 18.3 ± 0.1 | 1.15 ± 0.03 | 0.64 ± 0.02 | 0.049 ± 0.003 |
| B | 14.3 ± 0.8 | 0.68 ± 0.01 | 0.40 ± 0.06 | 1.90 ± 0.10 |
| C | 8.00 ± 1.08 | 1.01 ± 0.01 | 0.30 ± 0.02 | 0.90 ± 0.03 |
| D | 6.12 ± 0.26 | 0.62 ± 0.01 | 0.19 ± 0.01 | 1.83 ± 0.12 |
| E | 5.96 ± 0.61 | 0.36 ± 0.01 | 0.014 ± 0.005 | 0.30 ± 0.03 |
| F | 1.79 ± 0.08 | 0.32 ± 0.01 | 0.041 ± 0.019 | 0.44 ± 0.03 |
| Compound name | Rt (min) | Formula | Mass Mode | Theoretical Mass | Observed Mass | Mass Error (Da) | Mass Accuracy (ppm) |
|---|---|---|---|---|---|---|---|
| Protocatechuic acid (1) | 3.44 | C7H6O4 | N | 153.01824 | 153.01837 | 0.00013 | 0.8 |
| 5-O-Caffeoylquinic acid (2) | 5.03 | C16H18O9 | N | 353.08671 | 353.08804 | 0.00133 | 3.8 |
| 3-O-Caffeoylquinic acid (3) | 6.93 | C16H18O9 | N | 353.08671 | 353.08820 | 0.00149 | 4.2 |
| 4-O-Caffeoylquinic acid (4) | 7.34 | C16H18O9 | N | 353.08671 | 353.08838 | 0.00167 | 4.7 |
| Kaempferol 3-O-β-d-glucopyranosyl-7-O-α-l-rhamnopyranoside (5) | 9.71 | C27H30O15 | N | 593.15010 | 593.15179 | 0.00169 | 2.8 |
| Quercitrin (6) | 10.81 | C21H20O11 | N | 447.09219 | 447.09372 | 0.00153 | 3.4 |
| Afzelin (7) | 12.12 | C21H20O10 | N | 431.09727 | 431.09848 | 0.00121 | 2.8 |
| trans-Resveratrol (8) | 11.65 | C14H12O3 | P | 229.08592 | 229.08633 | 0.00041 | 1.8 |
| Helonioside A (9) | 12.06 | C32H38O17 | N | 693.20253 | 693.20441 | 0.00188 | 2.7 |
| Isoscutellarein-8-O-rhamnoside (10) | 13.08 | C21H20O10 | N | 431.09727 | 431.09845 | 0.00118 | 2.7 |
| Compound Number | Rt (min) | Regression Equation | r2 | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| 3 | 15.479 | y = (2.0 × 106)x + 13710 | 1.0000 | 1 - 250 | 0.96 | 2.92 |
| 4 | 17.416 | y = (2.0 × 106)x − 16531 | 1.0000 | 1 - 500 | 0.80 | 2.42 |
| 6 | 38.645 | y = (2.0 × 106)x + 26229 | 0.9997 | 1 - 500 | 2.78 | 8.44 |
| 8 | 45.640 | y = (7.0 × 106)x + 26716 | 0.9998 | 1 - 500 | 0.77 | 2.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.E.; Kim, J.A.; Whang, W.K. Chemical Constituents of Smilax china L. Stems and Their Inhibitory Activities against Glycation, Aldose Reductase, α-Glucosidase, and Lipase. Molecules 2017, 22, 451. https://doi.org/10.3390/molecules22030451
Lee HE, Kim JA, Whang WK. Chemical Constituents of Smilax china L. Stems and Their Inhibitory Activities against Glycation, Aldose Reductase, α-Glucosidase, and Lipase. Molecules. 2017; 22(3):451. https://doi.org/10.3390/molecules22030451
Chicago/Turabian StyleLee, Hee Eun, Jin Ah Kim, and Wan Kyunn Whang. 2017. "Chemical Constituents of Smilax china L. Stems and Their Inhibitory Activities against Glycation, Aldose Reductase, α-Glucosidase, and Lipase" Molecules 22, no. 3: 451. https://doi.org/10.3390/molecules22030451
APA StyleLee, H. E., Kim, J. A., & Whang, W. K. (2017). Chemical Constituents of Smilax china L. Stems and Their Inhibitory Activities against Glycation, Aldose Reductase, α-Glucosidase, and Lipase. Molecules, 22(3), 451. https://doi.org/10.3390/molecules22030451






