1. Introduction
The technology of liquid chromatography–tandem mass spectrometry (LC–MS) has both qualitative and quantitative uses for classifying the chemical components in complex chemical mixtures [
1]. These uses include identifying constituents and determining the structure of a compound by analyzing its fragmentation [
2]. Additionally, MS ion fragments provide abundant structural information. In recent years, LC–MS has been widely used in traditional Chinese medicine (TCM) research because of its high selectivity, high sensitivity, and ability to generate specific information, including cognate molecular mass and structural characteristics [
3]. TCM medicinal preparations generally contain a single herb or a mixture of two or more different types of medicinal herbs [
4]. In addition, the complex ingredients of TCM could promote their clinical effects and security; therefore, separation and analysis of those chemical compositions are vital for the modernization of TCM [
5]. Consequently, effective and specific methods for identifying multiple constituents of TCM have been developed.
For over a thousand years, TCM has held a critical role in treating various conditions in Asia. The worldwide population and the cost of traditional Chinese medicine therapy have grown dramatically in recent years [
6,
7]. Herbal products are composed of complex mixtures of organic materials that may come from some raw or processed parts of a plant, including the leaves, stems, flowers, roots, and seeds [
8]. Nevertheless, quality controls for these herbal products still rarely demonstrate batch-to-batch production consistency, even for products from the same industrial pharmaceutical company [
9]. In the Qing Dynasty, the classic TCM book
TCM Prescriptions, written by well-known ancient and modern physicians, originally described Xiang-Sha-Liu-Jun-Zi-Tang (XSLJZT), which includes
Amomum villosum and fresh costus roots (
Saussurea lappa), as having the function of promoting Qi circulation through the composition of Liu-Jun-Zi-Tang (LJZT) [
10]. XSLJZT is a phenomenally effective formula for cases involving spleen and stomach Qi deficiencies, with damp-cold stagnation inspiring the middle-jiao (middle burner). Symptoms and signs may include poor appetite, anorexia, general indigestion, abdominal distension or pain, vomiting or diarrhea, bloating after eating, and nausea [
11]. XSLJZT can significantly stimulate gastrointestinal motility and gastric emptying, improve the electrogastrogram, adjust gastrointestinal hormones, and reduce gastric sensitivity [
12]. According to Xiao’s research, XSLJZT is appropriate for the treatment of functional dyspepsia, due to the potential effects of multitarget therapy [
13].
The National Health Insurance Research Database (NHIRD) in Taiwan has surveyed the herbal formulation of XSLJZT, which was based mostly on the Chinese herbal formula used for colon cancer patients post-surgery [
14]. The herbal formulation XSLJZT consists of
Radix Aucklandiae (Chinese herbal name: Muxiang),
Fructus Amomi (Chinese herbal name: Sha-Ren),
Rhizoma Pinelliae (Chinese herbal name: Ban-Xia),
Pericarpium Citri Reticulatae (Chinese herbal name: Chenpi),
Radix Ginseng (Chinese herbal name: Ren-Shen),
Atractylodes Rhizoma (Chinese herbal name: Baizhu),
Poria cocos (Schw.)
Wolf (Chinese herbal name: Fu-Ling),
Glycyrrhizae Radix (Chinese herbal name: Gan-Cai), and
Ziziphus jujuba (Chinese herbal name: Da-Zao). The herbal formulation is derived from
Summarizing Songs on Popular Formulas by Xiu-yuan Chen, which is a classical TCM herbal formulation [
15].
Radix Aucklandiae specifically resolves stagnant Qi of the spleen, stomach, and intestines, which alleviates abdominal pain and discomfort [
16].
Fructus Amomi is commonly used to treat morning sickness, but it is particularly effective for reducing nausea.
Recent investigations confirmed the effects of Sha-Ren for relieving abdominal spasms, bloating, and pain [
17,
18].
Rhizoma Pinelliae has antiemetic, glandular, antitussive, secretion-inhibiting, and antitumor effects, according to modern pharmacological studies [
19].
Pericarpium Citri Reticulatae has a potent antineuroinflammatory ability that is attributed to the collective effects of nobiletin, hesperidin and tangeretin [
20]. In addition, research has indicated that hesperidin has a protective effect against cisplatin-induced renal injury in rats [
21]. Ginsenosides are found to be almost exclusively in the plant
Radix Ginseng, which has a long history of use in traditional medicine. Many studies discovered that ginsenosides have antioxidant properties, such as increasing internal antioxidant enzymes and acting as a free-radical scavenger [
22].
Atractylodes Rhizoma is the rootstock of
Atractylodes lancea (Thunb.) DC. or
Atractylodes chinensis (DC.) Koidz., and they are part of the
Asteraceae family. It has gastroprotective and neuroprotective activities as well as antibacterial, antiviral, anti-inflammatory and anti-allergic properties [
23]. The herb
Poria cocos (Schw.)
Wolf (
Polyporaceae) grows around the roots of pine trees, and it is a famous traditional East Asian medicinal plant in China, Japan, Korea, and North America [
24].
Poria cocos Wolf is used in the treatment of acute gastroenteric catarrh, chronic gastritis, edema, nephrosis, nausea, emesis, and dizziness [
25]. With a wide spectrum of antiviral activity, glycyrrhizic acid (GA) is considered to be the principal component in
Glycyrrhiza spp., and the roots have been used for the treatment of viral-induced hepatitis, cough, and skin diseases for thousands of years. [
26].
Ziziphus jujuba is an important plant in popular medicine, and has been shown to present beneficial nutritional and health-promoting effects [
27]. The major biologically active compounds include flavonoids, triterpene acids, polysaccharides, vitamin C, and phenolics [
28]. Jujube fruits have been discovered by recent phytochemical studies to have biological effects, such as anti-inflammatory, antioxidant, anti-cancer, hepatoprotective, and gastrointestinal protective activities [
28,
29,
30].
The popularity of TCM is increasing, but preparing TCMs is a time-consuming process. Therefore, herbal manufacturers produce instant herbal products to offer to consumers using TCM. In order to standardize high-quality herbal merchandise, quantitatively measuring the ingredients of TCM products with validated means is a long-term program in Taiwan. Under current regulations, it has been legal to add herbal powder to herbal pharmaceutical products to increase the contents of herbal ingredients [
9]. As a result, the plants’ raw powder, starch, and cellulose are deliberately intermixed with herbal extract for granulation and volume extension. On the other hand, the simple milled raw herbal powder can contain pesticide residues, heavy metals, and bacterial contamination. Those concerning problems have been regulated by the manufacturers themselves. Therefore, measurement of the major active biomarker contents and herbal additives in Chinese herbal pharmaceutical products is necessary for assessing the quantity [
31].
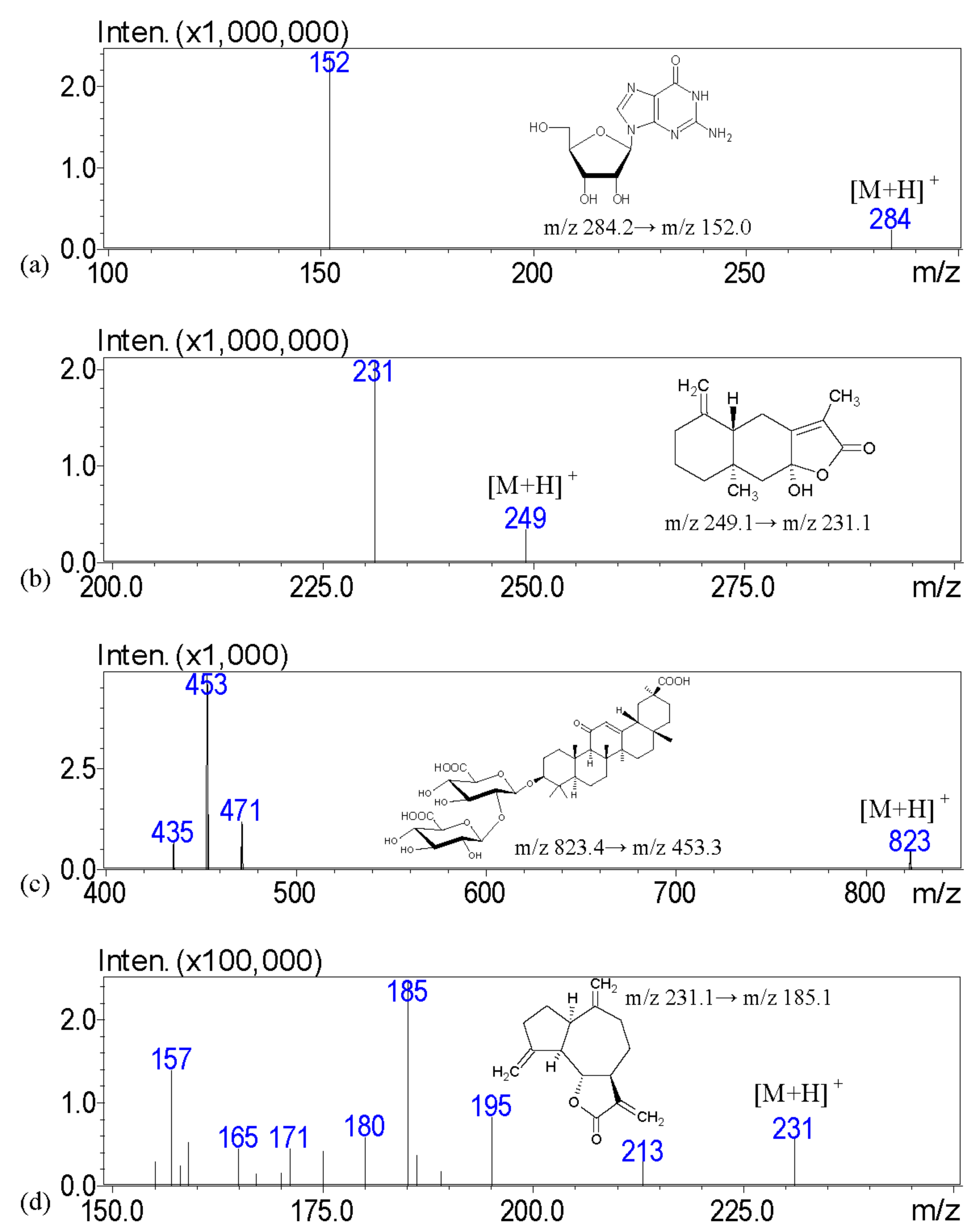
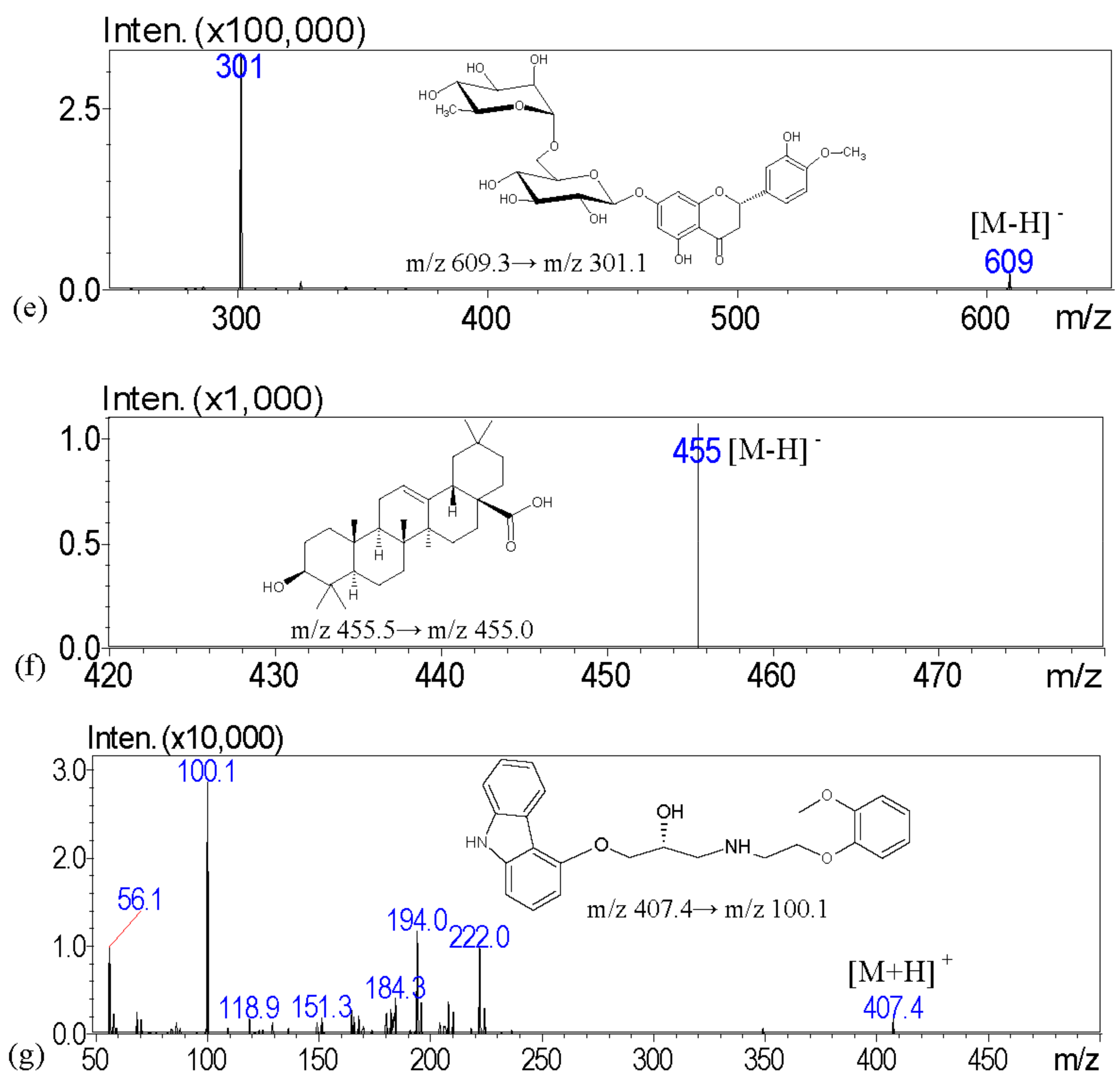
The aim of this investigation is to examine the quality of various brands of pharmaceutical products with scanning electron microscopy; light microscopy with Congo red and potassium iodide staining; a solubility test; and crude fiber analysis to examine the particle appearance, solubility, and the contents of crude fibers in order to identify the herbal formulation. To address these issues, we describe a high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS) assay with multiple reaction monitoring (MRM) for the measurement of major biomarkers of guanosine, atractylenolide III, glycyrrhizic acid, dehydrocostus lactone, hesperidin, and oleanolic acid for the chemical examination of XSLJZT. The tested Chinese herbal pharmaceutical products of XSLJZT are made by six manufacturers. The systemic chemical and physical methods that were developed provide a standard procedure for conducting quality control of Chinese herbal pharmaceutical products.
3. Materials and Methods
3.1. Reagents and Materials
Guanosine, atractylenolide III, glycyrrhizic acid, dehydrocostus lactone, hesperidin, oleanolic acid, and carvedilol were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). LC/MS-grade methanol, formic acid, and ammonium acetate were obtained from E. Merck (Darmstadt, Germany). Deionized water (Millipore, Bedford, MA, USA) was used throughout the entire experiment. Crushed XSLJZT herbs were purchased from a Chinese traditional herbal medicine store in Taipei, and prepared at the National Research Institute of Chinese Medicine, Taipei, Taiwan. The herbal pharmaceutical products of XSLJZT were purchased from six different pharmaceutical manufacturers in Taiwan, which included Sun Ten Pharmaceutical Co., Ltd. (Taipei, Taiwan), Kaiser Pharmaceutical Co., Ltd. (Tainan, Taiwan), Chuang Song-Zong Pharmaceutical Co., Ltd. (Kaohsiung, Taiwan), Koda Pharmaceutical Co., Ltd. (Taoyung, Taiwan), Sheng Chang Pharmaceutical Co., Ltd. (Taipei, Taiwan), and Jin-An Pharmaceutical Co., Ltd. (Chiayi, Taiwan). For the results of the analysis, coding was applied for the six manufacturers to preserve commercial confidentiality.
3.2. High-Performance Liquid Chromatography–Tandem Mass Spectrometry
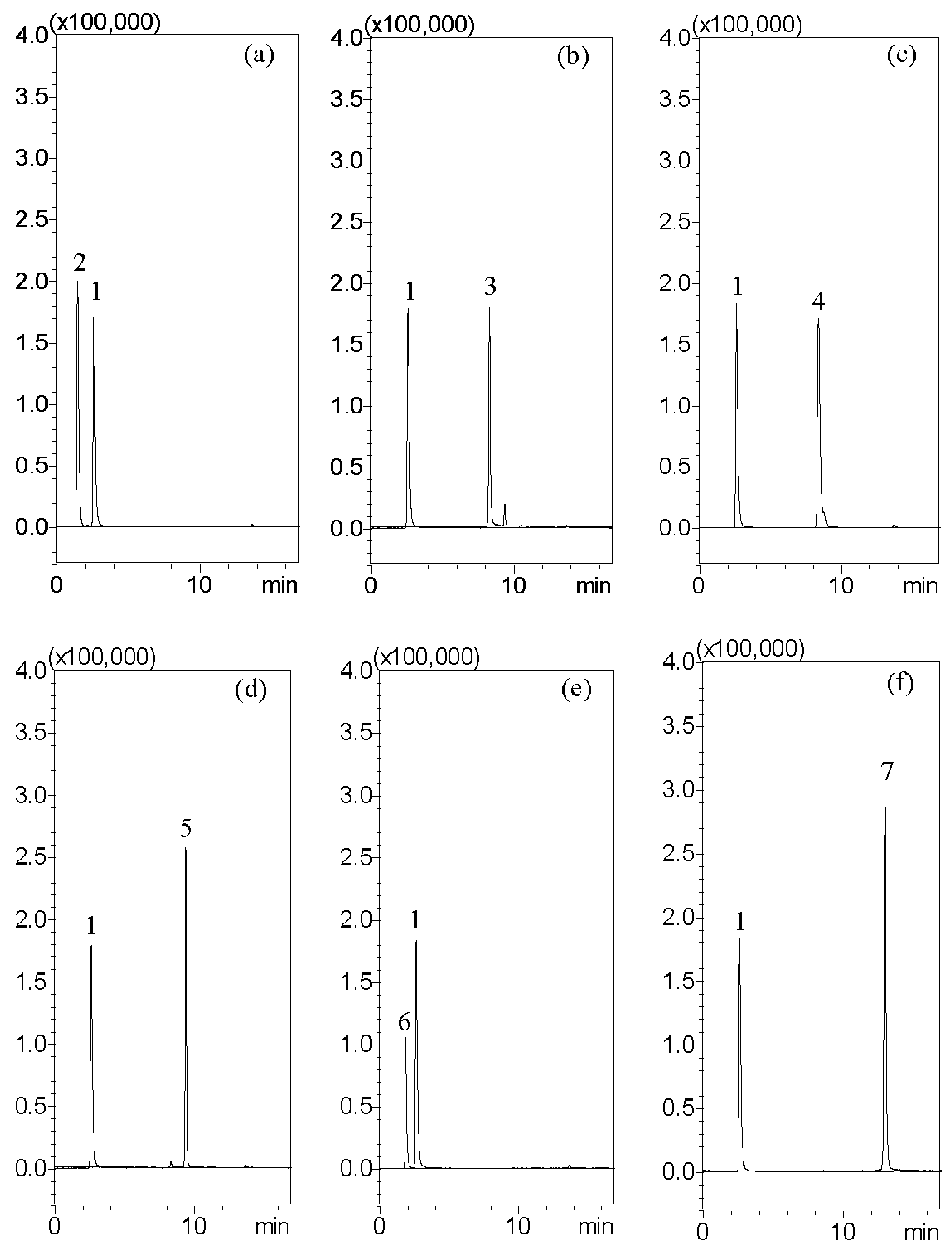
HPLC–MS analysis was carried out with a Shimadzu LCMS-8030 triple quadrupole mass spectrometer equipped with an electrospray ionization interface and integrated into the UPLC system (Shimadzu, Kyoto, Japan). The optimization of the instrument settings was as follows: interface voltage, 4.5 kV; desolvation line temperature, 250 °C; heat block temperature, 400 °C; desolvation gas, nitrogen; desolvation gas flow rate, 3 L/min; drying gas, nitrogen; drying gas flow rate, 15 L/min; collision gas, argon; and collision gas pressure, 230 kPa. Multiple reaction monitoring (MRM) mode was used in the MS spectrometer operating parameters. The chromatographic separation was accomplished using a Purospher® STAR RP-18 end-capped column (100 mm × 2.1 mm, two μm, Merck KGaA, Darmstadt, Germany). The column temperature was maintained at 40 °C. The following gradient program was used, along with a mobile phase consisting of 10 mM ammonium acetate in 0.1% formic acid (pH: 3.4) (solvent A) and methanol (solvent B): this initial term for 3 min in an isocratic elution composing with 60% solvent B; 3–8 min: 60–95% B; 8–13.5 min: 95–95% B; 13.5–15 min: 95–60% B; and 15–17 min: 60–60% B; v/v. The flow rate was 0.2 mL/min, and the injection volume was 10 μL.
3.3. Standard Solutions
The stock solutions were formed by dissolving 1 mg of guanosine, atractylenolide III, glycyrrhizic acid, dehydrocostus lactone, hesperidin, and oleanolic acid into 1 mL of 100% (v/v) methanol to a final concentration of 1.0 mg/mL, respectively. All of the stock solutions were stocked at −20 °C before use. To prepare a series of working standard solutions, the stock solution was diluted with 50% methanol to obtain the following concentrations: 50, 100, 250, 500, and 1000 ng/mL. Guanosine was assayed at concentrations of 100, 250, 500, 1000, and 2500 ng/mL. Working solutions for quality control (QC) samples with five level concentrations of 50, 100, 250, 500, and 1000 or 2500 ng/mL were prepared in the same manner. All of the solutions were kept at 4 °C and brought to room temperature before analysis.
3.4. Sample Preparation for Extracts of Herbal Pharmaceutical Powders
The classic TCM prescriptions written by well-known ancient and modern physicians originally described Xiang-Sha-Liu-Jun-Zi-Tang (XSLJZT), which is used after boiling herbs in water. Therefore, we followed the ancient recipe of boiling herbs to extract raw herbs practicing in the lab extraction. However, the herbal ingredients may not be exactly the same extraction in the lab, due to the complexities of herbal plants.
Radix Aucklandiae (7 g), Fructus Amomi (3 g), Radix ginseng (10 g), Atractylodes Rhizoma (20 g), Poria cocos (Schw.) Wolf (20 g), Glycyrrhizae radix (7 g), Pericarpium Citri Reticulatae (3 g), Rhizoma Pinelliae (12 g), and Ziziphus jujuba (four pieces) were extracted with 700 mL of boiling water for 60 min. The decoction was filtered, and the solution was freeze-dried under vacuum and dissolved in 100% MeOH for analysis. Each specimen was prepared using 0.1 g of Chinese pharmaceutical herbal powder or dried XSLJZT decoction powder steeped in 25 mL of methanol (4 mg/mL), which was then extracted in an ultrasonic oscillator for 20 min at room temperature and centrifuged at 13,000× g rpm for 10 min at 4 °C. The supernatant was collected through a 0.22 μm syringe filter, diluted to a suitable concentration, and infused into the HPLC–MS for analysis. However, Chinese medicine powder is produced with granulating via boiled soups, so the practicing method in this study using alcohol extraction is reasonable. Consequently, herbal preparation (A–F) and the lab extraction samples adopted the same extraction method.
3.5. Quantitative Determination of Active Compounds
Quantitative determination was completed by using the most intense ion detected. The interpolation method of the calibration curve was adopted to determine the relative concentration of active compounds in each sample, and each batch of samples correlated with the same calibration curve. Using back calculation, the content of active compounds in Xiang-Sha-Liu-Jun-Zi-Tang was calculated using the following formula. The content of active compounds in XSLJZT (mg/g) = [determined concentration (ng/mL)/concentration of sample (4 mg/mL)] × dilution ratio.
3.6. Method Validation
The HPLC–MS method was validated based on the recommendations published by the Food and Drug Administration (FDA) Guidance for Industry, Bioanalytical Method Validation [
38]. The correlation coefficients (
r2) of all of the calibration curves had good linearity, with values greater than 0.995. The limit of detection (LOD) was defined as the concentration that yields a signal-to-noise ratio of three, and the lower limit of quantification (LLOQ) was accepted as the lowest concentration of the linear regression. The intra-day and inter-day variations were determined by analyzing six replicates on the same day and on six successive days, respectively. The precision of the observed concentrations was calculated by relative standard deviation (RSD) (%) = (standard deviation (SD)/Cobs) × 100%. The accuracy was achieved by calculating the bias (%) = [(Cobs − Cnom)/Cnom] × 100%. Cobs represented the mean value of the observed concentrations, and Cnom represented the nominal concentration. The acceptable values of accuracy and precision were within ±15%, and the values of LLOQ were less than ±20%.
3.7. Physical Examination of Additives for Raw Herbal Powder
3.7.1. Estimation of Additives for Raw Herbal Powder
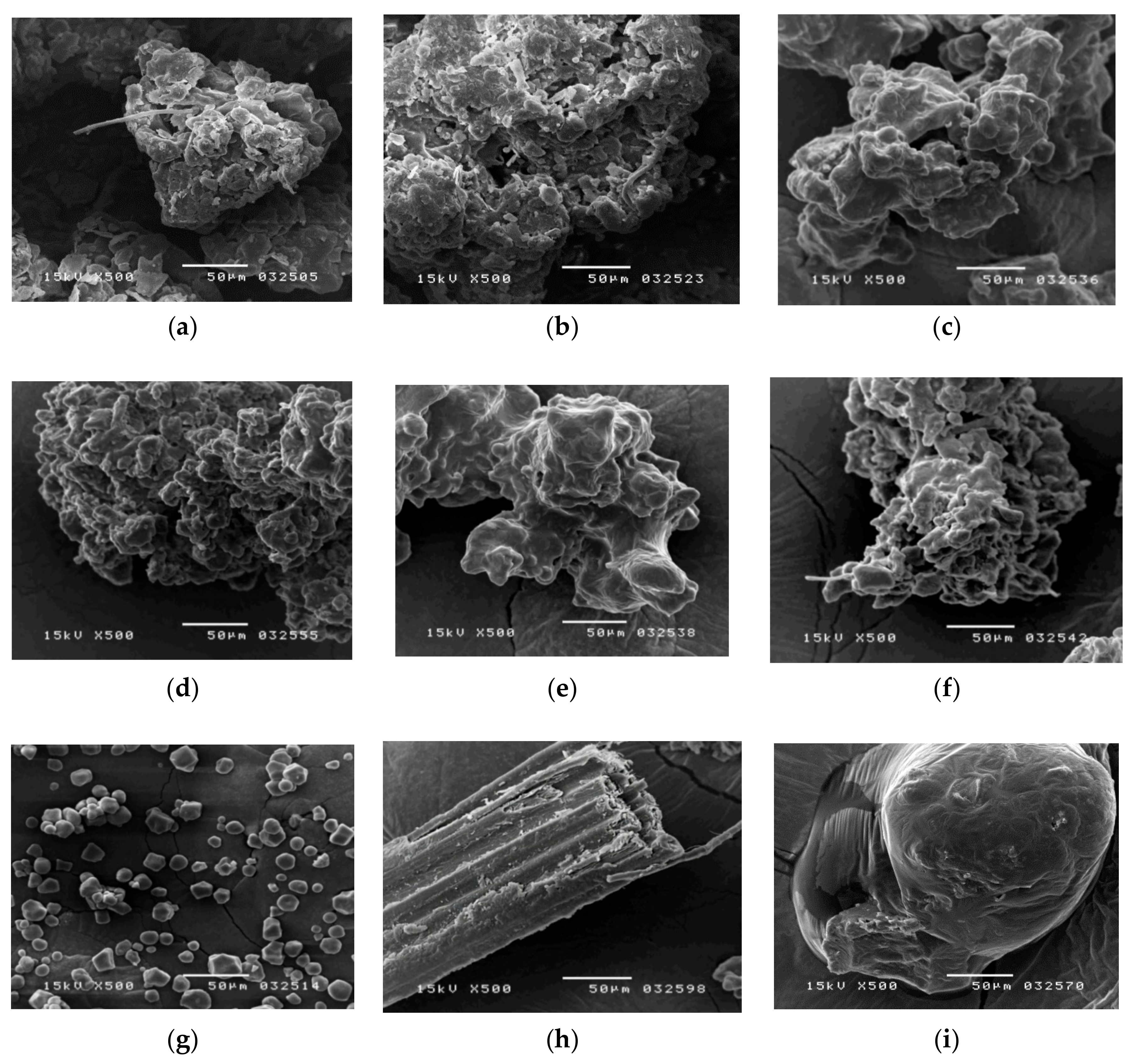
To observe the morphology of the samples, a scanning electron microscope (SEM) was used. The herbal pharmaceutical products of XSLJZT were purchased from six different pharmaceutical manufacturers in Taiwan. The crushed herbs of XSLJZT were obtained from a Chinese traditional herbal medicine shop in Taipei and formed a raw herbal powder in the National Research Institute of Chinese Medicine, Taipei, Taiwan. These herbs were filtered through a 60-mesh sieve after they were ground by the hammer mill (Hung Chuan RT-04, Taipei, Taiwan). Food-grade cornstarch (Sun Right Co., Ltd., New Taipei, Taiwan) was filtered through a 60-mesh sieve. For sample preparation, the herbal pharmaceutical powder was dried at 45 °C for 24 h in an oven (DO45, DENYNG Instruments Co., Ltd., New Taipei, Taiwan). Then the powder was fixed with double-sided adhesive tape on an aluminum stand, and coated with gold by a gold sputter package for 90 s in a high-vacuum evaporator (Ion Sputter JFC-1200, Jeol Ltd., Tokyo, Japan). A scanning electron microscope (JEOL JSM-5300, Jeol Ltd., Tokyo, Japan) was used to analyze the samples. The raw herbal powder, raw herbal extraction, and cornstarch were investigated in the same manner.
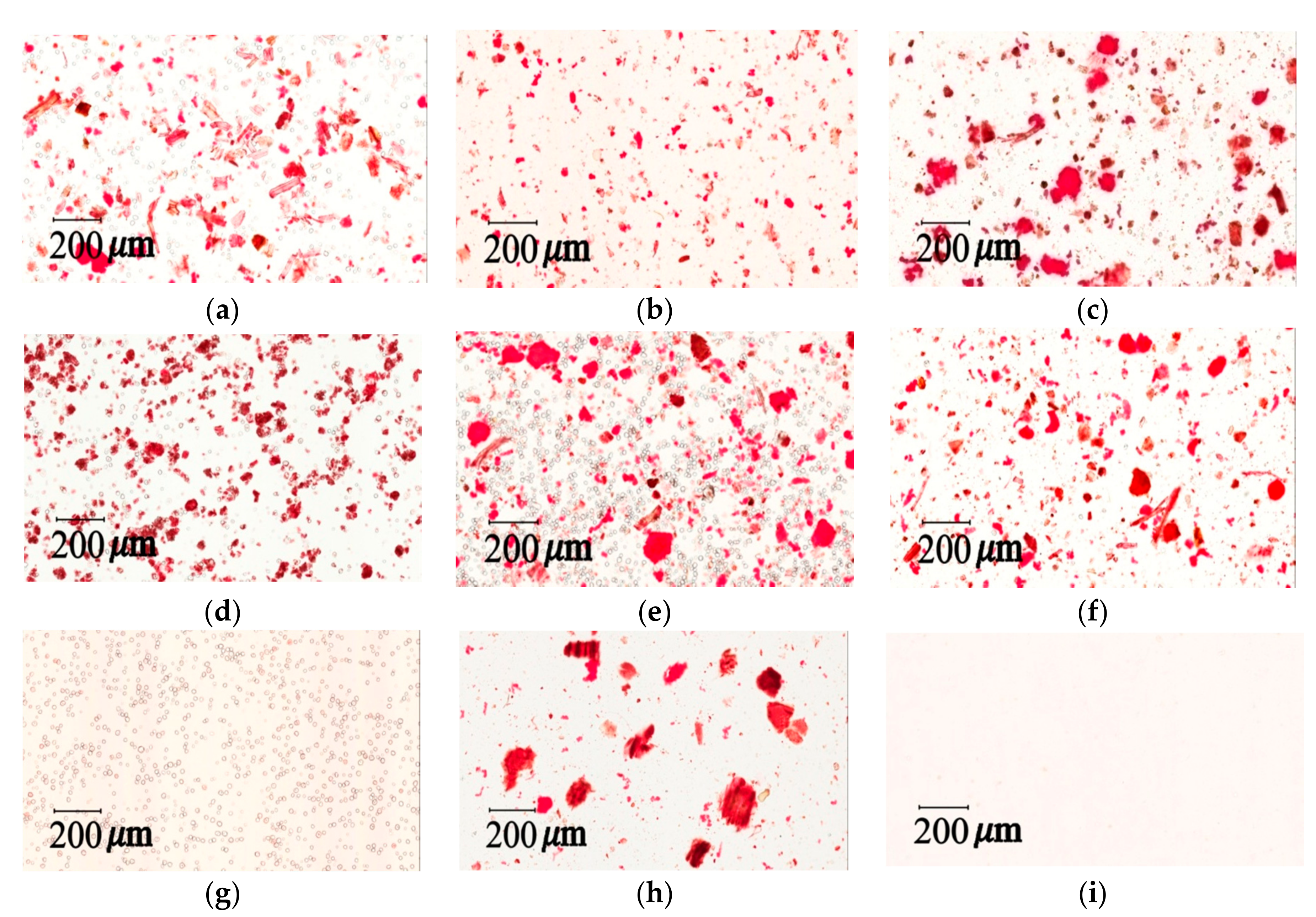
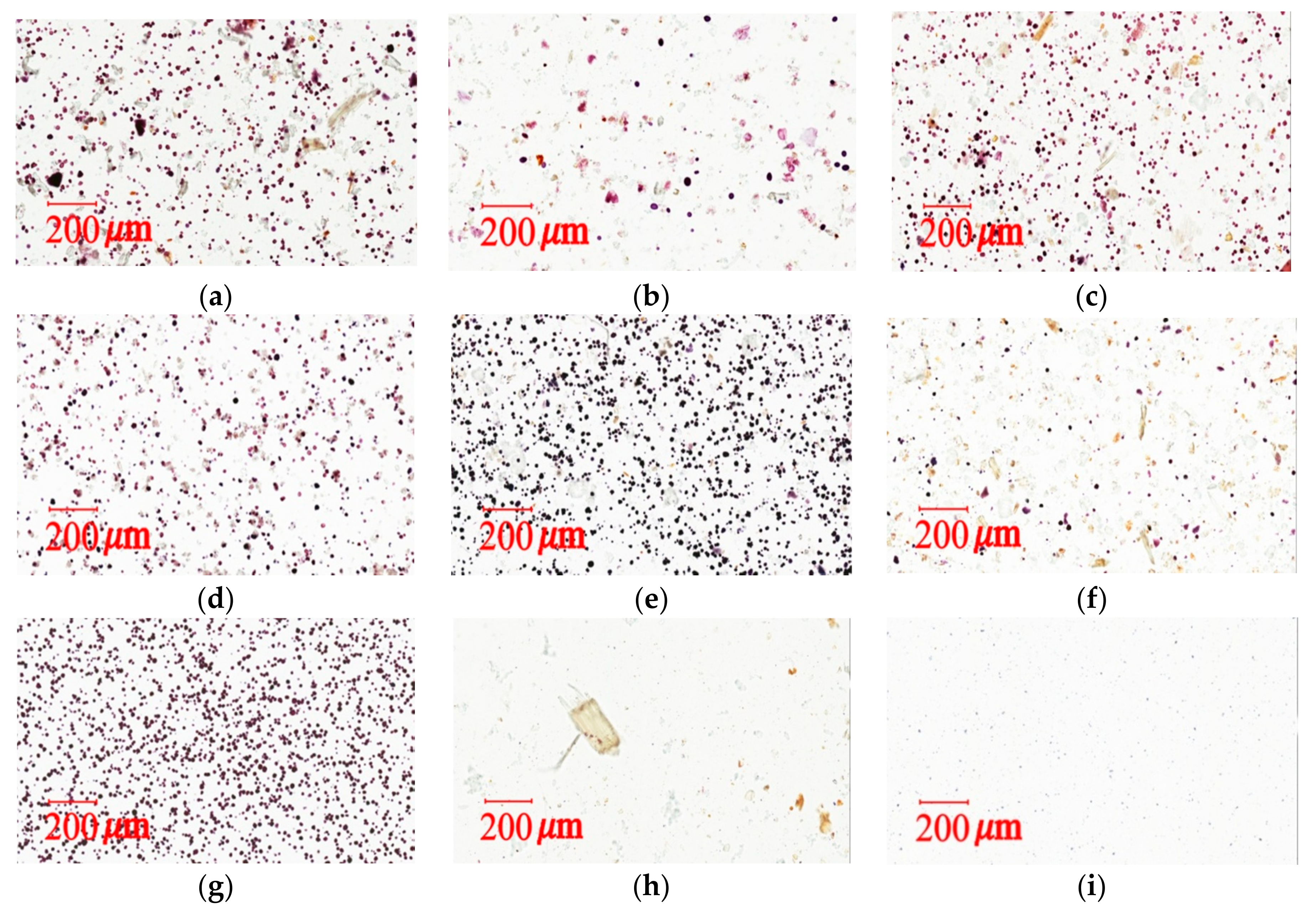
3.7.2. Light Microscopy Photographs of Congo Red and Iodine–KI Stained Samples
The iodine test for starch is used to determine the presence of starch in materials [
9,
31,
35,
39]. The affinity of Congo red is high for fibers. It has been widely used to test media additives, biological staining, plant mucin, and cellulose [
40]. Congo red and Iodine–KI staining tests can provide qualitative and quantitative measurements for cellulose fibers and cornstarch in herbal pharmaceutical products. Light microscopy images were taken with a light microscope (Aperio Scanscope CS system, Aperio, CA, USA). Samples were prepared in a mixed solution (glycerol/20% ethanol (1:1)) and placed on microslides by adding 1–2 drops of the solution. The preparation was then covered with a coverslip for viewing and stained with 0.1% Congo red or 2% iodine–KI solution.
The measurement of total raw herbal powder and cornstarch in six different herbal pharmaceutical XSLJZT products was determined by counting the number of staining spots for raw herbal powder and cornstarch. Acceptance criteria: The cornstarch is generally represented by rounded or spheroidal granules with diameters ranging from 10 μm–15 μm, and the raw herbal powder contained longed and fragmented cellulose fibers with diameters ranging from 20 μm–120 μm that could be clearly observed under the microscope after 10% iodine–KI and 0.1% Congo red staining.
3.7.3. Solubility and Swelling Test
The water solubility and swelling power index experiments of the herbal pharmaceutical powders were modified from previous reports [
9,
31,
35]. The respective brands of XSLJZT powder samples (0.45 g) were added to 30 mL of distilled water (30 g, 1.5%,
w/
w). These XSLJZT sample solutions were vortexed and heated to 55 °C, 65 °C, 75 °C, 85 °C, and 95 °C sequentially for one hour each in a circulating water bath (BH-230D, YIN DER Instruments Co., Ltd., New Taipei, Taiwan). After heating, the samples were cooled to room temperature and centrifuged at 13,000×
g at 4 °C for 20 min to separate the supernatant and sediment precipitations. The supernatant was collected and placed in a drying oven (DO45, DENGYNG Instruments Co., Ltd., New Taipei, Taiwan) at 100 °C; and the weight of the residue and the residue precipitate in the centrifuge tube were represented by W1 and W2, respectively. The solubility was calculated with the following formula: solubility = (W1/pharmaceutical herbal powder weight) × 100%. Swelling power = W2/[powder weight × (1 − Solubility/100)].
3.7.4. Crude Fiber Analysis
The crude fiber content of the XSLJZT powder samples were determined using a method from the Crude Fiber in Flours, Feeds, and Feedstuffs Method of the American Association of Cereal Chemists (AACC) [
41]. The XSLJZT herbal powder sample (2 g) was boiled for 30 min in 200 mL of 1.25% H
2SO
4, washed with hot distilled water, and filtered with a suction apparatus. Then, the sample was transferred to a boiled 1.25% NaOH solution and treated in the same manner. The residue was desiccated at 100 °C for 24 h in an oven to achieve constant weight and burned at 550–600 °C for 5–6 h in a muffle furnace (DF202, DENGYNG Instruments Co., Ltd., New Taipei City, Taiwan) until gray ash was collected. The crude fiber (%) was calculated by the formula: crude fiber (%) = (constant weight of residue − the weight of ash) × 100/weight of the sample.
3.8. Statistical Analysis
The microscopy photograph analysis was completed with an Aperio ScanScope CS (Aperio Technologies, Vista, CA, USA), and the number of particles was calculated by the software Aperio ImageScope (version 10.0). Statistical analyses were performed with SPSS Version 18.0 (SPSS, Chicago, IL, USA) and SigmaPlot 10.0 software. Data are expressed as the mean ± standard deviation. Analysis of variance was conducted by Student’s t test or a one-way ANOVA comparison adjustment, and statistically significant differences were defined as 𝑝 < 0.05.