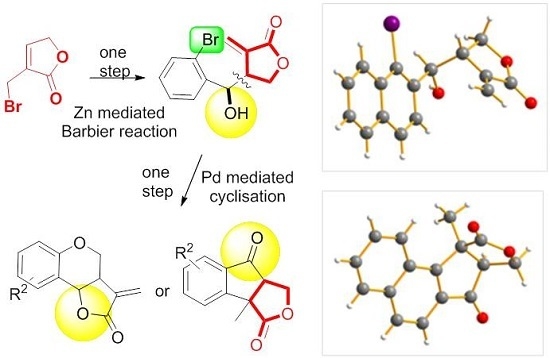
From α-Bromomethylbutenolide to Fused Tri(Tetra) Cyclic Dihydrofurandiones through Barbier Reaction–Heck Arylation Sequence
Abstract
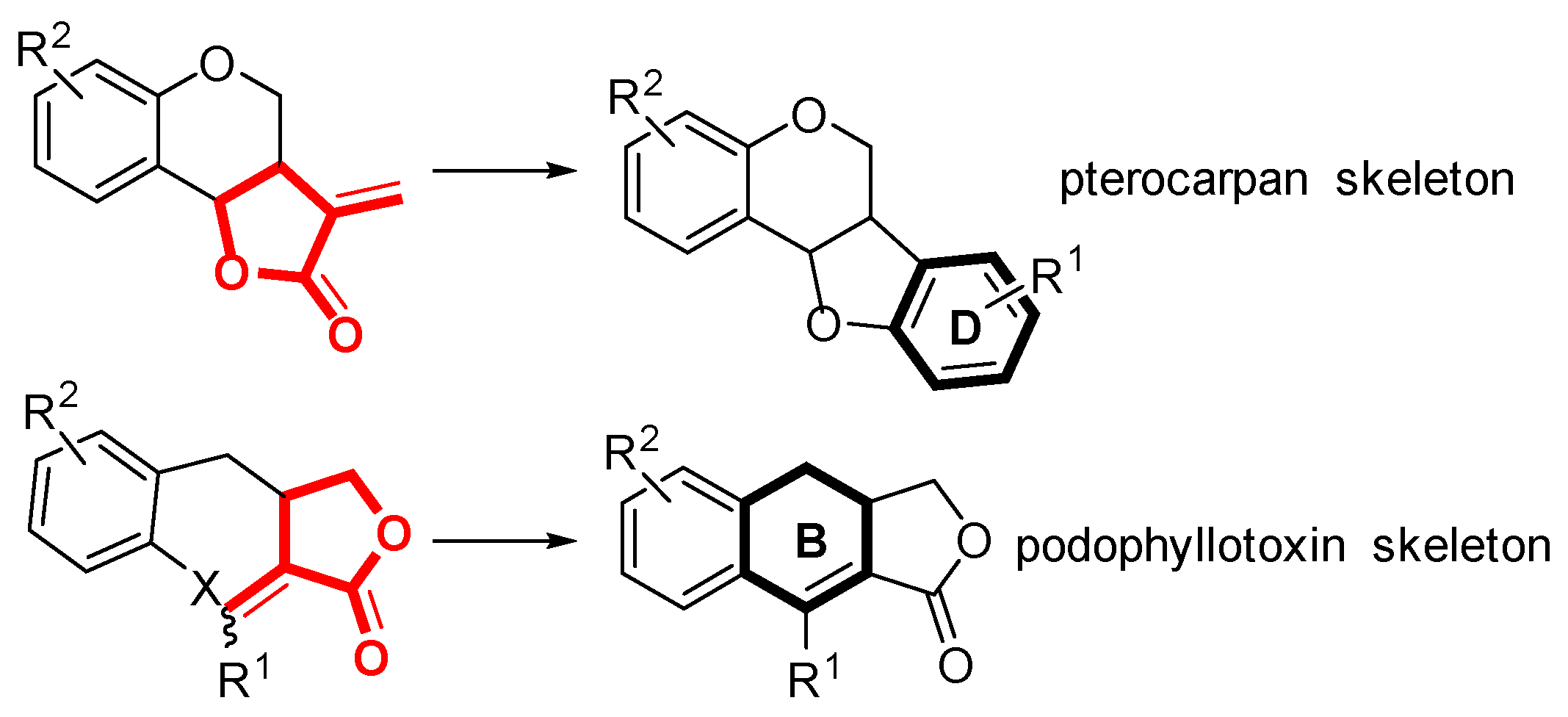
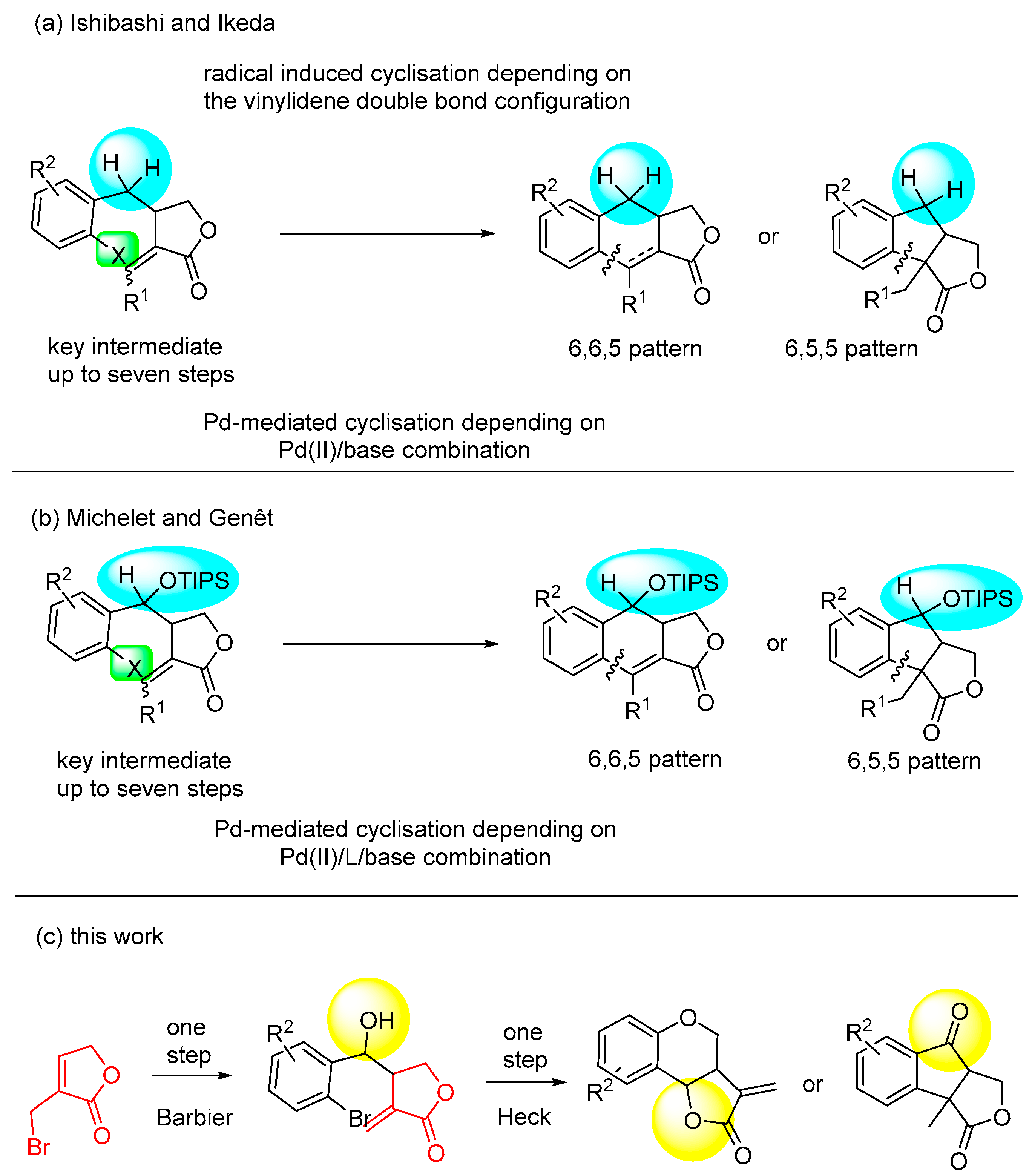
1. Introduction
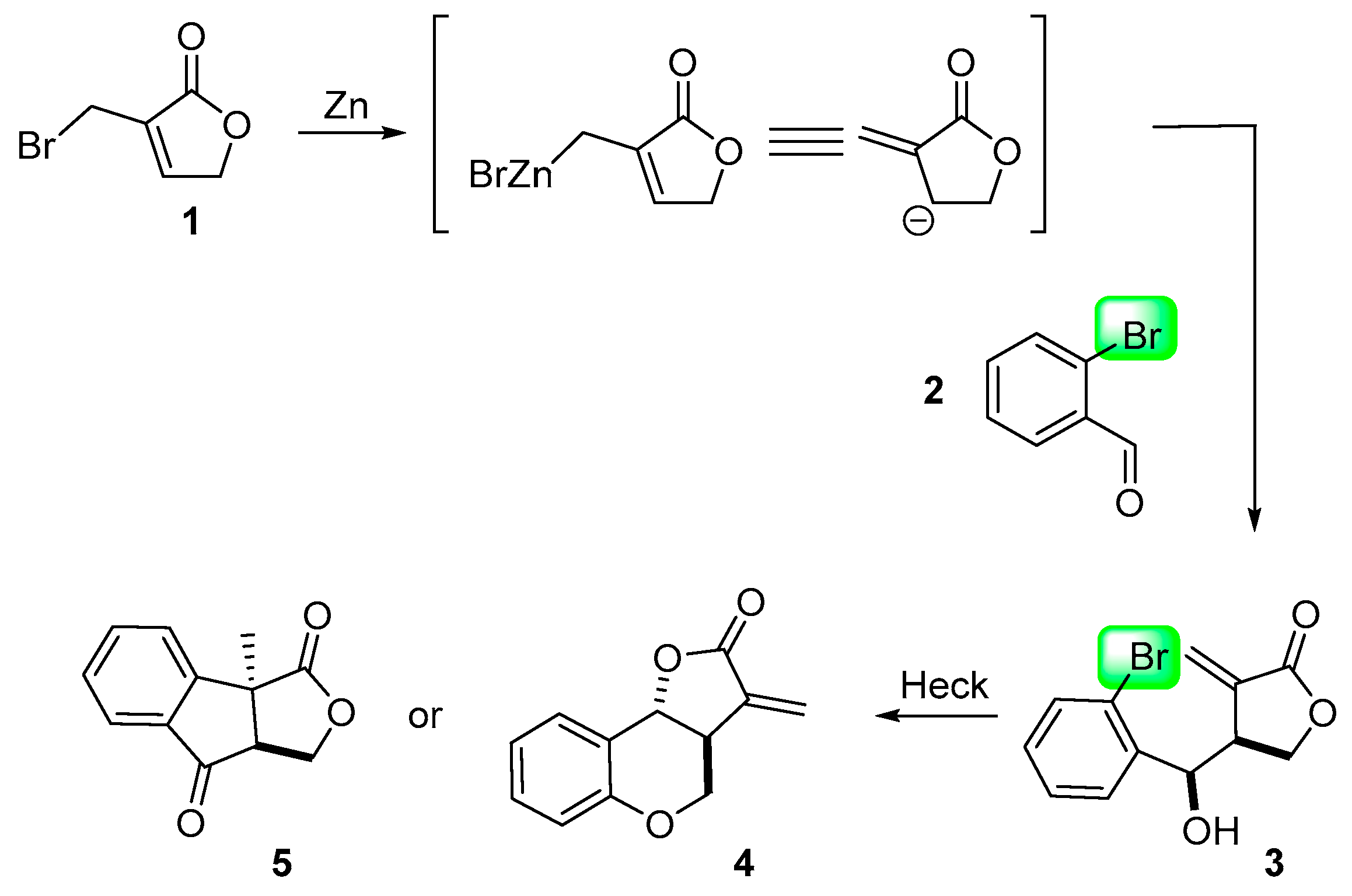
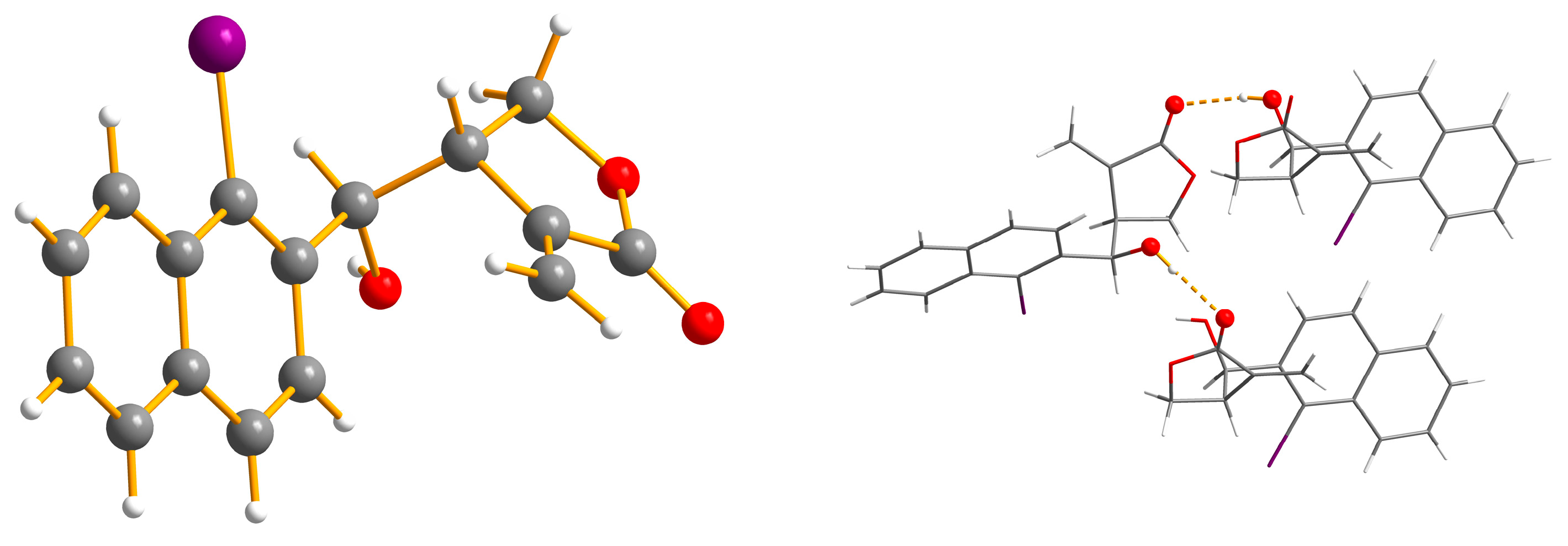
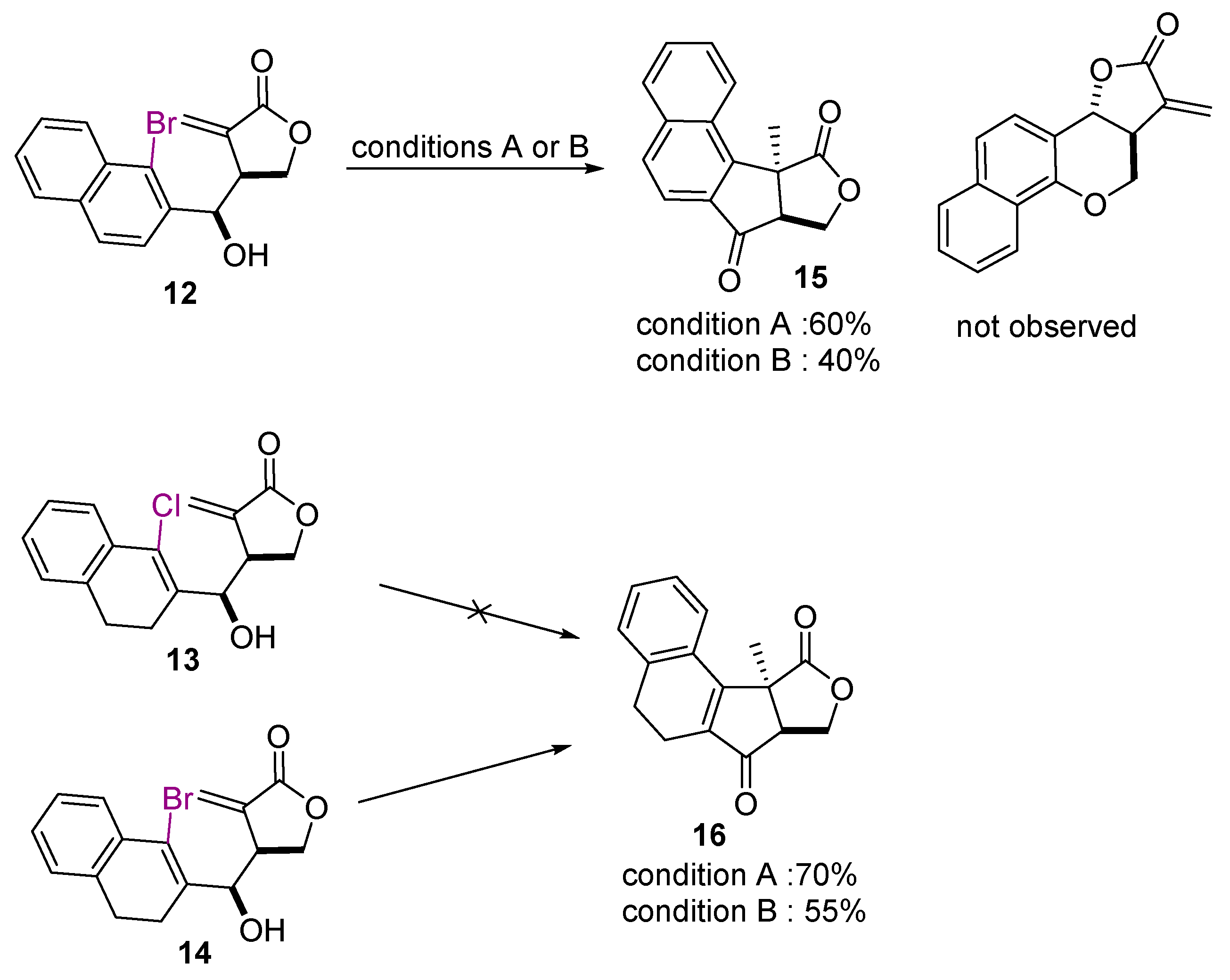
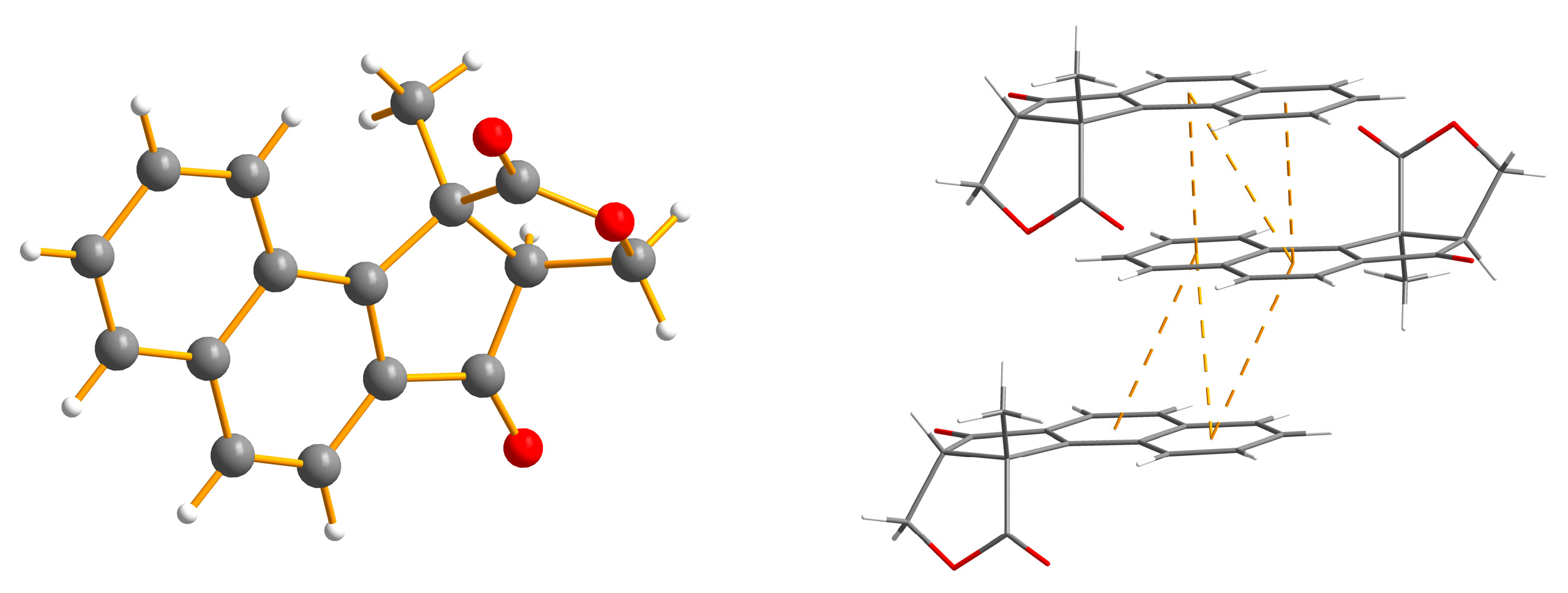
2. Results and Discussion
3. Materials and Methods
3.1. Methods
3.1.1. Representative Procedure for the Barbier Allylation Reaction of 3-Bromomethyl-5H-furan-2-one
3.1.2. Procedure A for Intramolecular Heck Reaction
3.1.3. Procedure B for Intramolecular Heck Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Westmeier, J.; Kress, S.; Pfaff, C.; Von Zezschwitz, P. Total synthesis of (R)-sarkomycin via asymmetric rhodium-catalyzed conjugate addition. J. Org. Chem. 2013, 78, 10718–10723. [Google Scholar] [CrossRef] [PubMed]
- Usuki, T.; Sato, M.; Hara, S.; Yoshimoto, Y.; Kondo, R.; Zimmermann, S.; Kaiser, M.; Brun, R.; Hamburger, M.; Adams, M. Antitrypanosomal structure–activity-relationship study of synthetic cynaropicrin derivatives. Biorg. Med. Chem. Lett. 2014, 24, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.M.; Talbot, E.P.A.; Clark, B.P. Stereoselective synthesis of β-(hydroxymethylaryl/alkyl)-α-methylene-γ-butyrolactones. Org. Lett. 2011, 13, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; He, Z.-T.; Yu, H.-J.; Fukui, Y.; Tian, P.; Lin, G.-Q. Zinc-mediated asymmetric allylation of chiral N-tert-butanesulfinyl aldimines with 3-bromomethyl-5H-furan-2-one. Synlett 2013, 24, 1649–1656. [Google Scholar] [CrossRef]
- Ferreira, M.; Bisol, T.B.; da Conceiçao, H.P.; Russo, T.V.C.; Bortoluzzi, A.J.; Sa, M.M. One-pot synthesis of α-ylidene δ-lactones from functionalized allylic bromides in a water–isopropanol medium. Synthesis 2017, 49, 667–676. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, Y.; Xie, L.; Xu, X. Pd-catalyzed diastereoselective allylation of aldehydes with 3-bromomethyl-5H-furan-2-one: Stereoselective synthesis of β-(hydroxymethylaryl/alkyl)-α-methylene-γ-butyrolactones with a syn configuration. Chem. Commun. 2013, 49, 4697–4699. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Schober, M.; Orthaber, A.; Faber, K. Asymmetric synthesis of β-substituted α-methylenebutyro-lactones via TRIP-catalyzed allylation: Mechanistic studies and application to the synthesis of (S)-(−)-hydroxymatairesinol. Adv. Synth. Catal. 2013, 355, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Mochida, K.; Kim, S.-W. Total synthesis of sophorapterocarpan A, maackiain, and anhydropisatin: Application of a 1,3-Michael-Claisen annulations to aromatic synthesis. J. Chem. Soc. Perkin Trans. 1989, 1, 1219–1224. [Google Scholar] [CrossRef]
- Goel, A.; Kumar, A.; Hemberger, Y.; Raghuvanshi, A.; Jeet, R.; Tiwari, G.; Knauer, M.; Kureel, J.; Singh, A.K.; Gautam, A.; et al. Synthesis, optical resolution, absolute configuration, and osteogenic activity of cis-pterocarpans. Org. Biomol. Chem. 2012, 10, 9583–9592. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Ito, K.; Tabuchi, M.; Ikeda, M. Studies on aconitum species. XIV. Deoxygenation of pseudokobusine to kobusine. Heterocycles 1991, 32, 1297–1300. [Google Scholar] [CrossRef]
- Ishibashi, H.; Ito, K.; Hirano, T.; Tabuchi, M.; Ikeda, M. Synthesis of podophyllotoxin derivatives by means of tributyltin hydride- or palladium-mediated cyclization of α-benzylidene-β-(o-bromobenzyl)-γ-lactones. Tetrahedron 1993, 49, 4173–4182. [Google Scholar] [CrossRef]
- Charruault, L.; Michelet, V.; Genêt, J.-P. Pd-catalyzed route to (±)-podophyllotoxin skeleton. Synthesis of the aryltetralin derivative. Tetrahedron Lett. 2002, 43, 4757–4760. [Google Scholar] [CrossRef]
- Galland, J.-C.; Dias, S.; Savignac, M.; Genêt, J.-P. Cycloisomerization of 1,6-enynes in organoaqueous medium: An efficient and eco-friendly access to furan derivatives. Synthesis of a key intermediate of podophyllotoxin. Tetrahedron 2001, 57, 5137–5148. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Sun, L.; Xie, L.; Xu, X. Zinc or indium-mediated Barbier-type allylation of aldehydes with 3-bromomethyl-5H-furan-2-one in aqueous media: An efficient synthesis method for α-methylene-γ-butyrolactone. Org. Biomol. Chem. 2012, 10, 3991–3998. [Google Scholar] [CrossRef] [PubMed]
- Santoso, H.; Casana, M.I.; Donner, C.D. Exploring O-stannyl ketyl and acyl radical cyclizations for the synthesis of γ-lactone-fused benzopyrans and benzofurans. Org. Biomol. Chem. 2014, 12, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Vaitla, J. Desulfonylative methenylation of β-keto sulfones. Org. Lett. 2015, 17, 4890–4893. [Google Scholar] [CrossRef] [PubMed]
- Donner, C.D.; Casana, M.I. Synthesis of novel pyranoquinones using an acyl radical cyclization strategy. Tetrahedron Lett. 2012, 53, 1105–1107. [Google Scholar] [CrossRef]
- Prim, D.; Campagne, J.-M.; Joseph, D.; Andrioletti, B. Palladium-catalysed reactions of aryl halides with soft, non-organometallic nucleophiles. Tetrahedron 2002, 58, 2041–2075. [Google Scholar] [CrossRef]
- Ashimori, A.; Bachand, B.; Overman, L.E.; Poon, D.J. Catalytic asymmetric synthesis of quaternary carbon centers. Exploratory investigations of intramolecular Heck reactions of (E)-α,β-unsaturated 2-haloanilides and analogues to form enantioenriched spirocyclic products. J. Am. Chem. Soc. 1998, 120, 6477–6487. [Google Scholar] [CrossRef]
- Coquerel, Y.; Bremond, P.; Rodriguez, J. Pd–H from Pd/C and triethylamine: Implications in palladium catalysed reactions involving amines. J. Organomet. Chem. 2007, 692, 4805–4808. [Google Scholar] [CrossRef]
- Jefford, C.W.; Rossier, J.-C.; Boukouvalas, J.; Sledeski, A.W.; Huang, P.-Z. A concise synthesis of siphonodictidine. J. Nat. Prod. 2004, 67, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Moise, J.; Arseniyadis, S.; Cossy, J. Cross-metathesis between α-methylene-γ-butyrolactone and olefins: A dramatic additive effect. Org. Lett. 2007, 9, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Boufroura, H.; Souibgui, A.; Gaucher, A.; Marrot, J.; Pieters, G.; Aloui, F.; Ben Hassine, B.; Clavier, G.; Prim, D. 3D shapes of aryl(dihydro)naphthothiophenes: A comprehensive and structural study. Org. Biomol. Chem. 2015, 13, 10844–10851. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Centre deposit numbers for compounds 12: 1565162 and 16: 1565163. (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html.
- Yang, H.S.; Qiao, X.X.; Cui, Q.; Xu, X.H. First synthesis of cedarmycin B. Chin. Chem. Lett. 2009, 20, 1023–1024. [Google Scholar] [CrossRef]
- Brunner, B.; Stogaitis, N.; Lautens, M. Synthesis of 1,2-dihydropyridines using vinyloxiranes as masked dienolates in imino-aldol reactions. Org. Lett. 2006, 8, 3473–3476. [Google Scholar] [CrossRef] [PubMed]
- Requet, A.; Souibgui, A.; Pieters, G.; Ferhi, S.; Letaieff, A.; Carlin-Sinclair, A.; Marque, S.; Marrot, J.; Ben Hassine, B.; Gaucher, A.; et al. Synthesis of partially hydrogenated oxa[5] and oxa[6]helicenes from β-chlorovinylaldehydes. Tetrahedron Lett. 2013, 54, 4721–4725. [Google Scholar] [CrossRef]
- Shunatona, H.P.; Früh, N.; Wang, Y.-M.; Rauniyar, V.; Toste, F.D. Enantioselective fluoroamination: 1,4-addition to conjugated dienes using anionic phase-transfer catalysis. Angew. Chem. Int. Ed. 2013, 52, 7724–7727. [Google Scholar] [CrossRef] [PubMed]
- Turrini, N.G.; Hall, M.; Faber, K. Enzymatic synthesis of optically active lactones via asymmetric bioreduction using ene-reductases from the old yellow enzyme family. Adv. Synth. Catal. 2015, 357, 1861–1871. [Google Scholar] [CrossRef]
- Smith, C.R. Activated zinc dust. Synlett 2009, 9, 1522–1523. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
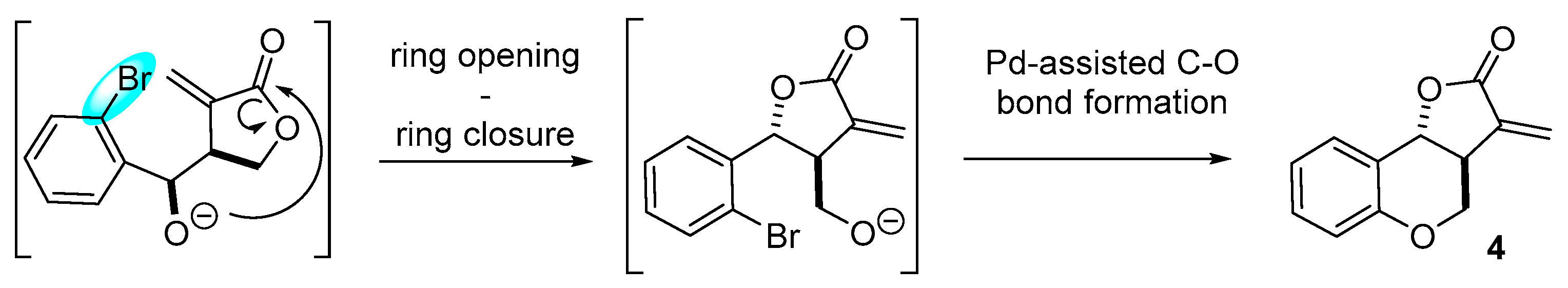
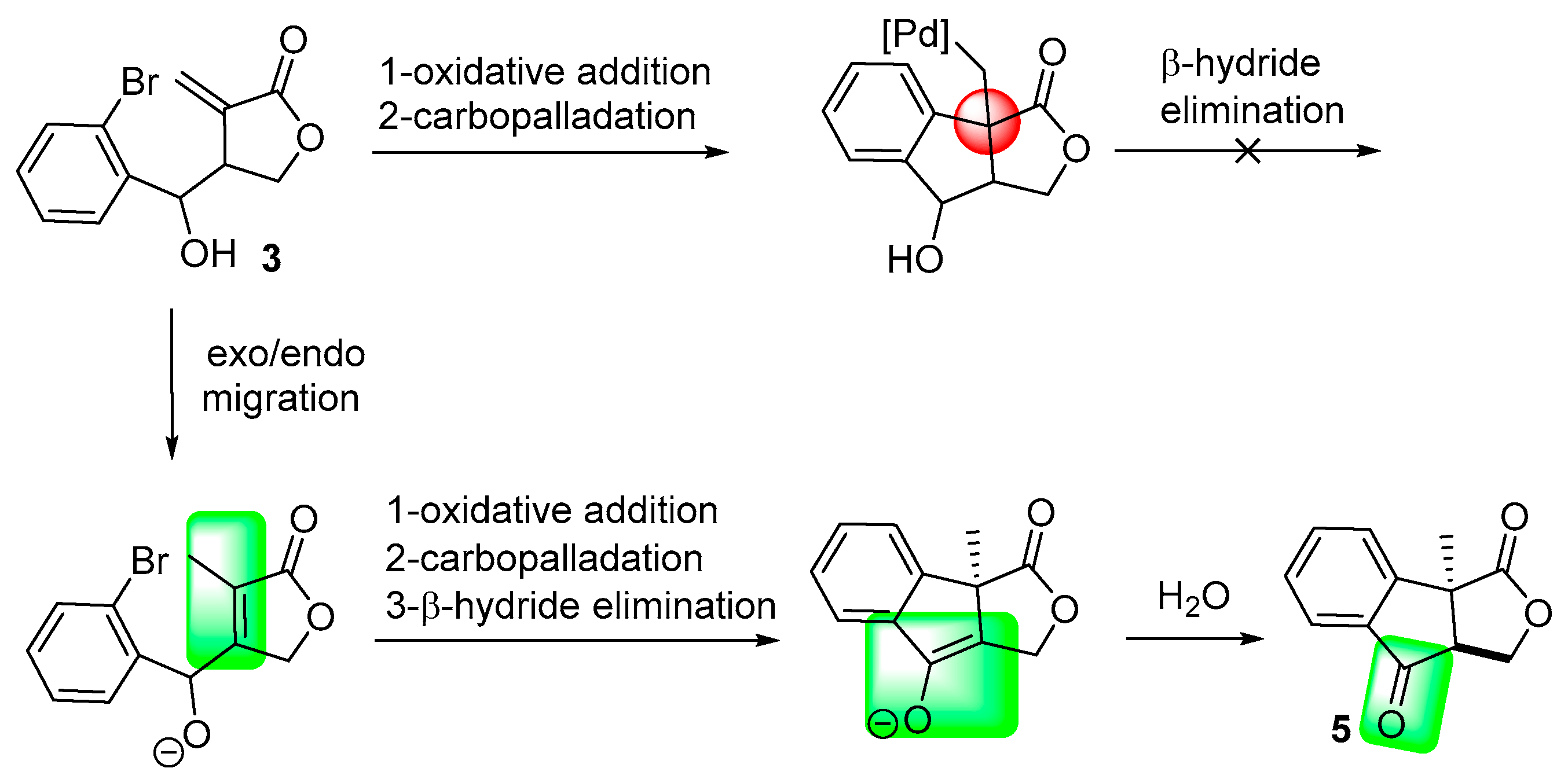
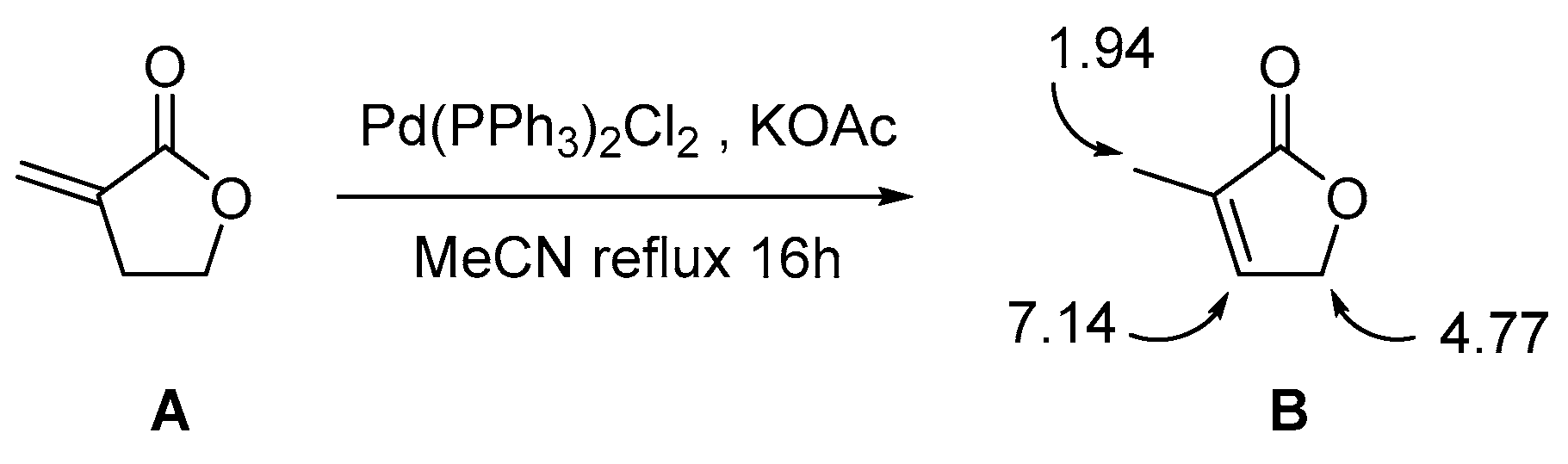

| Entry | Catalyst | Base | Additive | Solvent | Conditions | 3/4/5 a | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | Pd(PPh3)2Cl2 | K2CO3 | THF | 65 °C, 2 h | 1/1/0 | 4(20) | |
| 2 | Pd(PPh3)2Cl2 | K2CO3 | THF | 65 °C, 16 h | 0/1/0 | 4(60) | |
| 3 | Pd(PPh3)2Cl2 | K2CO3 | Ag2CO3 | THF | 65 °C, 16 h | - | - c |
| 4 | Pd(PPh3)2Cl2 | K2CO3 | MeCN | 90 °C, 16 h | 0/1/0 | 4(50) | |
| 5 | Pd(PPh3)2Cl2 | Cs2CO3 | MeCN | 90 °C, 2 h | - | - c | |
| 6 | - | K2CO3 | THF | 65 °C, 16 h | - | - d | |
| 7 | Pd(dppf)Cl2 | K2CO3 | MeCN | 90 °C, 2 h | 0.5/1/0 | 4(30) | |
| 8 | Pd(dppf)Cl2 | K2CO3 | THF | 65 °C, 16 h | 0/1/0 | 4(45) | |
| 9 | Pd(PPh3)2Cl2 | KOAc | THF | 65 °C, 16 h | 1/0/0.1 | nd | |
| 10 | Pd(PPh3)2Cl2 | KOAc | MeCN | 90 °C, 16 h | 0/0/1 | 5(40) | |
| 11 | Pd(PPh3)2Cl2 | KOAc | AgOAc | MeCN | 90 °C, 16 h | 0/0/1 | 5(14) |
| 12 | Pd(PPh3)2Cl2 | KOAc, K2CO3 b | THF | 65 °C, 16 h | 1/1.2/0.1 | - a |
| Entry | Starting Halide | Compound | Condition | α-Methylidene Butyrolactone | Product | Yield (%) | Dr a |
|---|---|---|---|---|---|---|---|
| 1 | 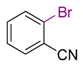 | 6 | THF, 18 h |  | 11 | - | - |
| 2 | 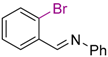 | 7 | THF, 18 h | 11 | - | - | |
| 3 | 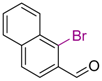 | 8 | THF, 18 h | 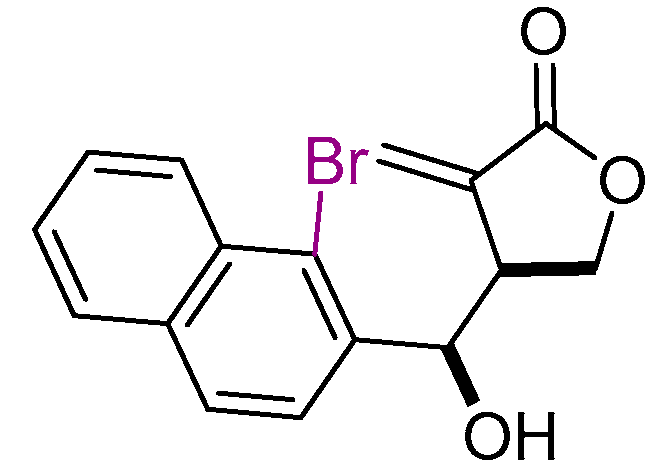 | 12 | 60 | 87/13 |
| 4 |  | 9, X = Cl | THF, 16 h |  | 13, X = Cl | 80 | 89/11 |
| 5 | 10, X = Br | 14, X = Br | 89 | 94/6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talbi, A.; Gaucher, A.; Bourdreux, F.; Marrot, J.; Efrit, M.L.; M’Rabet, H.; Prim, D. From α-Bromomethylbutenolide to Fused Tri(Tetra) Cyclic Dihydrofurandiones through Barbier Reaction–Heck Arylation Sequence. Molecules 2017, 22, 2171. https://doi.org/10.3390/molecules22122171
Talbi A, Gaucher A, Bourdreux F, Marrot J, Efrit ML, M’Rabet H, Prim D. From α-Bromomethylbutenolide to Fused Tri(Tetra) Cyclic Dihydrofurandiones through Barbier Reaction–Heck Arylation Sequence. Molecules. 2017; 22(12):2171. https://doi.org/10.3390/molecules22122171
Chicago/Turabian StyleTalbi, Arbia, Anne Gaucher, Flavien Bourdreux, Jérôme Marrot, Mohamed L. Efrit, Hédi M’Rabet, and Damien Prim. 2017. "From α-Bromomethylbutenolide to Fused Tri(Tetra) Cyclic Dihydrofurandiones through Barbier Reaction–Heck Arylation Sequence" Molecules 22, no. 12: 2171. https://doi.org/10.3390/molecules22122171
APA StyleTalbi, A., Gaucher, A., Bourdreux, F., Marrot, J., Efrit, M. L., M’Rabet, H., & Prim, D. (2017). From α-Bromomethylbutenolide to Fused Tri(Tetra) Cyclic Dihydrofurandiones through Barbier Reaction–Heck Arylation Sequence. Molecules, 22(12), 2171. https://doi.org/10.3390/molecules22122171