Nanoparticle/Metal–Organic Framework Composites for Catalytic Applications: Current Status and Perspective
Abstract
:1. Introduction
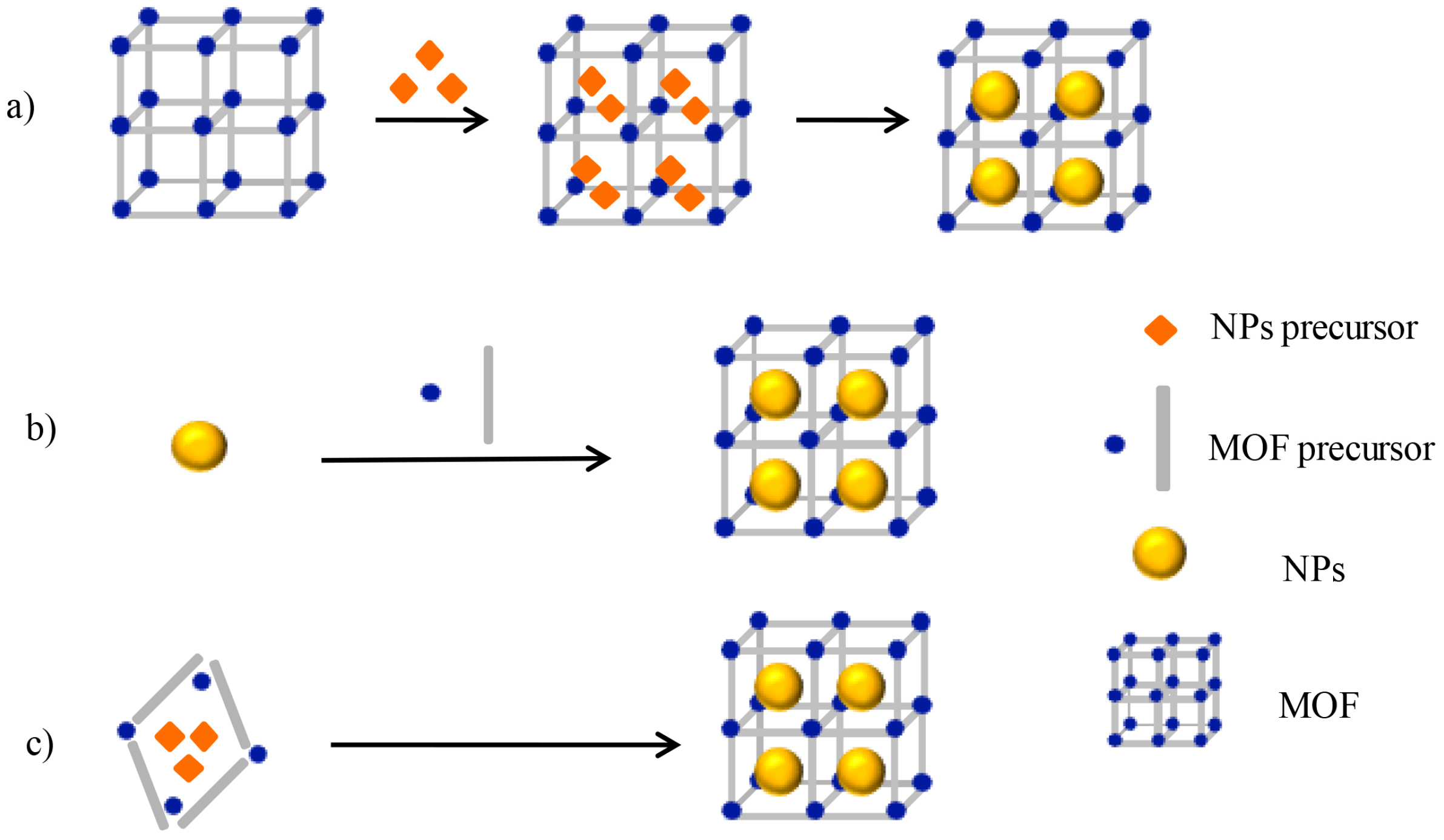
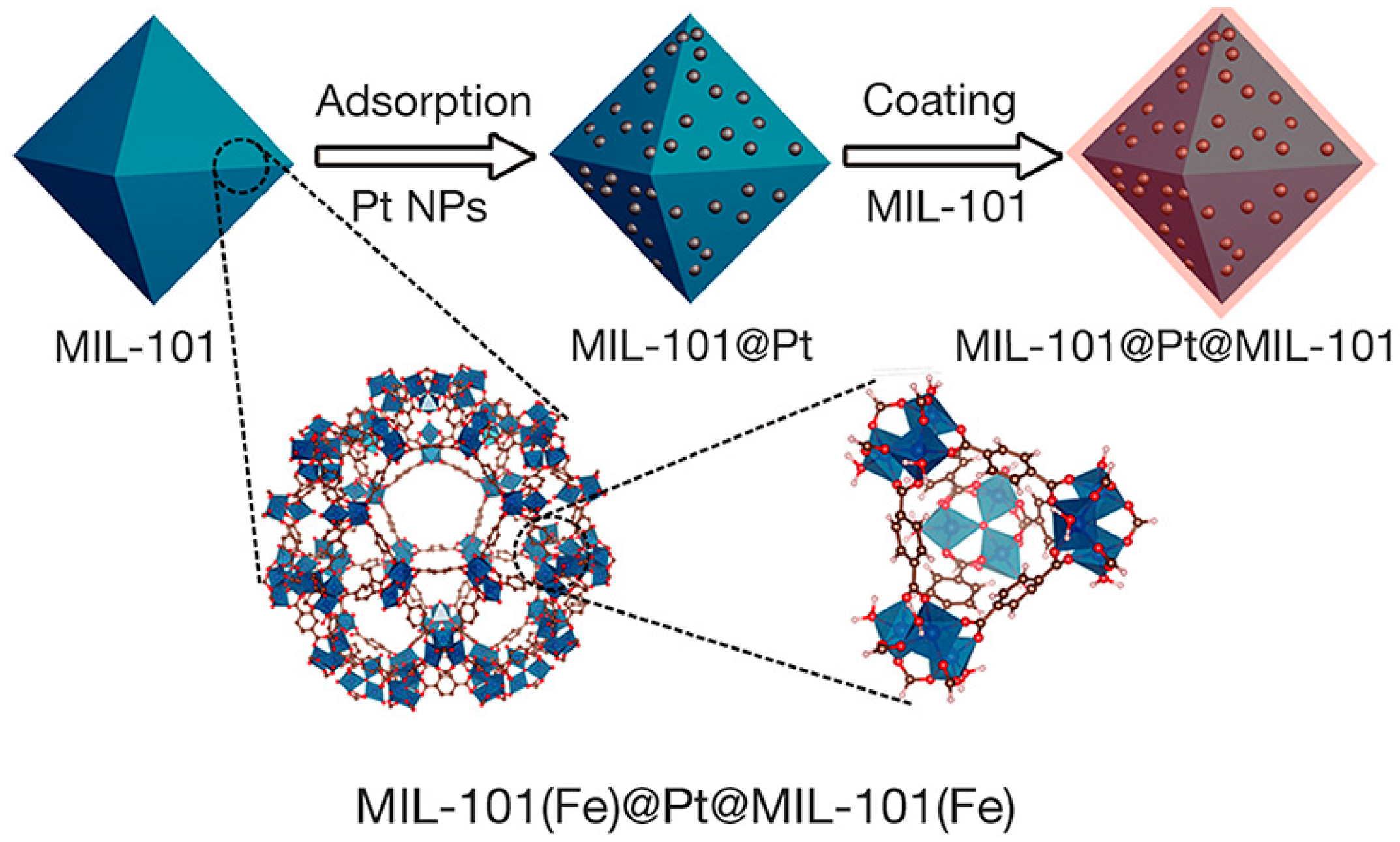
2. General Synthesis of Nanoparticle/MOF Composites
3. Catalysis Applications
3.1. Catalytic CO Oxidation
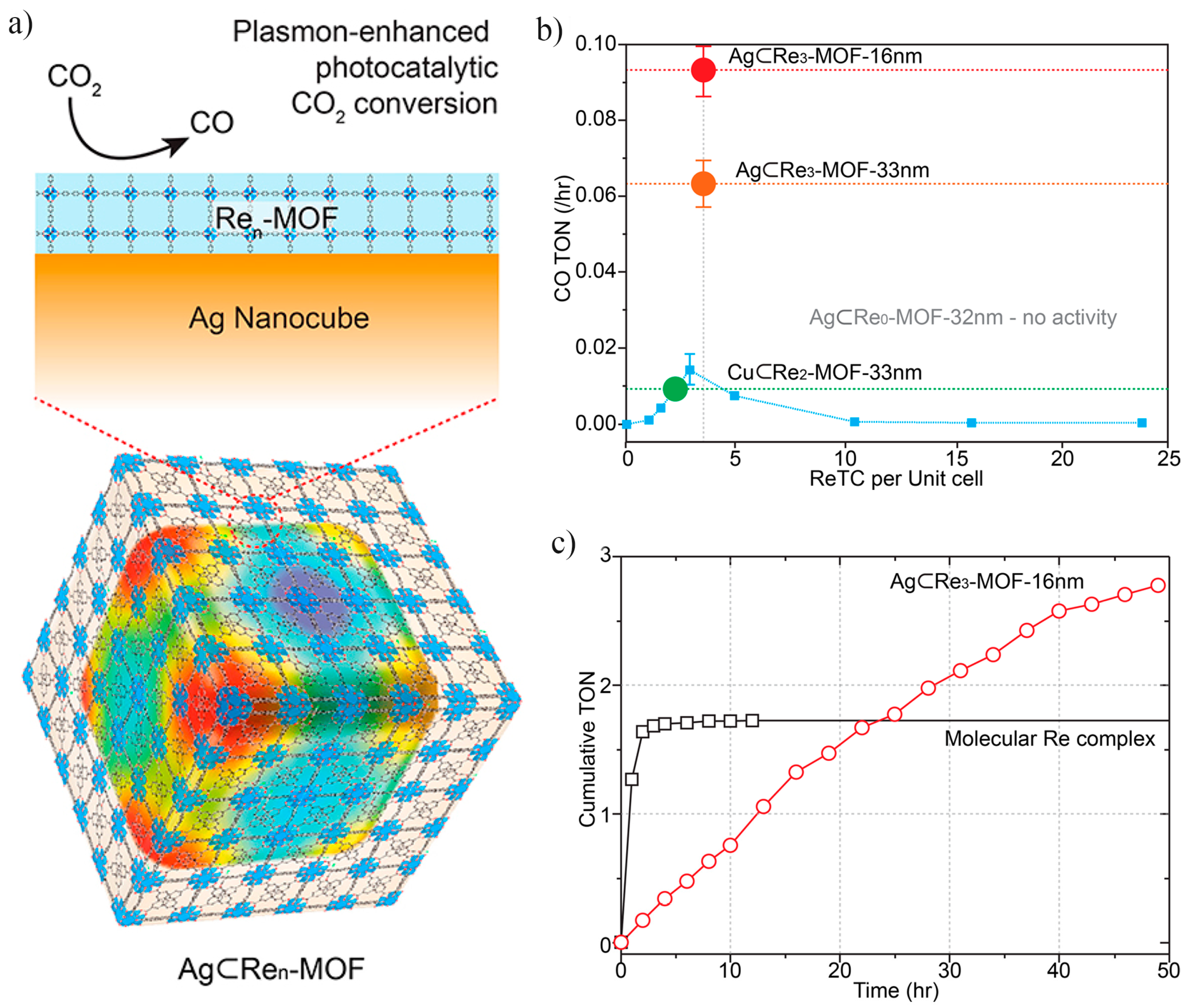
3.2. Catalytic CO2 Conversion
3.3. Catalytic Hydrogen Production
3.3.1. Catalytic Hydrogen Generation from Chemical Hydrides
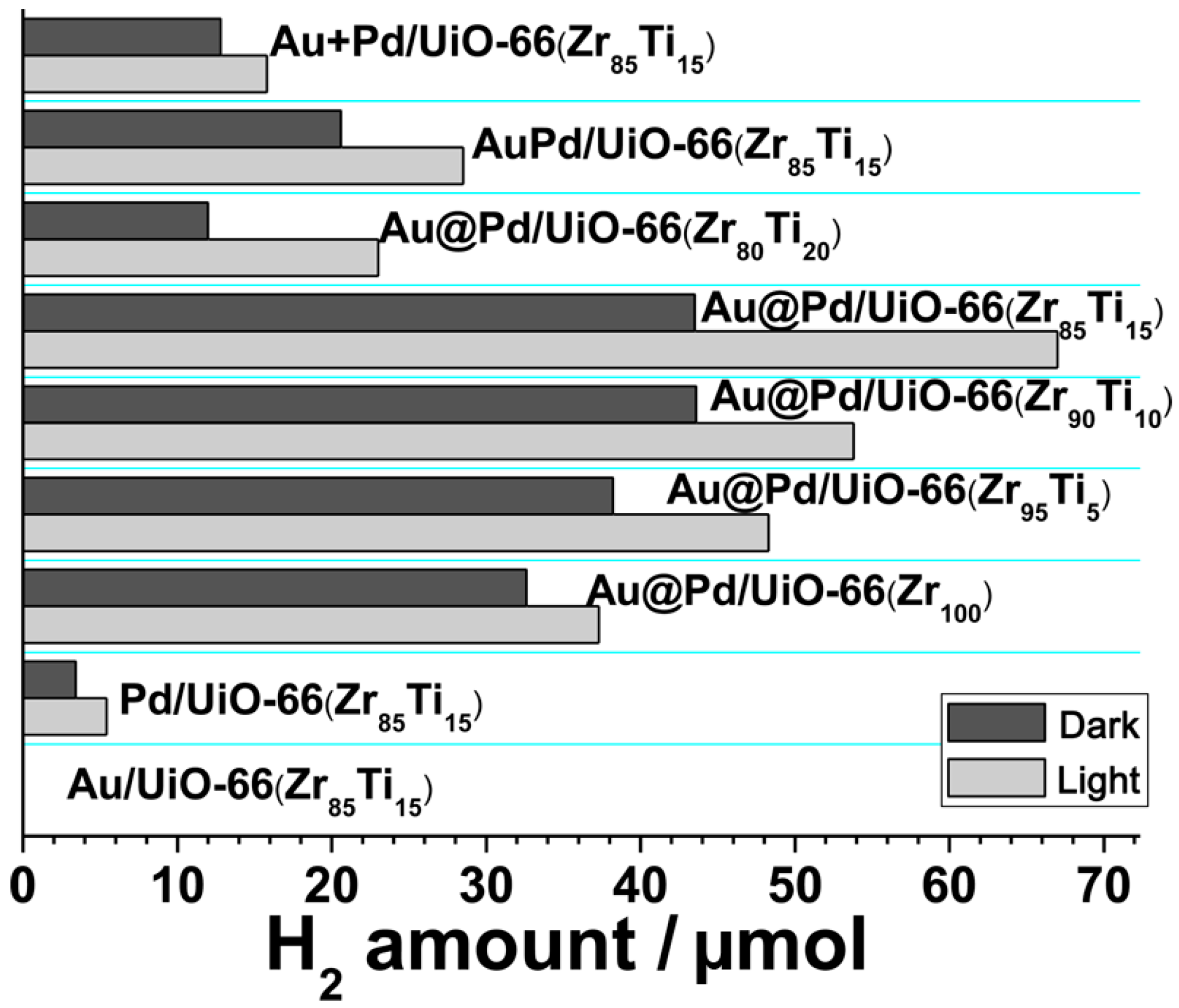
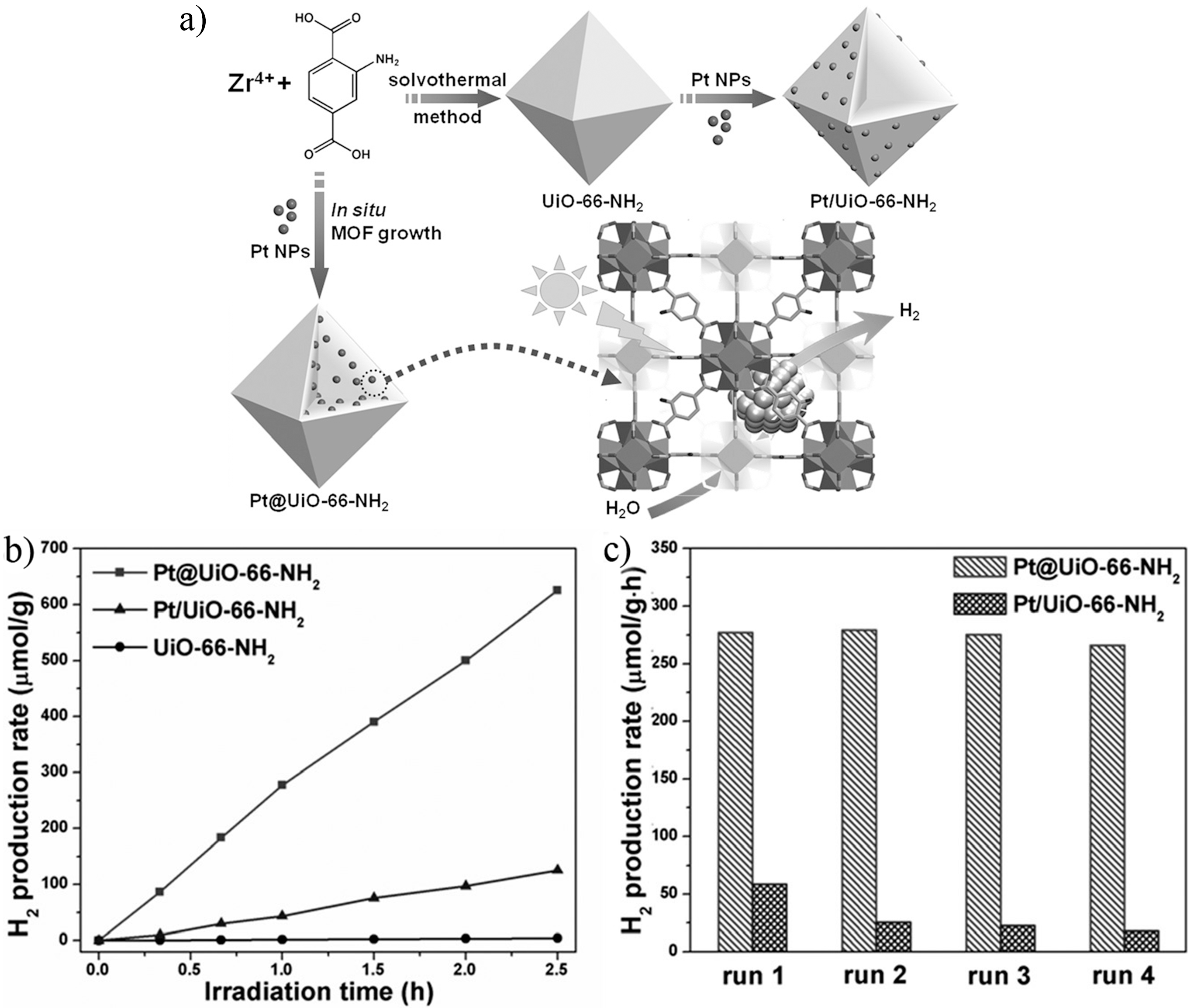
3.3.2. Catalytic Hydrogen Production from Water
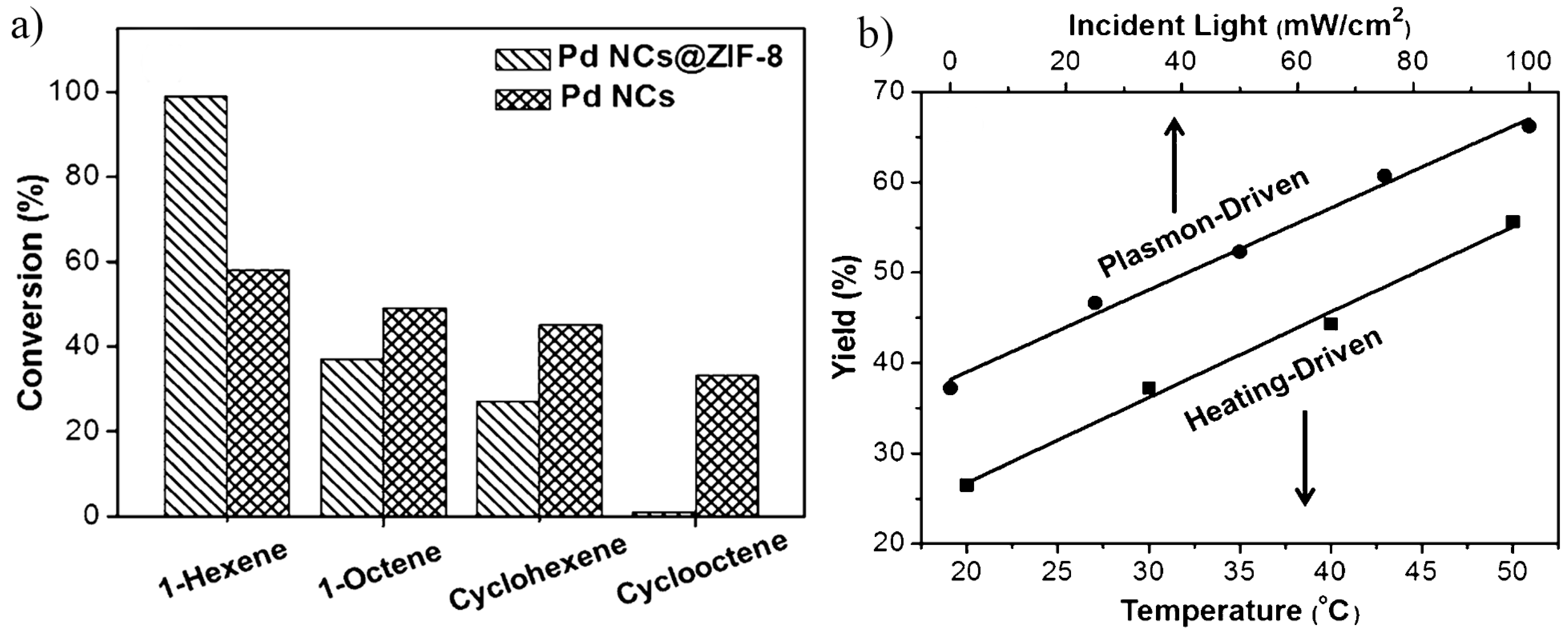
3.4. Organic Reactions
3.4.1. Oxidation of Alcohols and Hydrocarbons
3.4.2. Hydrogenation Reaction
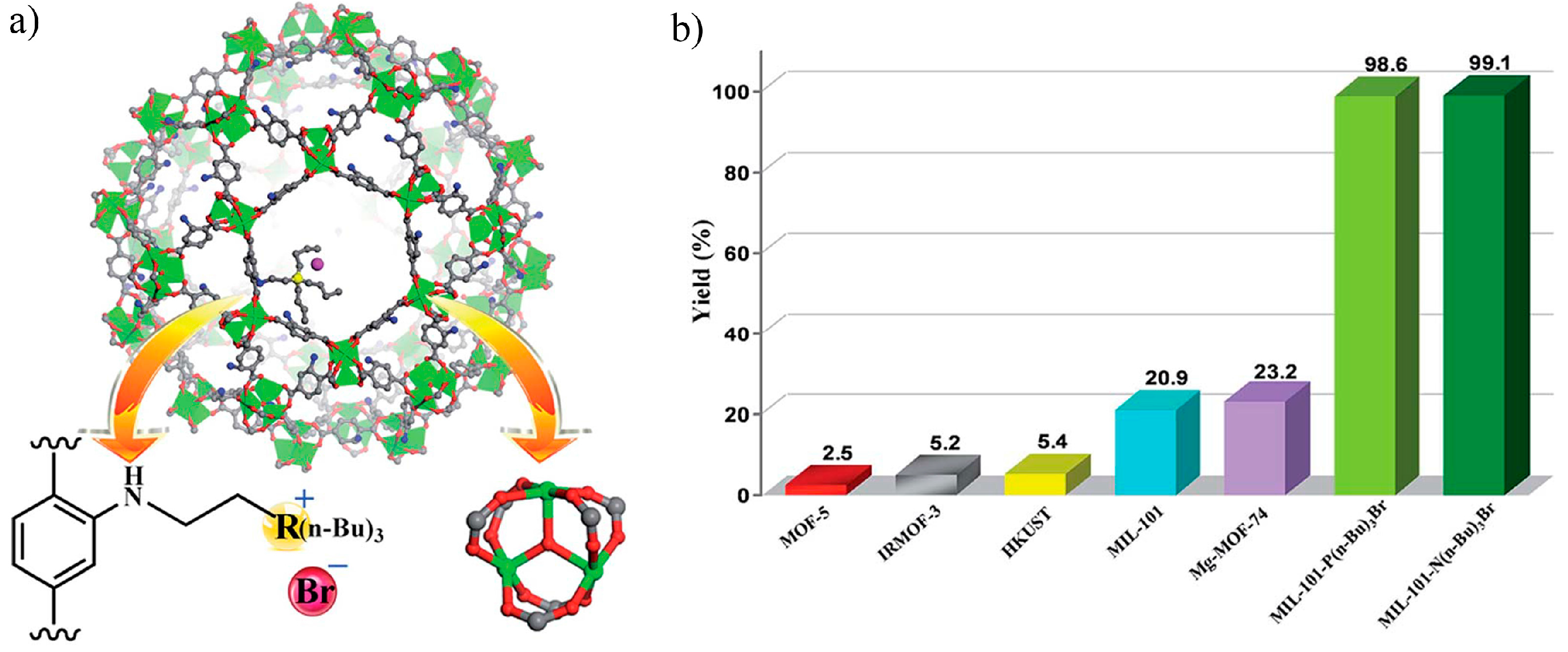
3.4.3. Catalytic C-C Coupling
3.5. Catalytic Remediation of Pollutants
3.5.1. Catalytic Degradation of Organic Pollutants
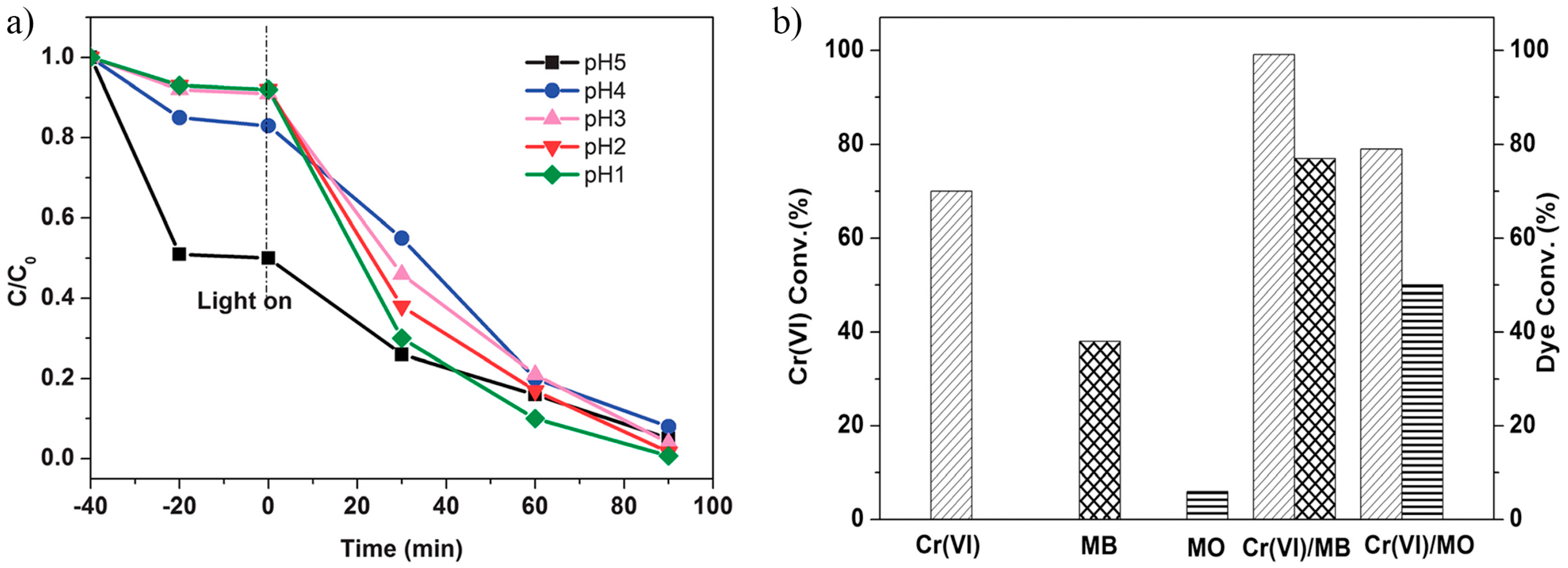
3.5.2. Catalytic Cr(VI) Reduction
4. Conclusions and Outlook
- (1)
- A principal advantage of MOFs is their designable structure with clear chemistry. When metal or metal oxide NPs are introduced, not clear chemistry may be caused because of not only various complex issues of NPs (like defects), but the interaction between MOFs and NPs. This is the most significant challenge for the future development with opportunities. In order to develop a size, structure, and location controllable preparation of NP/MOF composites, the interaction between metal or metal oxide and the MOF support needs to be further investigated. The functionalization and modification of MOFs needs to be also further investigated. The involvement of organic links in the catalytic reaction and the formation of intrinsic structure of MOFs with NPs generate many fundamental thermodynamic and kinetic issues for catalyst investigations. The reported studies are mostly limited within the experimental exploitation of MOFs for various reactions. Regarding the difficulty in the catalyst characterization, more theoretical studies are needed [45,161,162].
- (2)
- For the composites by the pre-synthesized NPs, the polymer capping agent, like PVP, has some negative effects on the catalytic properties. Alternatives to such agents should be investigated. In this regard, biomolecules, like peptide, can be a nice candidate [163,164,165]. Especially, the peptide metal composite can serve as an excellent nitrogen dopant, which will be good for the improvement of the electronic properties of the catalysts, especially, for photocatalyst and electro-photocatalyst.
- (3)
- Since MOFs are now mostly thermal sensitive materials, innovation in the post treatment or modification of the NP/MOF composites at low temperatures is extremely important. We attempted to use the room temperature electron reduction [166,167] for the preparation of noble metal/MOF composite catalyst. The present result shows the NPs cannot disperse well into the pores of MOFs. Further improvement is required. Other new approaches are also needed.
- (4)
- (5)
- The development of MOF based solid acid and solid base will create more opportunities for NP/MOF composite catalysts [170].
- (6)
- MOF itself has been demonstrated to be an excellent single site catalyst, with which metal nodes in MOFs mimic homogeneous catalysts not only functionally but also mechanistically [171,172]. It provides a blueprint for the development of advanced heterogeneous catalysts with similar degrees of tunability to their homogeneous counterparts. It could open new ways for the investigations of NP/MOF catalysts or MOF supported NP catalysts, which possess characteristics and advantages of both heterogeneous and homogeneous catalysts.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, X.W.; Sun, T.J.; Hu, J.L.; Wang, S.D. Composites of metal–organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Ricco, R.; Malfatti, L.; Takahashi, M.; Hill, A.J.; Falcaro, P. Applications of magnetic metal-organic framework composites. J. Mater. Chem. A 2013, 1, 13033–13045. [Google Scholar] [CrossRef]
- Li, S.Z.; Huo, F.W. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef] [PubMed]
- Kitao, T.; Zhang, Y.Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of MOFs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.D.; Zhao, M. Incorporation of Molecular Catalysts in Metal-Organic Frameworks for Highly Efficient Heterogeneous Catalysis. Adv. Mater. 2017, 29, 1605446. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mu, C.; Yan, B.; Qin, X.; Shen, C.; Xue, H.; Pang, H. Nanoparticle/MOF composites: Preparations and applications. Mater. Horiz. 2017, 4, 557–569. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal Organic Frameworks as Versatile Hosts of Au Nanoparticles in Heterogeneous Catalysis. ACS Catal. 2017, 7, 2896–2919. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Du, D.Y.; Qin, J.S.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Recent advances in porous polyoxometalate-based metal-organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, M.; Chen, X.; Yue, B.; He, H. Heterogeneous Catalysis of Polyoxometalate Based Organic-Inorganic Hybrids. Materials 2015, 8, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- Fujie, K.; Otsubo, K.; Ikeda, R.; Yamada, T.; Kitagawa, H. Low temperature ionic conductor: Ionic liquid incorporated within a metal–organic framework. Chem. Sci. 2015, 6, 4306. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Li, Y. Metal–organic-framework-based catalysts for hydrogenation reactions. Chin. J. Catal. 2017, 38, 1108–1126. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, C.-J. Cu3(BTC)2: CO oxidation over MOF based catalysts. Chem. Commun. 2011, 47, 2167–2169. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Du, X.D.; Li, J.; Guo, X.X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B Environ. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, Y.Q.; Li, J.; Wang, P. Photocatalytic CO2 reduction in metal–organic frameworks: A mini review. J. Mol. Struct. 2015, 1083, 127–136. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Dai, T.L.; Zhang, F.; Zhang, J.; Chu, G.; Quan, C.S. Fe3O4@UiO-66-NH2 core-shell nanohybrid as stable heterogeneous catalyst for Knoevenagel condensation. Chin. J. Catal. 2016, 37, 2106–2113. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal-organic frameworks catalyzed C-C and C-heteroatom coupling reactions. Chem. Soc. Rev. 2015, 44, 1922–1947. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Fixation of carbon dioxide into dimethyl carbonate over titanium-based zeolitic thiophene-benzimidazolate framework. Sci. Rep. 2017, 7, 655. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Titanium-based zeolitic imidazolate framework for chemical fixation of carbon dioxide. Green Chem. 2016, 18, 4855–4858. [Google Scholar] [CrossRef]
- Liu, Y.X.; Geng, Y.Y.; Yan, M.Y.; Huang, J. Stable ABTS Immobilized in the MIL-100(Fe) Metal-Organic Framework as an Efficient Mediator for Laccase-Catalyzed Decolorization. Molecules 2017, 22, 920. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Q.; Yu, S.H.; Jiang, H.L. Pd Nanocubes@ ZIF-8: Integration of Plasmon-Driven Photothermal Conversion with a Metal-Organic Framework for Efficient and Selective Catalysis. Angew. Chem. Int. Ed. 2016, 55, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, R.; Huang, J.; Shi, Y.; Zhang, D.; Zhong, X.; Wang, D.; Wu, Y.; Li, Y. Platinum-nickel frame within metal-organic framework fabricated in situ for hydrogen enrichment and molecular sieving. Nat. Commun. 2015, 6, 8248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Xu, Q. Metal-organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Zhou, Y.; Zhao, Y.; Liu, C.J. Recent progresses in the size and structure control of MOF supported noble metal catalysts. Catal. Today 2016, 263, 61–68. [Google Scholar] [CrossRef]
- Aijaz, A.; Karkamkar, A.; Choi, Y.J.; Tsumori, N.; Ronnebro, E.; Autrey, T.; Shioyama, H.; Xu, Q. Immobilizing highly catalytically active Pt nanoparticles inside the pores of metal-organic framework: A double solvents approach. J. Am. Chem. Soc. 2012, 134, 13926–13929. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Li, J.; Xu, Q. Immobilizing metal nanoparticles to metal-organic frameworks with size and location control for optimizing catalytic performance. J. Am. Chem. Soc. 2013, 135, 10210–10213. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Pachfule, P.; Xu, Q. High Catalytic Performance of MIL-101-Immobilized NiRu Alloy Nanoparticles towards the Hydrolytic Dehydrogenation of Ammonia Borane. Eur. J. Inorg. Chem. 2016, 2016, 4353–4357. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Q.L.; Xu, Q. Highly active AuCo alloy nanoparticles encapsulated in the pores of metal-organic frameworks for hydrolytic dehydrogenation of ammonia borane. Chem. Commun. 2014, 50, 5899–5901. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Q.L.; Xu, Q. Non-noble bimetallic CuCo nanoparticles encapsulated in the pores of metal-organic frameworks: Synergetic catalysis in the hydrolysis of ammonia borane for hydrogen generation. Catal. Sci. Technol. 2015, 5, 525–530. [Google Scholar] [CrossRef]
- Zhen, W.; Gao, F.; Tian, B.; Ding, P.; Deng, Y.; Li, Z.; Gao, H.; Lu, G. Enhancing activity for carbon dioxide methanation by encapsulating (1 1 1) facet Ni particle in metal–organic frameworks at low temperature. J. Catal. 2017, 348, 200–211. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, J.; Bradshaw, D. Synthesis and applications of metal-organic framework-quantum dot (QD@MOF) composites. Coord. Chem. Rev. 2016, 307, 267–291. [Google Scholar] [CrossRef]
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal-organic framework. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.D.; Shang, Q.; Xiong, Y.; Zhang, Q.; Luo, Y.; Yu, S.H.; Jiang, H.L. Boosting photocatalytic hydrogen production of a metal-organic framework decorated with platinum nanoparticles: The platinum location matters. Angew. Chem. Int. Ed. 2016, 128, 9535–9539. [Google Scholar] [CrossRef]
- Vetter, S.; Haffer, S.; Wagner, T.; Tiemann, M. Nanostructured Co3O4 as a CO gas sensor: Temperature-dependent behavior. Sens. Actuators B Chem. 2015, 206, 133–138. [Google Scholar] [CrossRef]
- Koop, J.; Deutschmann, O. Detailed surface reaction mechanism for Pt-catalyzed abatement of automotive exhaust gases. Appl. Catal. B Environ. 2009, 91, 47–58. [Google Scholar] [CrossRef]
- Miao, Y.X.; Li, W.C.; Sun, Q.; Shi, L.; He, L.; Wang, J.; Deng, G.M.; Lu, A.H. Nanogold supported on manganese oxide doped alumina microspheres as a highly active and selective catalyst for CO oxidation in a H2-rich stream. Chem. Commun. 2015, 51, 17728–17731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.; Liu, C.J. Perspective on CO oxidation over Pd-based catalysts. Catal. Sci. Technol. 2015, 5, 69–81. [Google Scholar] [CrossRef]
- Jiang, H.L.; Liu, B.; Akita, T.; Haruta, M.; Sakurai, H.; Xu, Q. Au@ ZIF-8: CO oxidation over gold nanoparticles deposited to metal−organic framework. J. Am. Chem. Soc. 2009, 131, 11302–11303. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Fernandez, E.V.; Pieters, C.; van der Linden, B.; Juan-Alcañiz, J.; Serra-Crespo, P.; Verhoeven, M.W.G.M.; Niemantsverdriet, H.; Gascon, J.; Kapteijn, F. Highly dispersed platinum in metal organic framework NH2-MIL-101(Al) containing phosphotungstic acid–Characterization and catalytic performance. J. Catal. 2012, 289, 42–52. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Zhou, K.; Liu, H.; Yadian, B.; Wei, J.; Zhu, H.; Huang, Y. Highly dispersed Au nanoparticles immobilized on Zr-based metal–organic frameworks as heterostructured catalyst for CO oxidation. J. Mater. Chem. A 2013, 1, 14294. [Google Scholar] [CrossRef]
- Liang, Q.; Zhao, Z.; Liu, J.; Wei, Y.C.; Jiang, G.Y.; Duan, A.J. Pd Nanoparticles deposited on metal-organic framework of MIL-53 (Al) an active catalyst for CO oxidation. Acta Phys.-Chim. Sin. 2014, 30, 129–134. [Google Scholar] [CrossRef]
- Zhuang, G.L.; Bai, J.Q.; Zhou, X.; Gao, Y.F.; Huang, H.L.; Cui, H.Q.; Zhong, X.; Zhong, C.L.; Wang, J.G. The Effect of N-Containing Supports on Catalytic CO Oxidation Activity over Highly Dispersed Pt/UiO-67. Eur. J. Inorg. Chem. 2017, 172–178. [Google Scholar] [CrossRef]
- Lin, A.; Ibrahim, A.A.; Arab, P.; El-Kaderi, H.M.; El-Shall, M.S. Palladium Nanoparticles Supported on Ce-Metal-Organic Framework for Efficient CO Oxidation and Low-Temperature CO2 Capture. ACS Appl. Mater. Interfaces 2017, 9, 17961–17968. [Google Scholar] [CrossRef] [PubMed]
- El-Shall, M.S.; Abdelsayed, V.; Khder, A.E.R.S.; Hassan, H.M.A.; El-Kaderi, H.M.; Reich, T.E. Metallic and bimetallic nanocatalysts incorporated into highly porous coordination polymer MIL-101. J. Mater. Chem. 2009, 19, 7625. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, C.; Liu, C.J. Enhanced CO oxidation over thermal treated Ag/Cu-BTC. Catal. Commun. 2013, 38, 74–76. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Zhang, R.; He, D.; Liu, H.; Liao, S. Metal-organic framework as a host for synthesis of nanoscale Co3O4 as an active catalyst for CO oxidation. Catal. Commun. 2011, 12, 875–879. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Jiang, Z.; Wang, X.; Wang, X.; Zhang, S.; Han, P.; Yang, C. Enzymatic conversion of carbon dioxide. Chem. Soc. Rev. 2015, 44, 5981–6000. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Cometto, C.; Ishitani, O.; Robert, M. Electrons, photons, protons and earth-abundant metal complexes for molecular catalysis of CO2 reduction. ACS Catal. 2017, 7, 70–88. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal-Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal-Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Luo, X.; Xu, Z.; Ge, J. Monodispersed gold nanoparticles supported on a zirconium-based porous metal-organic framework and their high catalytic ability for the reverse water-gas shift reaction. Chem. Commun. 2017, 53, 7953–7956. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, M.; Ding, F.; Song, C.; Zhang, G.; Guo, X. Hydrothermally stable MOFs for CO2 hydrogenation over iron-based catalyst to light olefins. J. CO2 Util. 2016, 15, 89–95. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Kafizas, A.; Zafeiratos, S.; Petit, C. CO2 capture and photocatalytic reduction using bifunctional TiO2/MOF nanocomposites under UV-vis irradiation. Appl. Catal. B Environ. 2017, 210, 131–140. [Google Scholar] [CrossRef]
- Li, R.; Hu, J.; Deng, M.; Wang, H.; Wang, X.; Hu, Y.; Jiang, H.L.; Jiang, J.; Zhang, Q.; Xie, Y.; Xiong, Y. Integration of an inorganic semiconductor with a metal-organic framework: A platform for enhanced gaseous photocatalytic reactions. Adv. Mater. 2014, 26, 4783–4788. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, D.; Li, Z. Self-assembly of CPO-27-Mg/TiO2 nanocomposite with enhanced performance for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2016, 183, 47–52. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B Environ. 2015, 162, 494–500. [Google Scholar] [CrossRef]
- Liu, Q.; Low, Z.X.; Li, L.; Razmjou, A.; Wang, K.; Yao, J.; Wang, H. ZIF-8/Zn2GeO4 nanorods with an enhanced CO2 adsorption property in an aqueous medium for photocatalytic synthesis of liquid fuel. J. Mater. Chem. A 2013, 1, 11563. [Google Scholar] [CrossRef]
- Liu, S.; Chen, F.; Li, S.; Peng, X.; Xiong, Y. Enhanced photocatalytic conversion of greenhouse gas CO2 into solar fuels over g-C3N4 nanotubes with decorated transparent ZIF-8 nanoclusters. Appl. Catal. B Environ. 2017, 211, 1–10. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, D.; Rungtaweevoranit, B.; Trickett, C.A.; Barmanbek, J.T.; Alshammari, A.S.; Yang, P.; Yaghi, O.M. Plasmon-Enhanced Photocatalytic CO2 Conversion within Metal-Organic Frameworks under Visible Light. J. Am. Chem. Soc. 2017, 139, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Z.; Liu, H.; Wang, Y. Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H 2 evolution and CO2 reduction. Appl. Catal. B Environ. 2017, 200, 448–457. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent Progress in Metal-Organic Frameworks for Applications in Electrocatalytic and Photocatalytic Water Splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.H.; Wang, J.Q.; Sun, J.; Huang, Y.; Zhang, J.P.; Zhang, X.P.; Zhang, S.J. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach. Green Chem. 2015, 17, 108–122. [Google Scholar] [CrossRef]
- Sołtys-Brzostek, K.; Terlecki, M.; Sokołowski, K.; Lewiński, J. Chemical fixation and conversion of CO2 into cyclic and cage-type metal carbonates. Coord. Chem. Rev. 2017, 334, 199–231. [Google Scholar] [CrossRef]
- Luo, Q.X.; Ji, M.; Lu, M.H.; Hao, C.; Qiu, J.S.; Li, Y.Q. Organic electron-rich N-heterocyclic compound as a chemical bridge: Building a Brönsted acidic ionic liquid confined in MIL-101 nanocages. J. Mater. Chem. A 2013, 1, 6530–6534. [Google Scholar] [CrossRef]
- Liang, J.; Chen, R.P.; Wang, X.Y.; Liu, T.T.; Wang, X.S.; Huang, Y.B.; Cao, R. Postsynthetic ionization of an imidazole-containing metal–organic framework for the cycloaddition of carbon dioxide and epoxides. Chem. Sci. 2017, 8, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.G.; Yao, B.J.; Jiang, W.L.; Li, J.T.; Fu, Q.J.; Li, Y.A.; Liu, Z.H.; Ma, J.P.; Dong, Y.B. Bifunctional Imidazolium-Based Ionic Liquid Decorated UiO-67 Type MOF for Selective CO2 Adsorption and Catalytic Property for CO2 Cycloaddition with Epoxides. Inorg. Chem. 2017, 56, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, B.; Liu, K.; Zhang, X.; Zou, W.; Yang, Y.; Li, G.; Shi, Z.; Feng, S. Bifunctional MOF heterogeneous catalysts based on the synergy of dual functional sites for efficient conversion of CO2 under mild and co-catalyst free conditions. J. Mater. Chem. A 2015, 3, 23136–23142. [Google Scholar] [CrossRef]
- Tharun, J.; Bhin, K.M.; Roshan, R.; Kim, D.W.; Kathalikkattil, A.C.; Babu, R.; Ahn, H.Y.; Won, Y.S.; Park, D.W. Ionic liquid tethered post functionalized ZIF-90 framework for the cycloaddition of propylene oxide and CO2. Green Chem. 2016, 18, 2479–2487. [Google Scholar] [CrossRef]
- Gu, X.; Lu, Z.H.; Jiang, H.L.; Akita, T.; Xu, Q. Synergistic catalysis of metal-organic framework-immobilized Au-Pd nanoparticles in dehydrogenation of formic acid for chemical hydrogen storage. J. Am. Chem. Soc. 2011, 133, 11822–11825. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Z.; Aranishi, K.; Xu, Q. ZIF-8 immobilized nickel nanoparticles: Highly effective catalysts for hydrogen generation from hydrolysis of ammonia borane. Chem. Commun. 2012, 48, 3173–3175. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Xu, Q. Metal-Organic Framework Supported Bimetallic Ni@Pt Nanoparticles as High-performance Catalysts for Hydrogen Generation from Hydrazine in Aqueous Solution. ChemCatChem 2013, 5, 3000–3004. [Google Scholar] [CrossRef]
- Cao, N.; Liu, T.; Su, J.; Wu, X.; Luo, W.; Cheng, G. Ruthenium supported on MIL-101 as an efficient catalyst for hydrogen generation from hydrolysis of amine boranes. New J. Chem. 2014, 38, 4032. [Google Scholar] [CrossRef]
- Cao, N.; Yang, L.; Dai, H.; Liu, T.; Su, J.; Wu, X.; Luo, W.; Cheng, G. Immobilization of ultrafine bimetallic Ni-Pt nanoparticles inside the pores of metal-organic frameworks as efficient catalysts for dehydrogenation of alkaline solution of hydrazine. Inorg. Chem. 2014, 53, 10122–10128. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Cao, N.; Yang, L.; Su, J.; Luo, W.; Cheng, G. AgPd nanoparticles supported on MIL-101 as high performance catalysts for catalytic dehydrogenation of formic acid. J. Mater. Chem. A 2014, 2, 11060. [Google Scholar] [CrossRef]
- Xia, B.; Cao, N.; Dai, H.; Su, J.; Wu, X.; Luo, W.; Cheng, G. Bimetallic Nickel-Rhodium Nanoparticles Supported on ZIF-8 as Highly Efficient Catalysts for Hydrogen Generation from Hydrazine in Alkaline Solution. ChemCatChem 2014, 6, 2549–2552. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Xu, Q.; Yu, S.H.; Jiang, H.L. Tiny Pd@Co core-shell nanoparticles confined inside a metal-organic framework for highly efficient catalysis. Small 2015, 11, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Xia, B.; Wen, L.; Du, C.; Su, J.; Luo, W.; Cheng, G. Synergistic catalysis of AgPd@ZIF-8 on dehydrogenation of formic acid. Appl. Catal. B Environ. 2015, 165, 57–62. [Google Scholar] [CrossRef]
- Ke, F.; Wang, L.; Zhu, J. An efficient room temperature core-shell AgPd@MOF catalyst for hydrogen production from formic acid. Nanoscale 2015, 7, 8321–8325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cao, N.; Luo, W.; Cheng, G. Nanoscale MIL-101 supported RhNi nanoparticles: An efficient catalyst for hydrogen generation from hydrous hydrazine. J. Mater. Chem. A 2015, 3, 12468–12475. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, N.; Su, J.; Luo, W.; Cheng, G. NiIr Nanoparticles Immobilized on the Pores of MIL-101 as Highly Efficient Catalyst toward Hydrogen Generation from Hydrous Hydrazine. ACS Sustain. Chem. Eng. 2015, 3, 1086–1093. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, X.; Liu, P.; Wang, T.; Su, H. Controlling catalytic dehydrogenation of formic acid over low-cost transition metal-substituted AuPd nanoparticles immobilized by functionalized metal–organic frameworks at room temperature. J. Mater. Chem. A 2016, 4, 16645–16652. [Google Scholar] [CrossRef]
- Gao, S.T.; Liu, W.; Feng, C.; Shang, N.Z.; Wang, C. A Ag-Pd alloy supported on an amine-functionalized UiO-66 as an efficient synergetic catalyst for the dehydrogenation of formic acid at room temperature. Catal. Sci. Technol. 2016, 6, 869–874. [Google Scholar] [CrossRef]
- Wen, M.; Cui, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Non-Noble-Metal Nanoparticle Supported on Metal-Organic Framework as an Efficient and Durable Catalyst for Promoting H2 Production from Ammonia Borane under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2016, 8, 21278–21284. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Kuwahara, Y.; Mori, K.; Yamashita, H. Enhancement of Catalytic Activity Over AuPd Nanoparticles Loaded Metal Organic Framework under Visible Light Irradiation. Top. Catal. 2016, 59, 1765–1771. [Google Scholar] [CrossRef]
- Liu, P.; Gu, X.; Kang, K.; Zhang, H.; Cheng, J.; Su, H. Highly Efficient Catalytic Hydrogen Evolution from Ammonia Borane Using the Synergistic Effect of Crystallinity and Size of Noble-Metal-Free Nanoparticles Supported by Porous Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 10759–10767. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Plasmonic Au@Pd Nanoparticles Supported on a Basic Metal–Organic Framework: Synergic Boosting of H2 Production from Formic Acid. ACS Energy Lett. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Toyao, T.; Saito, M.; Horiuchi, Y.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Matsuoka, M. Efficient hydrogen production and photocatalytic reduction of nitrobenzene over a visible-light-responsive metal–organic framework photocatalyst. Catal. Sci. Technol. 2013, 3, 2092. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kamegawa, T.; Yamashita, H. Amine-functionalized MIL-101(Cr) with imbedded platinum nanoparticles as a durable photocatalyst for hydrogen production from water. Chem. Commun. 2014, 50, 11645–11648. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Ma, J.; Lu, G. Small-sized Ni(1 1 1) particles in metal-organic frameworks with low over-potential for visible photocatalytic hydrogen generation. Appl. Catal. B Environ. 2016, 190, 12–25. [Google Scholar] [CrossRef]
- He, J.; Yan, Z.; Wang, J.; Xie, J.; Jiang, L.; Shi, Y.; Yuan, F.; Yu, F.; Sun, Y. Significantly enhanced photocatalytic hydrogen evolution under visible light over CdS embedded on metal-organic frameworks. Chem. Commun. 2013, 49, 6761–6763. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Luo, M.; Liu, Y.; Liang, R.; Jing, F.; Wu, L. Noble-metal-free MoS2 co-catalyst decorated UiO-66/CdS hybrids for efficient photocatalytic H2 production. Appl. Catal. B Environ. 2015, 166–167, 445–453. [Google Scholar] [CrossRef]
- Peters, A.W.; Li, Z.; Farha, O.K.; Hupp, J.T. Toward Inexpensive Photocatalytic Hydrogen Evolution: A Nickel Sulfide Catalyst Supported on a High-Stability Metal-Organic Framework. ACS Appl. Mater. Interfaces 2016, 8, 20675–20681. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Jin, Z.; Yang, H.; Lu, G.; Bi, Y. Peculiar synergetic effect of MoS2 quantum dots and graphene on Metal-Organic Frameworks for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2017, 210, 45–56. [Google Scholar] [CrossRef]
- Lin, R.; Shen, L.; Ren, Z.; Wu, W.; Tan, Y.; Fu, H.; Zhang, J.; Wu, L. Enhanced photocatalytic hydrogen production activity via dual modification of MOF and reduced graphene oxide on CdS. Chem. Commun. 2014, 50, 8533–8535. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gu, L.; Zhou, J.; Liu, X.; Teng, F.; Li, C.; Shen, Y.; Yuan, Y. Quasi-Polymeric Metal-Organic Framework UiO-66/g-C3N4 Heterojunctions for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Adv. Mater. Interfaces 2015, 2, 1500037. [Google Scholar] [CrossRef]
- Kong, X.J.; Lin, Z.; Zhang, Z.M.; Zhang, T.; Lin, W. Hierarchical Integration of Photosensitizing Metal-Organic Framework and Nickel-Containing Polyoxometalates for Efficient Visible-Light Driven Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 6411–6416. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Li, Y.; Tang, Z.; Jiang, H. Metal−organic framework supported gold nanoparticles as a highly active heterogeneous catalyst for aerobic oxidation of alcohols. J. Phys. Chem. C 2010, 114, 13362–13369. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Luque, R.; Li, Y. Metal−organic framework encapsulated Pd nanoparticles: Towards advanced heterogeneous catalysts. Chem. Sci. 2014, 5, 3708–3714. [Google Scholar] [CrossRef]
- Liu, H.; Chang, L.; Chen, L.; Li, Y. In situ one-step synthesis of metal–organic framework encapsulated naked Pt nanoparticles without additional reductants. J. Mater. Chem. A 2015, 3, 8028–8033. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. CdS-decorated UiO–66(NH2) nanocomposites fabricated by a facile photodeposition process: An efficient and stable visible-light-driven photocatalyst for selective oxidation of alcohols. J. Mater. Chem. A 2013, 1, 11473. [Google Scholar] [CrossRef]
- Liu, L.; Song, Y.; Chong, H.; Yang, S.; Xiang, J.; Jin, S.; Kang, X.; Zhang, J.; Yu, H.; Zhu, M. Size-confined growth of atom-precise nanoclusters in metal-organic frameworks and their catalytic applications. Nanoscale 2016, 8, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lu, J.Y.; Chen, Q.Y.; Qu, L.L.; Lu, Y.Q.; Gao, G.F. Eu-Based MOF/graphene oxide composite: A novel photocatalyst for the oxidation of benzyl alcohol using water as oxygen source. New J. Chem. 2017, 41, 3882–3886. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, X.; Li, Y. Controlled growth of dense and ordered metal-organic framework nanoparticles on graphene oxide. Chem. Commun. 2015, 51, 3874–3877. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shen, M.N.; Zhao, X.J.; Wang, P.C.; Lu, M. Polyoxometalate-Based Metal-Organic Frameworks as Catalysts for the Selective Oxidation of Alcohols in Micellar Systems. ChemPlusChem 2014, 79, 872–878. [Google Scholar] [CrossRef]
- Luan, Y.; Qi, Y.; Gao, H.; Zheng, N.; Wang, G. Synthesis of an amino-functionalized metal–organic framework at a nanoscale level for gold nanoparticle deposition and catalysis. J. Mater. Chem. A 2014, 2, 20588–20596. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Aerobic oxidation of alcohols in visible light on Pd-grafted Ti cluster. Tetrahedron 2017, 73, 5577–5580. [Google Scholar] [CrossRef]
- Sun, Z.; Li, G.; Liu, L.; Liu, H.O. Au nanoparticles supported on Cr-based metal-organic framework as bimetallic catalyst for selective oxidation of cyclohexane to cyclohexanone and cyclohexanol. Catal. Commun. 2012, 27, 200–205. [Google Scholar] [CrossRef]
- Long, J.; Liu, H.; Wu, S.; Liao, S.; Li, Y. Selective Oxidation of Saturated Hydrocarbons Using Au–Pd Alloy Nanoparticles Supported on Metal–Organic Frameworks. ACS Catal. 2013, 3, 647–654. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Chen, X.; Wu, D.; Yi, Z.; Cao, R. Bimetallic alloy nanocrystals encapsulated in ZIF-8 for synergistic catalysis of ethylene oxidative degradation. Chem. Commun. 2014, 50, 10115–10117. [Google Scholar] [CrossRef] [PubMed]
- Granadeiro, C.M.; Barbosa, A.D.; Silva, P.; Paz, F.A.A.; Saini, V.K.; Pires, J.; Castro, B.; Balula, S.S.; Cunha-Silva, L. Monovacant polyoxometalates incorporated into MIL-101 (Cr): Novel heterogeneous catalysts for liquid phase oxidation. Appl. Catal. A: Gen. 2013, 453, 316–326. [Google Scholar] [CrossRef]
- Tong, J.; Wang, W.; Su, L.; Li, Q.; Liu, F.; Ma, W.; Lei, Z.; Bo, L. Highly selective oxidation of cyclohexene to 2-cyclohexene-1-one over polyoxometalate/metal-organic framework hybrids with greatly improved performance. Catal. Sci. Technol. 2017, 7, 222–230. [Google Scholar] [CrossRef]
- Aguado, S.; El-Jamal, S.; Meunier, F.; Canivet, J.; Farrusseng, D. A Pt/Al2O3-supported metal-organic framework film as the size-selective core-shell hydrogenation catalyst. Chem. Commun. 2016, 52, 7161–7163. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, S.; Fan, Y. A novel strategy for producing highly dispersed Pd particles on ZIF-8 through the occupation and unoccupation of carboxyl groups and its application in selective diene hydrogenation. J. Catal. 2015, 327, 54–57. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, G.; Cui, C.; Liu, Y.; Li, S.; Yan, W.; Xing, C.; Chi, Y.R.; Yang, Y.; Huo, F. A family of metal-organic frameworks exhibiting size-selective catalysis with encapsulated noble-metal nanoparticles. Adv. Mater. 2014, 26, 4056–4060. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Li, S.; Cui, C.; Wu, J.; Chen, H.; Huo, F. Designable Yolk–Shell Nanoparticle@MOF Petalous Heterostructures. Chem. Mater. 2014, 26, 1119–1125. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Zhan, W.; Cao, Z.; Chen, J.; Jiang, Q.; Jiang, Y.; Xie, Z.; Kuang, Q.; Zheng, L. Controlled Encapsulation of Flower-like Rh-Ni Alloys with MOFs via Tunable Template Dealloying for Enhanced Selective Hydrogenation of Alkyne. ACS Appl. Mater. Interfaces 2016, 8, 31059–31066. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, B.; Qiu, X.; Wang, X.; Luque, R.; Li, Y. Seed-mediated growth of MOF-encapsulated Pd@Ag core-shell nanoparticles: Toward advanced room temperature nanocatalysts. Chem. Sci. 2016, 7, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Tan, X.; Dou, R.; Pei, Y.; Qiao, M.; Sun, B.; Zong, B. Ru–B nanoparticles on metal–organic frameworks as excellent catalysts for hydrogenation of benzene to cyclohexane under mild reaction conditions. Green Chem. 2016, 18, 2216–2221. [Google Scholar] [CrossRef]
- Chen, L.; Huang, W.; Wang, X.; Chen, Z.; Yang, X.; Luque, R.; Li, Y. Catalytically active designer crown-jewel Pd-based nanostructures encapsulated in metal-organic frameworks. Chem. Commun. 2017, 53, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Chen, X.D.; Liu, H.L.; Li, Y.W. Encapsulation of Mono- or Bimetal Nanoparticles Inside Metal-Organic Frameworks via In situ Incorporation of Metal Precursors. Small 2015, 11, 2642–2648. [Google Scholar] [CrossRef] [PubMed]
- Hermannsdorfer, J.; Friedrich, M.; Miyajima, N.; Albuquerque, R.Q.; Kummel, S.; Kempe, R. Ni/Pd@MIL-101: Synergistic catalysis with cavity-conform Ni/Pd nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 11473–11477. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Huang, N.; Wang, J.; Wang, T. Geometric effect in the highly selective hydrogenation of 3-methylcrotonaldehyde over Pt@ZIF-8 core–shell catalysts. Catal. Sci. Technol. 2017, 7, 2601–2608. [Google Scholar] [CrossRef]
- Zhao, M.; Yuan, K.; Wang, Y.; Li, G.; Guo, J.; Gu, L.; Hu, W.; Zhao, H.; Tang, Z. Metal-organic frameworks as selectivity regulators for hydrogenation reactions. Nature 2016, 539, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Parthasarathi, R.; Davis, R.W.; El Gabaly, F.; Sale, K.L.; Simmons, B.A.; Singh, S.; Allendorf, M.D. MOF-Based Catalysts for Selective Hydrogenolysis of Carbon–Oxygen Ether Bonds. ACS Catal. 2015, 6, 55–59. [Google Scholar] [CrossRef]
- Aijaz, A.; Zhu, Q.L.; Tsumori, N.; Akita, T.; Xu, Q. Surfactant-free Pd nanoparticles immobilized to a metal-organic framework with size- and location-dependent catalytic selectivity. Chem. Commun. 2015, 51, 2577–2580. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X. Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, J.; Li, X.; Yang, Y.; Yang, Q.; Li, C. Assembly of ZIF nanostructures around free Pt nanoparticles: Efficient size-selective catalysts for hydrogenation of alkenes under mild conditions. Chem. Commun. 2013, 49, 3330–3332. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.S.; Mondal, J.; Banerjee, B.; Mondal, P.; Bhaumik, A.; Islam, S.M. Pd-grafted porous metal–organic framework material as an efficient and reusable heterogeneous catalyst for C–C coupling reactions in water. Appl. Catal. A Gen. 2014, 469, 320–327. [Google Scholar] [CrossRef]
- Pourkhosravani, M.; Dehghanpour, S.; Farzaneh, F. Palladium Nanoparticles Supported on Zirconium Metal Organic Framework as an Efficient Heterogeneous Catalyst for the Suzuki–Miyaura Coupling Reaction. Catal. Lett. 2015, 146, 499–508. [Google Scholar] [CrossRef]
- Sun, D.; Li, Z. Double-Solvent Method to Pd Nanoclusters Encapsulated inside the Cavity of NH2–Uio-66(Zr) for Efficient Visible-Light-Promoted Suzuki Coupling Reaction. J. Phys. Chem. C 2016, 120, 19744–19750. [Google Scholar] [CrossRef]
- Yang, Y.; Cong, D.; Hao, S. Template-Directed Ordered Mesoporous Silica@Palladium-Containing Zinc Metal-Organic Framework Composites as Highly Efficient Suzuki Coupling Catalysts. ChemCatChem 2016, 8, 900–905. [Google Scholar] [CrossRef]
- Augustyniak, A.W.; Zawartka, W.; Navarro, J.A.; Trzeciak, A.M. Palladium nanoparticles supported on a nickel pyrazolate metal organic framework as a catalyst for Suzuki and carbonylative Suzuki couplings. Dalton Trans. 2016, 45, 13525–13531. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Shen, L.; Jing, F.; Qin, N.; Wu, L. Preparation of MIL-53(Fe)-Reduced Graphene Oxide Nanocomposites by a Simple Self-Assembly Strategy for Increasing Interfacial Contact: Efficient Visible-Light Photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 9507–9515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Liu, J.; Leong, S.; Lin, X.C.; Wei, J.; Kong, B.; Xu, Y.F.; Low, Z.X.; Yao, J.F.; Wang, H.T. Rapid Construction of ZnO@ZIF-8 Heterostructures with Size-Selective Photocatalysis Properties. ACS Appl. Mater. Interfaces 2016, 8, 9080–9087. [Google Scholar] [CrossRef] [PubMed]
- Araya, T.; Jia, M.; Yang, J.; Zhao, P.; Cai, K.; Ma, W.; Huang, Y. Resin modified MIL-53 (Fe) MOF for improvement of photocatalytic performance. Appl. Catal. B Environ. 2017, 203, 768–777. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Li, H. Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl. Catal. B Environ. 2015, 174–175, 445–454. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Dong, H.; Chen, X.; Leng, L.; Wu, Z.; Peng, L. In situ synthesis of In2S3@MIL-125(Ti) core–shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl. Catal. B Environ. 2016, 186, 19–29. [Google Scholar] [CrossRef]
- Hu, L.; Deng, G.; Lu, W.; Pang, S.; Hu, X. Deposition of CdS nanoparticles on MIL-53(Fe) metal-organic framework with enhanced photocatalytic degradation of RhB under visible light irradiation. Appl. Surf. Sci. 2017, 410, 401–413. [Google Scholar] [CrossRef]
- Hong, J.; Chen, C.; Bedoya, F.E.; Kelsall, G.H.; O’Hare, D.; Petit, C. Carbon nitride nanosheet/metal–organic framework nanocomposites with synergistic photocatalytic activities. Catal. Sci. Technol. 2016, 6, 5042–5051. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Catalytic chromium reduction using formic acid and metal nanoparticles immobilized in a metal-organic framework. Chem. Commun. 2013, 49, 3327–3329. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Vellingiri, K.; Kim, K.H.; Paul, A.K.; Deep, A. Efficient photocatalytic degradation of rhodamine 6G with a quantum dot-metal organic framework nanocomposite. Chemosphere 2016, 154, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.R.; Wu, M.K.; Zhao, W.N.; Liu, P.F.; Yi, F.Y.; Li, G.C.; Tao, K.; Han, L. In Situ Growth of Metal–Organic Framework on BiOBr 2D Material with Excellent Photocatalytic Activity for Dye Degradation. Cryst. Growth Des. 2017, 17, 2309–2313. [Google Scholar] [CrossRef]
- Jahurul Islam, M.; Kim, H.K.; Amaranatha Reddy, D.; Kim, Y.; Ma, R.; Baek, H.; Kim, J.; Kim, T.K. Hierarchical BiOI nanostructures supported on a metal organic framework as efficient photocatalysts for degradation of organic pollutants in water. Dalton Trans. 2017, 46, 6013–6023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.R.; Liu, P.F.; Wu, M.K.; Zhao, W.N.; Li, G.C.; Tao, K.; Yi, F.Y.; Han, L. Enhanced photocatalytic performance of BiOBr/NH2-MIL-125(Ti) composite for dye degradation under visible light. Dalton Trans. 2016, 45, 17521–17529. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, X.; Wang, S.; Niu, H.; Cai, Y. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants. Environ. Sci. Technol. 2015, 49, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, Z.; Zhou, L.; Wang, D.; Li, G. Synthesis of nanoscale titania embedded in MIL-101 for the adsorption and degradation of volatile pollutants with thermal desorption gas chromatography and mass spectrometry detection. J. Sep. Sci. 2014, 37, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Liu, W.J.; Han, H.X.; Yu, H.Q. Hydrophilic swellable metal–organic framework encapsulated Pd nanoparticles as an efficient catalyst for Cr(VI) reduction. J. Mater. Chem. A 2016, 4, 11680–11687. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, J.; Lu, S.; Cen, C.; Chen, Y.; Ren, L.; Feng, W.; Fang, Z. Zirconium metal-organic framework supported highly-dispersed nanosized BiVO4for enhanced visible-light photocatalytic applications. J. Chem. Technol. Biotechnol. 2016, 91, 2785–2792. [Google Scholar] [CrossRef]
- Zhang, C.F.; Qiu, L.G.; Ke, F.; Zhu, Y.J.; Yuan, Y.P.; Xu, G.S.; Jiang, X. A novel magnetic recyclable photocatalyst based on a core–shell metal–organic framework Fe3O4@MIL-100(Fe) for the decolorization of methylene blue dye. J. Mater. Chem. A 2013, 1, 14329. [Google Scholar] [CrossRef]
- Shen, L.; Wu, W.; Liang, R.; Lin, R.; Wu, L. Highly dispersed palladium nanoparticles anchored on UiO-66(NH(2)) metal-organic framework as a reusable and dual functional visible-light-driven photocatalyst. Nanoscale 2013, 5, 9374–9382. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.H.; Janssens, N.; Sree, S.P.; Wiktor, C.; Gobechiya, E.; Fischer, R.A.; Kirschhock, C.E.; Martens, J.A. Local transformation of ZIF-8 powders and coatings into ZnO nanorods for photocatalytic application. Nanoscale 2014, 6, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.; Bhaskaran, B.; Kumar, A.; Singh, G.; Kumar, A.; Rath, N.P. Metal-organic framework MIL-101 supported bimetallic Pd–Cu nanocrystals as efficient catalysts for chromium reduction and conversion of carbon dioxide at room temperature. New J. Chem. 2016, 40, 3109–3118. [Google Scholar] [CrossRef]
- Sha, Z.; Wu, J. Enhanced visible-light photocatalytic performance of BiOBr/UiO-66(Zr) composite for dye degradation with the assistance of UiO-66. RSC Adv. 2015, 5, 39592–39600. [Google Scholar] [CrossRef]
- Shen, L.; Huang, L.; Liang, S.; Liang, R.; Qin, N.; Wu, L. Electrostatically derived self-assembly of NH2-mediated zirconium MOFs with graphene for photocatalytic reduction of Cr(vi). RSC Adv. 2014, 4, 2546–2549. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Zhang, M.H.; Huang, X.W.; Chen, Y.F. DFT insights into the adsorption of NH3-SCR related small gases in Mn-MOF-74. Phys. Chem. Chem. Phys. 2016, 18, 28854–28863. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Johnson, J.K. Screening Lewis pair moieties for catalytic hydrogenation of CO2 in functionalized UiO-66. ACS Catal. 2015, 5, 6219–6229. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Wang, W.; Anderson, C.; Wang, Z.; Wu, W.; Cui, H.; Liu, C.-J. Peptide-templated noble metal catalysts: Syntheses and applications. Chem. Sci. 2017, 8, 3310–3324. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Yang, M.; Zhong, C.-J.; Liu, C.-J. Highly active and stable Pt (111) catalysts synthesized by peptide Assisted room temperature electron reduction for oxygen reduction reaction. Nano Energy 2016, 25, 26–33. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Wang, J.; Zhong, C.-J.; Liu, C.-J. Highly active and stable Pt-Pd alloy catalysts synthesized by room temperature electron reduction for oxygen reduction reaction. Adv. Sci. 2017, 4, 1600486. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Li, M.; Wang, J.; Zhou, X.; Guo, Q.; Yan, J.; Li, Y. Cold plasma applications for green catalyst preparation: Current status and perspective. Chin. J. Catal. 2016, 37, 340–348. [Google Scholar] [CrossRef]
- Qin, L.; Li, Z.; Hu, Q.; Xu, Z.; Guo, X.; Zhang, G. One-pot assembly of metal/organic-acid sites on amine-functionalized ligands of MOFs for photocatalytic hydrogen peroxide splitting. Chem. Commun. 2016, 52, 7110–7113. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, P.; Li, Z.; Xu, Z.; Wang, F.; Ou, H.; Zhang, J.; Zhang, G.; Zeng, E. Ultrathin metal–organic framework membrane production by gel–vapour deposition. Nat. Commun. 2017, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal−organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Metzger, E.D.; Comito, R.J.; Hendon, C.H.; Dinca, M. Mechanism of single-site molecule-like catalytic ethylene dimerization in Ni-MFU-4l. J. Am. Chem. Soc. 2017, 139, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Chaemchuen, S.; Luo, Z.; Zhou, K.; Mousavi, B.; Phatanasri, S.; Jaroniec, M.; Verpoort, F. Defect formation in metal–organic frameworks initiated by the crystal growth-rate and effect on catalytic performance. J. Catal. 2017, 354, 84–91. [Google Scholar] [CrossRef]


| Active Species (wt %) | MOF | T50 a (°C) | T100 b (°C) | Reference |
|---|---|---|---|---|
| Au (5%) | ZIF-8 | 170 | 210 | [41] |
| Au (4%) | UIO-66 | 155 | 225 | [43] |
| Pt (5%) | NH2-MIL-101(Al) | 170 | 207 | [42] |
| Pt (5%) | MIL-101(Cr) | 118 | 150 | [28] |
| Pt (5%) | N-UiO-67 | 100 | 120 | [45] |
| Ag (5%) | Cu3(BTC)2 | 100 | 120 | [48] |
| Pd (2.7%) | MIL-53(Al) | 100 | 115 | [44] |
| Pd (2.9%) | MIL-101(Cr) | 92 | 107 | [47] |
| Pd (5%) | Ce-MOF | 77 | 96 | [46] |
| Co3O4 | ZIF-8 | 58 | 80 | [49] |
| Catalyst | Chemical Hydrides | T (°C) | TOF (h−1) | Refernence |
|---|---|---|---|---|
| AuPd@ED-MIL-101 | HCOOH | 90 | 106 | [74] |
| Ag20Pd80@MIL-101 | HCOOH | 80 | 848 | [79] |
| Ag18Pd82@ZIF-8 | HCOOH | 80 | 580 | [82] |
| Au28Pd47Co25/MIL-101–NH2 | HCOOH | 25 | 347 | [86] |
| Ag25Pd75@NH2-UIO-66 | HCOOH | 25 | 103 | [87] |
| Au@Pd/NH2-UiO-66(Zr85Ti15) | HCOOH | 30 | 200 | [91] |
| Ni@ZIF-8 | NH3BH3 | 25 | 504 | [75] |
| Ru@MIL-101 | NH3BH3 | 25 | 10680 | [77] |
| Ni@MIL-101 | NH3BH3 | 25 | 3238 | [88] |
| Pd@Co@MIL-101 | NH3BH3 | 30 | 3060 | [81] |
| Au7Ni93 @MIL-101 | NH3BH3 | 25 | 3972 | [29] |
| Ru30Ni70@MIL-101 | NH3BH3 | 25 | 16363 | [30] |
| Au6Co94@MIL-101 | NH3BH3 | 25 | 1410 | [31] |
| Cu30Co70@MIL-101 | NH3BH3 | 25 | 1176 | [32] |
| CuCo@MIL-101 | NH3BH3 | 25 | 3102 | [90] |
| FeCo@MIL-101 | NH3BH3 | 25 | 3048 | [90] |
| NiCo@MIL-101 | NH3BH3 | 25 | 2658 | [90] |
| Ni80Pt20@ZIF-8 | Hydrazine | 50 | 90 | [76] |
| Ni66Rh34@ZIF-8 | Hydrazine | 50 | 140 | [80] |
| Ni88Pt12@MIL-101 | Hydrazine | 50 | 375 | [78] |
| Ni42Rh58@MIL-101 | Hydrazine | 50 | 344 | [84] |
| Ni85Ir15@MIL-101 | Hydrazine | 50 | 464 | [85] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.-j. Nanoparticle/Metal–Organic Framework Composites for Catalytic Applications: Current Status and Perspective. Molecules 2017, 22, 2103. https://doi.org/10.3390/molecules22122103
Xiang W, Zhang Y, Lin H, Liu C-j. Nanoparticle/Metal–Organic Framework Composites for Catalytic Applications: Current Status and Perspective. Molecules. 2017; 22(12):2103. https://doi.org/10.3390/molecules22122103
Chicago/Turabian StyleXiang, Wenlong, Yueping Zhang, Hongfei Lin, and Chang-jun Liu. 2017. "Nanoparticle/Metal–Organic Framework Composites for Catalytic Applications: Current Status and Perspective" Molecules 22, no. 12: 2103. https://doi.org/10.3390/molecules22122103
APA StyleXiang, W., Zhang, Y., Lin, H., & Liu, C.-j. (2017). Nanoparticle/Metal–Organic Framework Composites for Catalytic Applications: Current Status and Perspective. Molecules, 22(12), 2103. https://doi.org/10.3390/molecules22122103






