Design, Synthesis, and Fungicidal Activity of Novel Thiosemicarbazide Derivatives Containing Piperidine Fragments
Abstract
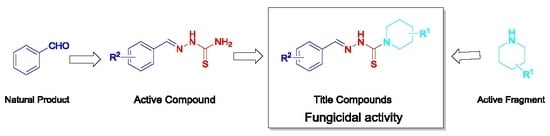
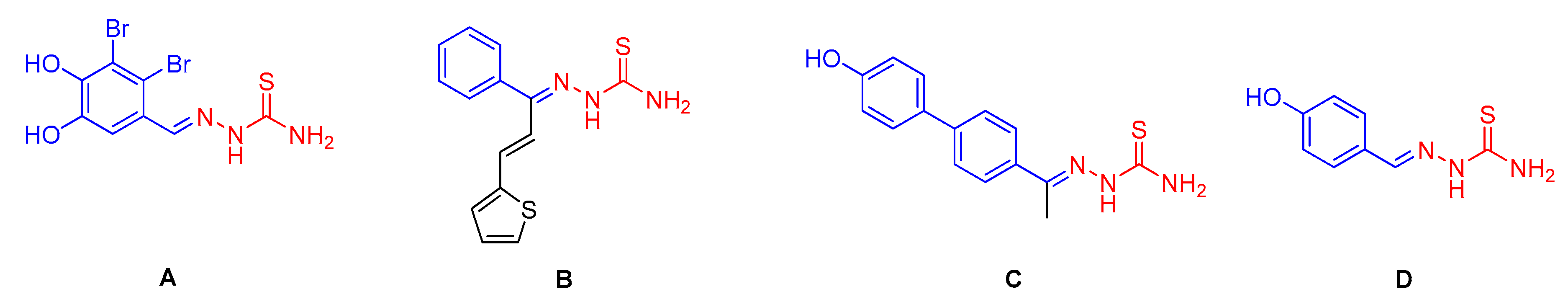
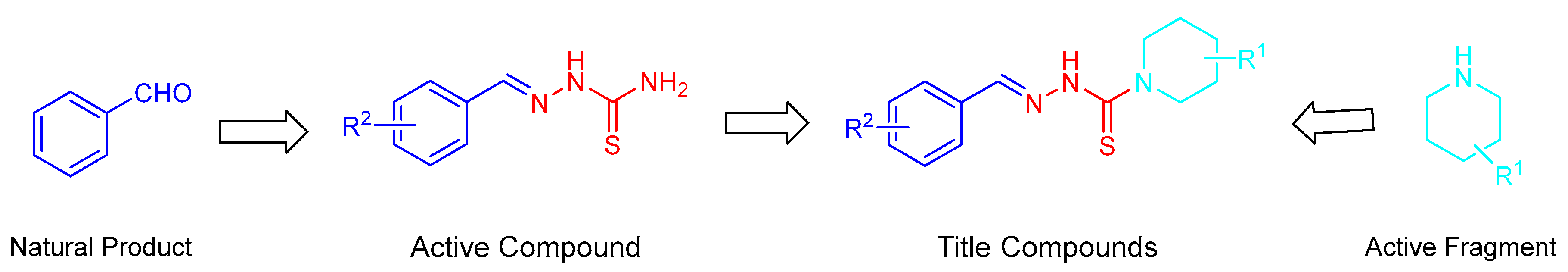
:1. Introduction
2. Results and Discussion
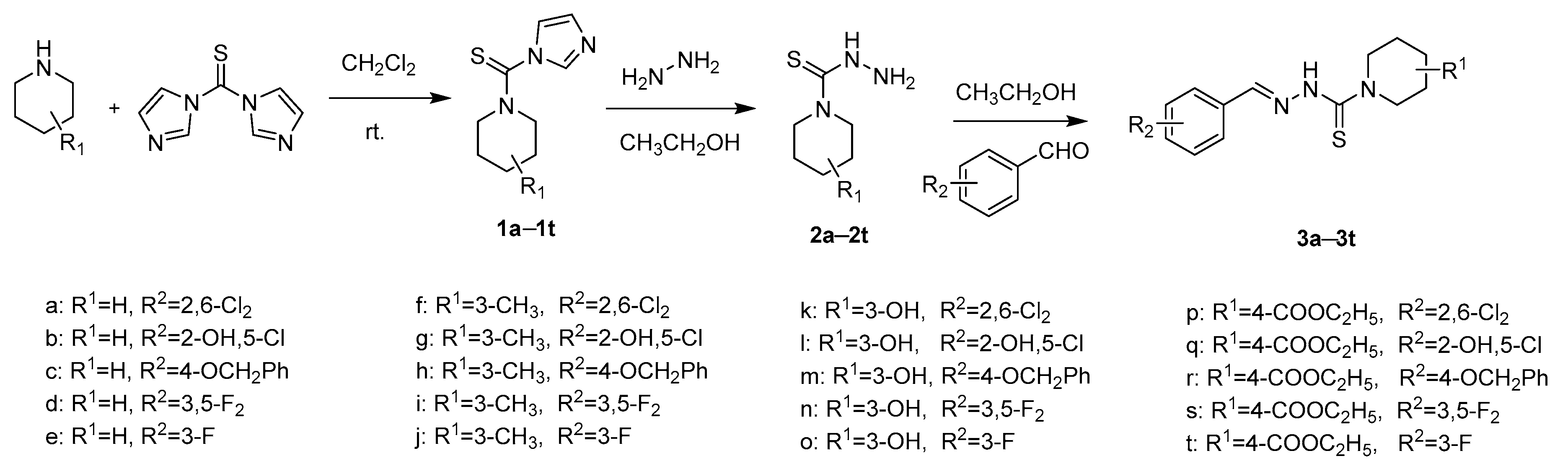
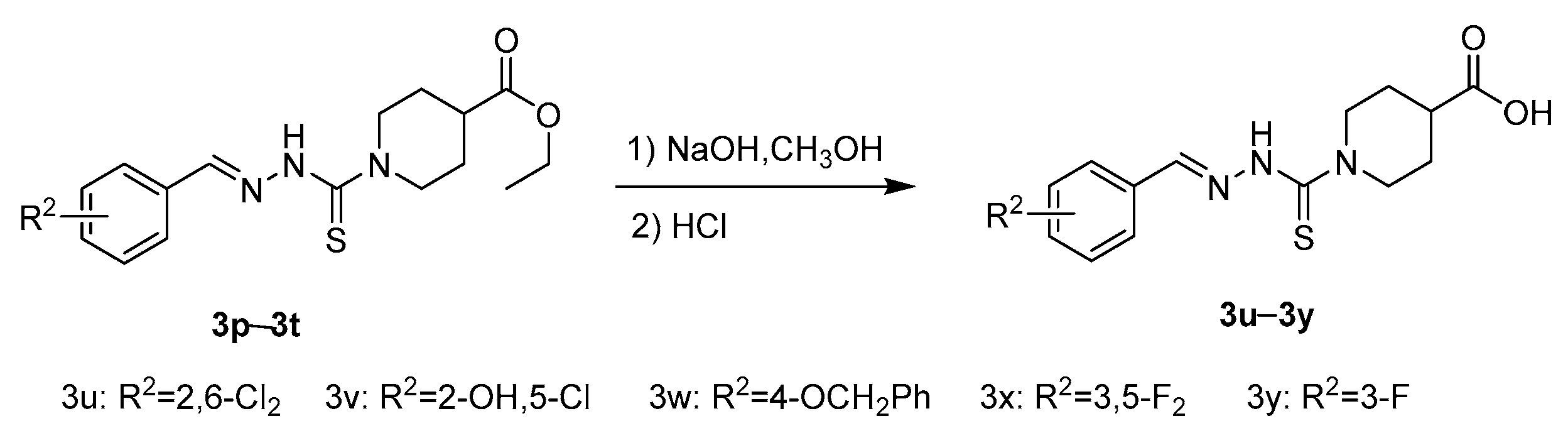
2.1. Chemistry
2.2. Fungicidal Activities
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Benzaldehyde Thiosemicarbazide Analogues 3a–3y
3.2.1. General Procedure for the Preparation of Intermediates 1
3.2.2. General Procedure for the Preparation of Intermediates 2
3.2.3. General Procedure of Benzaldehyde Thiosemicarbazide Analogues 3a–3t
3.2.4. General Procedure of Benzaldehyde Thiosemicarbazide Analoges 3u–3y
3.3. Biological Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wodnicka, A.; Huzar, E.; Krawczyk, M.; Kwiecien, H. Synthesis and antifungal activity of new salicylic acid derivatives. Pol. J. Chem. Technol. 2017, 19, 143–148. [Google Scholar] [CrossRef]
- Da Rocha Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of salicylic acid to reduce Penicillium expansum inoculum and preserve apple fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Stefan, B.; Arantxa, E.; Julia, K.; Carl, F.N. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Ji, H.B.; Wang, L.F. Advance in synthesis of natural benzaldehyde. Fine Chem. 2010, 6, 579–583. [Google Scholar]
- Eva, N.; Karel, W.; Jiri, K.; Karel, P.; Lenka, S. Design, synthesis, and biological evaluation of isothiosemicarbazones with antimycobacterial activity. Arch. Pharm. Chem. Life Sci. 2017, 350, e1700020. [Google Scholar]
- Cao, Y.H.; Zhang, Z.H.; Lin, F.K.; Lai, D.W.; Yao, Y.R.; Xie, B.Y.; Zhou, L.G. Secondary metabolites of endophytic fungus acremonium implicatum and their biological activities. Nat. Prod. Res. Dev. 2016, 28, 182–187. [Google Scholar]
- Maruthachalam, M.; Ganesan, A.; Gunasekaran, R.; Chinnasamy, J. Synthesis, characterization, DNA binding, DNA cleavage, antioxidant and in vitro cytotoxicity studies of ruthenium(II) complexes containing hydrazone ligands. J. Coord. Chem. 2016, 69, 3545–3559. [Google Scholar]
- Haraguchi, S.K.; Silva, A.A.; Vidotti, G.J.; dos Santos, P.V.; Garcia, F.P.; Pedroso, R.B.; Nakamura, C.V.; de Oliveira, C.M.; da Silva, C.C. Antitrypanasomal activity of novel benzaldehyde-thiosemicarbazone derivatives from kaurenoic acid. Molecules 2011, 16, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Augeri, D.J.; Bencivenga, A.F.; Blanden, A.; Carpizo, D.R.; Gilleran, J.A.; Kimball, S.D.; Loh, S.N.; Yu, X. Preparation of Thiosemicarbazone Derivatives Useful for Treatment of Cancer. Patent WO 2016123242, 4 August 2016. [Google Scholar]
- Chibale, K.; Greenbaum, D.C.; McKerrow, J.H. Thiosemicarbazones and Other Compounds as Antiparasitic Compounds, Their Preparation, and Methods of Their Use. Patent WO 2005087211, 22 September 2005. [Google Scholar]
- Shi, D.Y.; Wang, L.J.; Guo, C.L.; Jiang, B.; Wang, S.Y.; Zhao, Y. Process for Preparation of Bromophenol Amino Thiourea Compounds, and Pharmaceutical Activity. Patent CN 106748939, 31 May 2017. [Google Scholar]
- Song, S.C.; Zhu, G.X.; Chen, Z.Y.; You, A.; Qi, J.; Liang, H.; Pan, W.L. Alkyl Substituted Benzaldehyde or Acetophenone Thiosemicarbazone Derivatives as Tyrosinase Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Aging and Skin Pigmentation. Patent CN 105439926, 30 March 2016. [Google Scholar]
- Song, S.C.; You, A.; Zhu, G.X.; Yi, Z.; Qi, J.; Chen, Z.Y.; Liang, H.; Pan, W.L. Aryl-Substituted Benzaldehyde or Acetophenone Thiosemicarbazone Derivative as Tyrosinase Inhibitor and Their Preparation. Patent CN 105294527, 3 February 2016. [Google Scholar]
- Tang, X.R.; Yang, J.; Gao, S.M.; Liu, H.; Gao, Y.; Li, W.Y.; Zeng, Y.; Xu, Z.H.; Zhang, Y.; Wang, L. Thiosemicarbazone Derivative, Its Preparation and Application in Controlling Plant Pathogenic Fungi. Patent CN 105384722, 9 March 2016. [Google Scholar]
- Liu, C.L.; Dong, X.W.; Zhang, Z.; Lei, J.; Chen, H.H.; Zhao, J.D. Semicarbazone Compounds as SOD1 Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Diseases Caused by Copper Metabolic Disorders. Patent CN 105017197, 4 November 2015. [Google Scholar]
- Wang, W.; Yao, J.; Cao, W.; Liu, X.D. Prepration of Hydrazinethioamide Compound from Aminothiourea, Hydroxybenzaldehyde and Aromatic Diamine. Patent CN 103709082, 9 April 2014. [Google Scholar]
- Wang, Z.; Dong, W.; Xu, Y.; Liang, P.; Yang, X.L. Synthesis of substituted benzylidene hydrazinecarbothioamide (hydrazinecarboxamide, nitrohydrazinecarboximidamide) and their inhibitory activity on tyrosinase of diamondback moth Plutella xylostella (L.). Nongyaoxue Xuebao 2010, 12, 264–268. [Google Scholar]
- Meena, P.; Nemaysh, V.; Khatri, M.; Manral, A.; Luthra, P.M.; Tiwari, M. Synthesis, biological evaluation and molecular docking study of novel piperidine and piperazine derivatives as multi-targeted agents to treat Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Zhang, X.; Xu, Y.; Xu, G.; Liu, X.; Yang, X.; Zhang, X.; Ling, Y. Synthesis and fungicidal activity of pyrazole derivatives containing 1,2,3,4-tetrahydroquinoline. Chem. Cent. J. 2016, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.N.; Zheng, M.Y.; Miao, F.X.; Yu, J.Q.; Li, S.H.; Li, F.N.; Qu, N. Synthesis and anti-hepatoma activities of N-(piperidine-4-yl)benzamide derivatives. Yingyong Huaxue 2016, 33, 661–667. [Google Scholar]
- Pivatto, M.; Baccini, L.R.; Sharma, A.; Nakabashi, M.; Danuello, A.; Viegas, C.J.; Garcia, C.R.S.; Bolzani, V.S. Antimalarial activity of piperidine alkaloids from Senna spectabilis and semisynthetic derivatives. J. Braz. Chem. Soc. 2014, 25, 1900–1906. [Google Scholar]
- Rajput, A.P.; Nagarale, D.V. Synthesis, characterization and antimicrobial study of piperidine-2,6-diones derivatives. Pharm. Chem. 2016, 8, 182–186. [Google Scholar]
- Li, F.Y.; Zhu, Y.J.; Fan, Z.J.; Xu, J.H.; Guo, X.F.; Zong, G.N.; Song, H.B.; Chen, L.; Song, Y.Q.; Qian, X.L. Synthesis, crystal structure and biological activity of 2-(1-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carbonyl)piperidine-4-yl)-N-isopropyl-1,3-thiazole-4-carboxamide. Chin. J. Struct. Chem. 2015, 34, 659–666. [Google Scholar]
- Shaw, S.A.; Balasubramanian, B.; Bonacorsi, S.; Cortes, J.C.; Cao, K.; Chen, B.C.; Dai, J.; Decicco, C.; Goswami, A.; Guo, Z.W. Synthesis of biologically active piperidine metabolites of clopidogrel: determination of structure and analyte development. J. Org. Chem. 2015, 80, 7019–7032. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, X.C.; Yu, M.C.; Xiao, L.; Zhang, X.J.; Sun, H.J.; Chen, H.; Pan, G.X.; Yan, Y.R.; Wang, S.C. Discovery, synthesis, biological evaluation and structure-based optimization of novel piperidine derivatives as acetylcholine-binding protein ligands. Acta Pharmacol. Sin. 2017, 38, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Pasteris, R.J.; Hanagan, M.A.; Bisaha, J.J.; Finkelstein, B.L.; Hoffman, L.E.; Gregory, V.; Andreassi, J.L.; Sweigard, J.A.; Klyashchitsky, B.A.; Henry, Y.T.; et al. Discovery of oxathiapiprolin, a new oomycete fungicide that targets an oxysterol binding protein. Bioorg. Med. Chem. 2016, 24, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, K.; Iwamoto, S.; Shigeta, Y.; Hirokawa, Y.; Oowada, S.; Nakamura, T.; Ishiwata, N. Preparation of Pyrazolone Compounds as Thrombopoietin Receptor Activators. U.S. Patent 2007142308, 13 December 2007. [Google Scholar]
- Du, S.; Tian, Z.; Yang, D.; Li, X.; Li, H.; Jia, C.; Che, C.; Wang, M.; Qin, Z. Synthesis, antifungal activity and structure-activity relationships of novel 3-(difluoromethyl)-1-methyl-1h-pyrazole-4-carboxylic acid amides. Molecules 2015, 20, 8395–8408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3a–3y are available from the authors. |



| Compounds | R1 | R2 | Inhibitory Rate (%)/50 μg/mL | |||||
|---|---|---|---|---|---|---|---|---|
| P. a | R. s | V. m | B. c | A. s | G. g | |||
| 3a | H | 2,6-Cl2 | 20 | 80 | 86 | 78 | 70 | 61 |
| 3b | H | 2-OH,5-Cl | 99 | 93 | 86 | 72 | 44 | 99 |
| 3c | H | 4-OCH2Ph | 31 | 76 | 79 | 50 | 56 | 53 |
| 3d | H | 3,5-F2 | 49 | 87 | 88 | 64 | 70 | 98 |
| 3e | H | 3-F | 55 | 91 | 92 | 60 | 71 | 97 |
| 3f | 3-CH3 | 2,6-Cl2 | 52 | 90 | 84 | 69 | 63 | 54 |
| 3g | 3-CH3 | 2-OH,5-Cl | 65 | 68 | 94 | 58 | 60 | 100 |
| 3h | 3-CH3 | 4-OCH2Ph | 17 | 74 | 77 | 61 | 45 | 61 |
| 3i | 3-CH3 | 3,5-F2 | 30 | 78 | 83 | 52 | 66 | 81 |
| 3j | 3-CH3 | 3-F | 55 | 95 | 96 | 62 | 73 | 100 |
| 3k | 3-OH | 2,6-Cl2 | 46 | 89 | 62 | 29 | 38 | 34 |
| 3l | 3-OH | 2-OH,5-Cl | 98 | 79 | 39 | 52 | 43 | 63 |
| 3m | 3-OH | 4-OCH2Ph | 29 | 76 | 57 | 58 | 47 | 28 |
| 3n | 3-OH | 3,5-F2 | 36 | 52 | 49 | 17 | 31 | 22 |
| 3o | 3-OH | 3-F | 27 | 67 | 47 | 8 | 23 | 11 |
| 3p | 4-COOC2H5 | 2,6-Cl2 | 47 | 80 | 78 | 48 | 52 | 76 |
| 3q | 4-COOC2H5 | 2-OH,5-Cl | 48 | 61 | 65 | 49 | 47 | 31 |
| 3r | 4-COOC2H5 | 4-OCH2Ph | 15 | 71 | 61 | 52 | 32 | 98 |
| 3s | 4-COOC2H5 | 3,5-F2 | 17 | 40 | 65 | 7 | 32 | 1 |
| 3t | 4-COOC2H5 | 3-F | 35 | 82 | 70 | 37 | 54 | 55 |
| 3u | 4-COOH | 2,6-Cl2 | 14 | 75 | 40 | 25 | 17 | 15 |
| 3v | 4-COOH | 2-OH,5-Cl | 11 | 66 | 96 | 75 | 54 | 30 |
| 3w | 4-COOH | 4-OCH2Ph | 22 | 74 | 67 | 61 | 42 | 42 |
| 3x | 4-COOH | 3,5-F2 | 0 | 12 | 38 | 51 | 23 | 7 |
| 3y | 4-COOH | 3-F | 0 | 51 | 35 | 51 | 43 | 0 |
| Fluopicolide | - | - | 99 | 43 | 63 | 43 | 58 | 95 |
| Compounds | R1 | R2 | EC50 (μg/mL) | |||
|---|---|---|---|---|---|---|
| R. s | V. m | G. g | P. a | |||
| 3a | H | 2,6-Cl2 | 15.2 | 10.5 | NT a | NT |
| 3b | H | 2-OH,5-Cl | 9.6 | 2.3 | 9.3 | 1.6 |
| 3c | H | 4-OCH2Ph | 2.2 | 4.6 | NT | NT |
| 3d | H | 3,5-F2 | 11.6 | 12.2 | 17.2 | NT |
| 3e | H | 3-F | 8.4 | 18.1 | 14.9 | NT |
| 3f | 3-CH3 | 2,6-Cl2 | 2.1 | 35.1 | NT | NT |
| 3g | 3-CH3 | 2-OH,5-Cl | NT | 2.8 | 10.1 | NT |
| 3i | 3-CH3 | 3,5-F2 | 8.3 | 5.3 | 18.1 | NT |
| 3j | 3-CH3 | 3-F | 8.0 | 9.0 | 16.5 | NT |
| 3l | 3-OH | 2-OH,5-Cl | 26.3 | NT | NT | 4.3 |
| 3q | 4-COOC2H5 | 2,6-Cl2 | 9.2 | 14.3 | 48.9 | NT |
| 3r | 4-COOC2H5 | 4-OCH2Ph | NT | NT | 72.0 | NT |
| 3t | 4-COOC2H5 | 3-F | 10.6 | NT | NT | NT |
| 3v | 4-COOH | 2-OH,5-Cl | NT | 9.9 | NT | NT |
| 3w | 4-COOH | 4-OCH2Ph | NT | NT | 59.5 | NT |
| 3z | 2-OH,5-Cl | >30.0 | 17.2 | 24.8 | 22.8 | |
| Azoxystrobin | NT | 0.01 | NT | 16.9 | ||
| Pyraclostrobin | 0.03 | 0.01 | 0.19 | NT | ||
| Fluopicolide | NT | NT | NT | 1.0 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lei, P.; Sun, T.; Jin, X.; Yang, X.; Ling, Y. Design, Synthesis, and Fungicidal Activity of Novel Thiosemicarbazide Derivatives Containing Piperidine Fragments. Molecules 2017, 22, 2085. https://doi.org/10.3390/molecules22122085
Zhang X, Lei P, Sun T, Jin X, Yang X, Ling Y. Design, Synthesis, and Fungicidal Activity of Novel Thiosemicarbazide Derivatives Containing Piperidine Fragments. Molecules. 2017; 22(12):2085. https://doi.org/10.3390/molecules22122085
Chicago/Turabian StyleZhang, Xuebo, Peng Lei, Tengda Sun, Xiaoyu Jin, Xinling Yang, and Yun Ling. 2017. "Design, Synthesis, and Fungicidal Activity of Novel Thiosemicarbazide Derivatives Containing Piperidine Fragments" Molecules 22, no. 12: 2085. https://doi.org/10.3390/molecules22122085
APA StyleZhang, X., Lei, P., Sun, T., Jin, X., Yang, X., & Ling, Y. (2017). Design, Synthesis, and Fungicidal Activity of Novel Thiosemicarbazide Derivatives Containing Piperidine Fragments. Molecules, 22(12), 2085. https://doi.org/10.3390/molecules22122085