Synthesis of Nitrogen Heterocycles Using Samarium(II) Iodide
Abstract
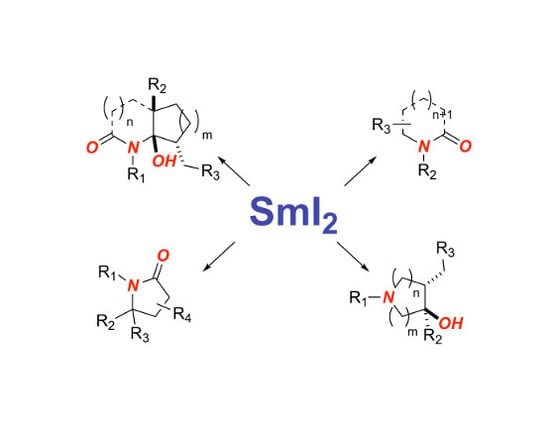
1. Introduction
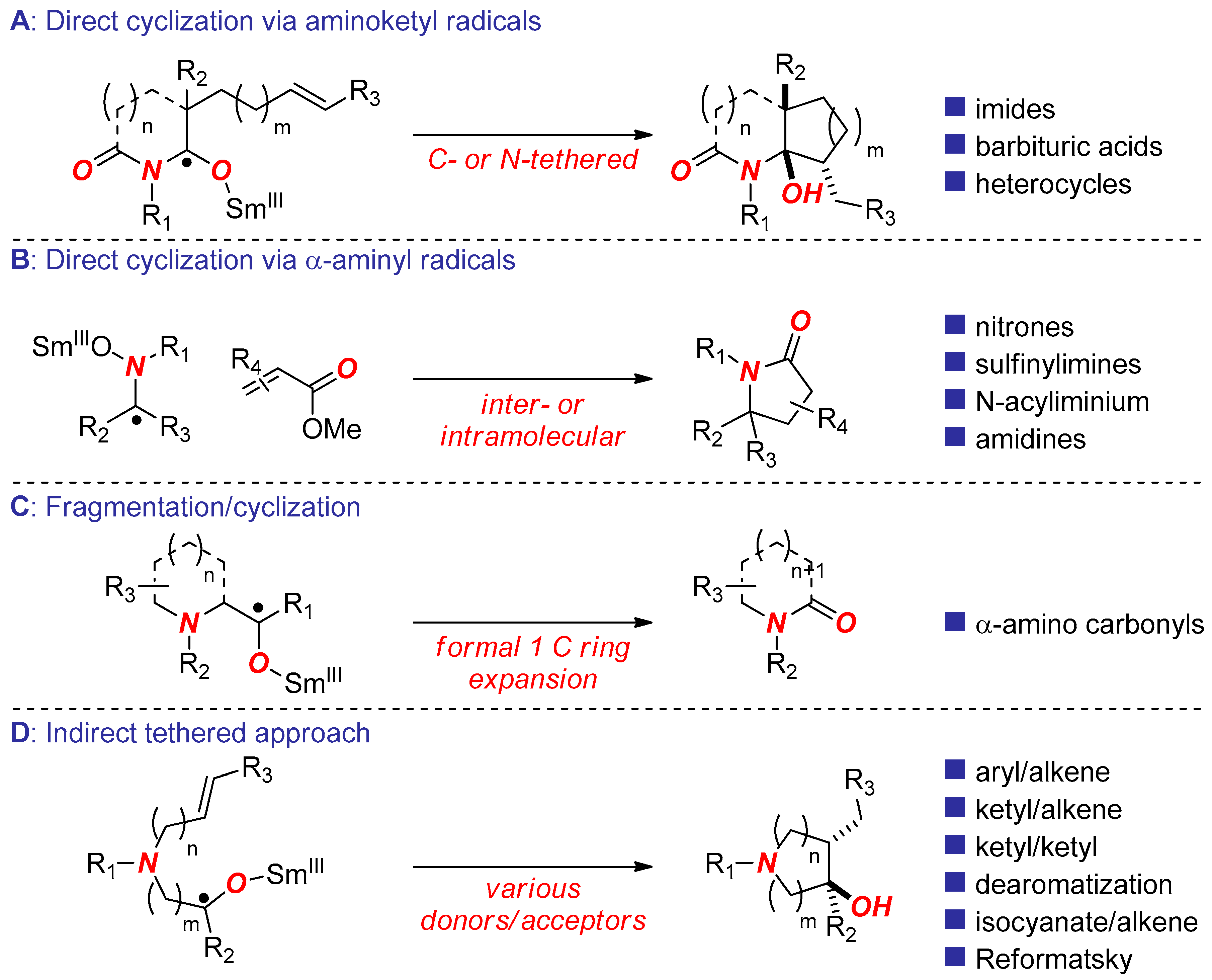
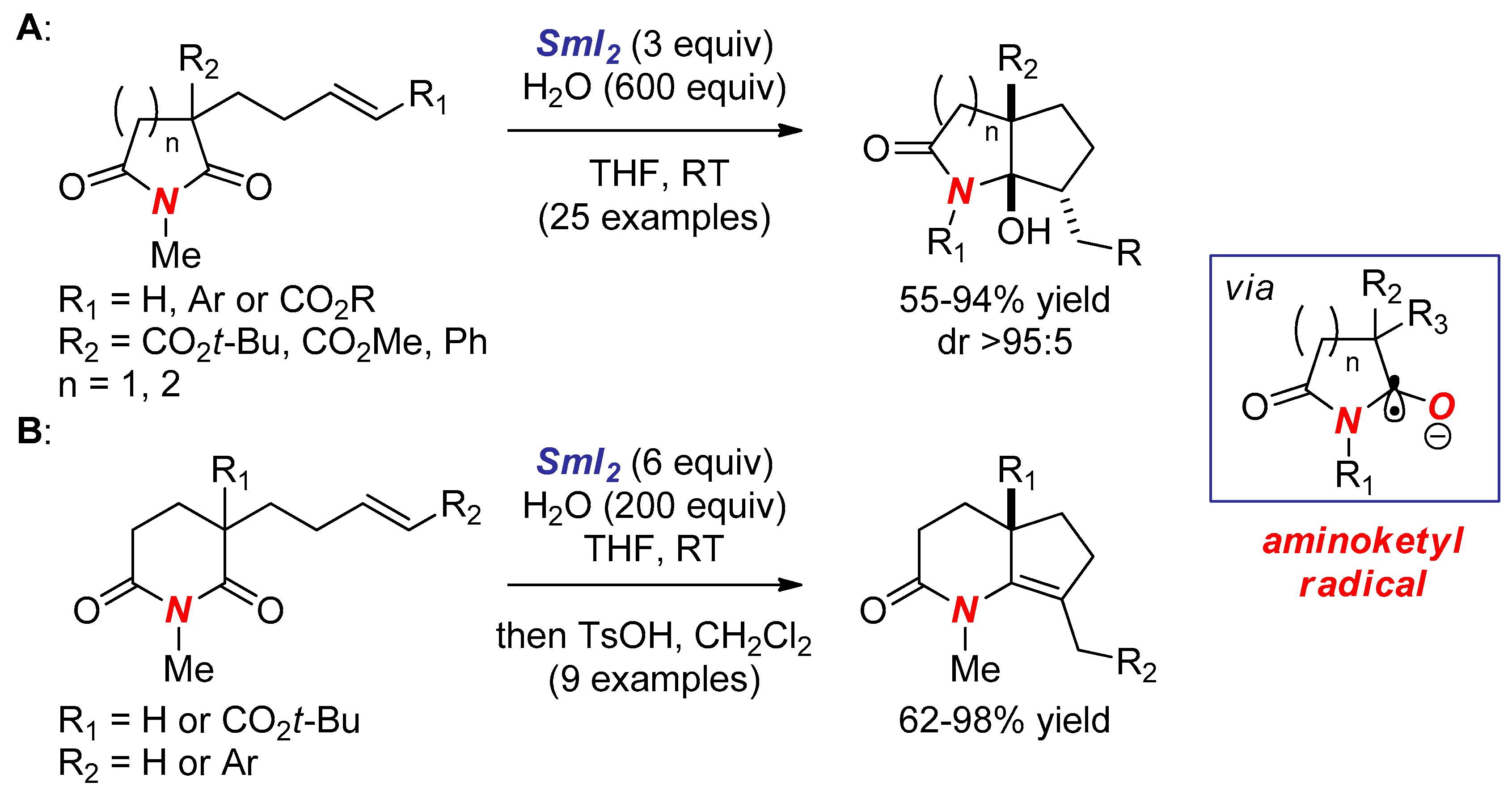
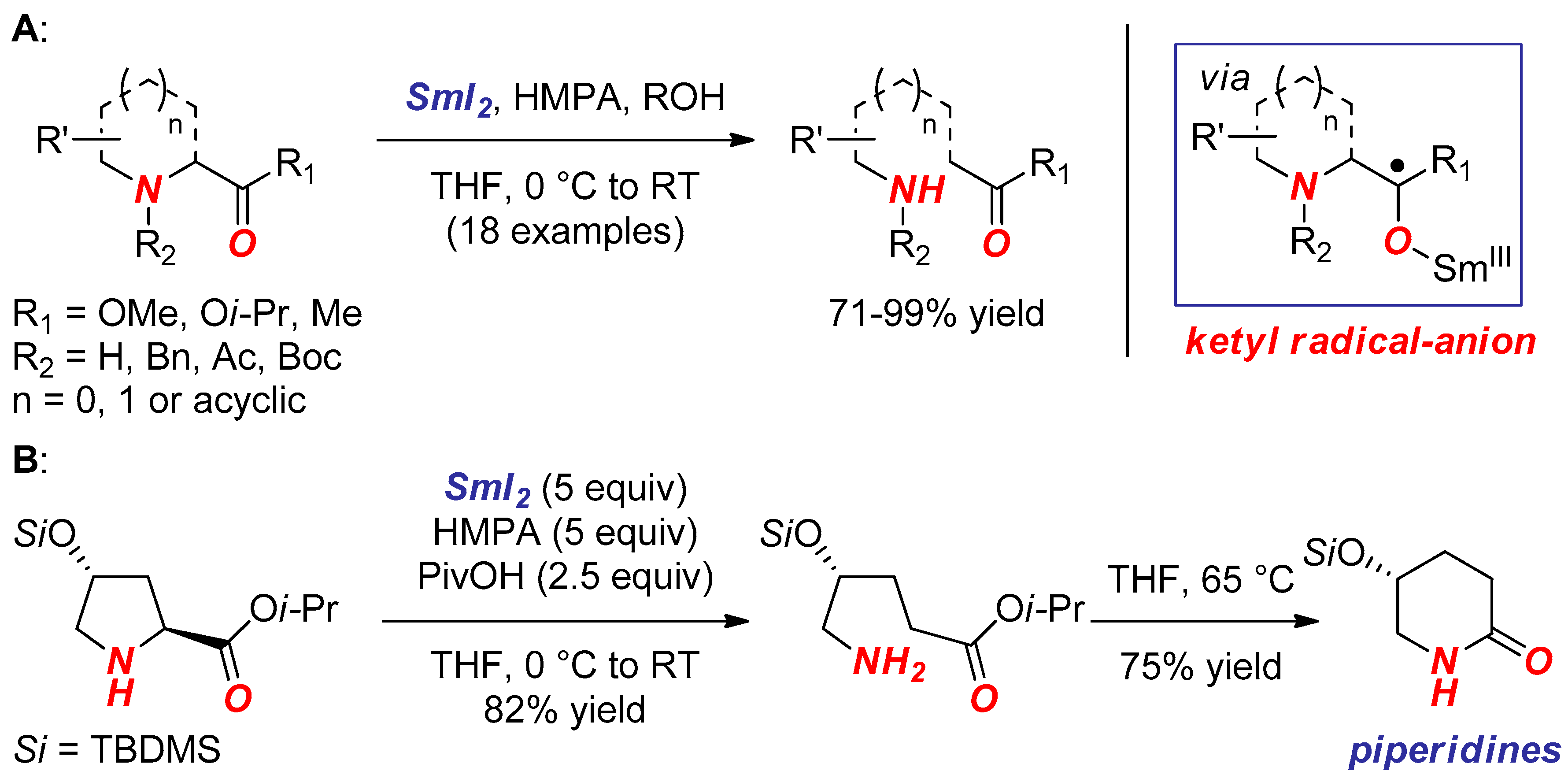
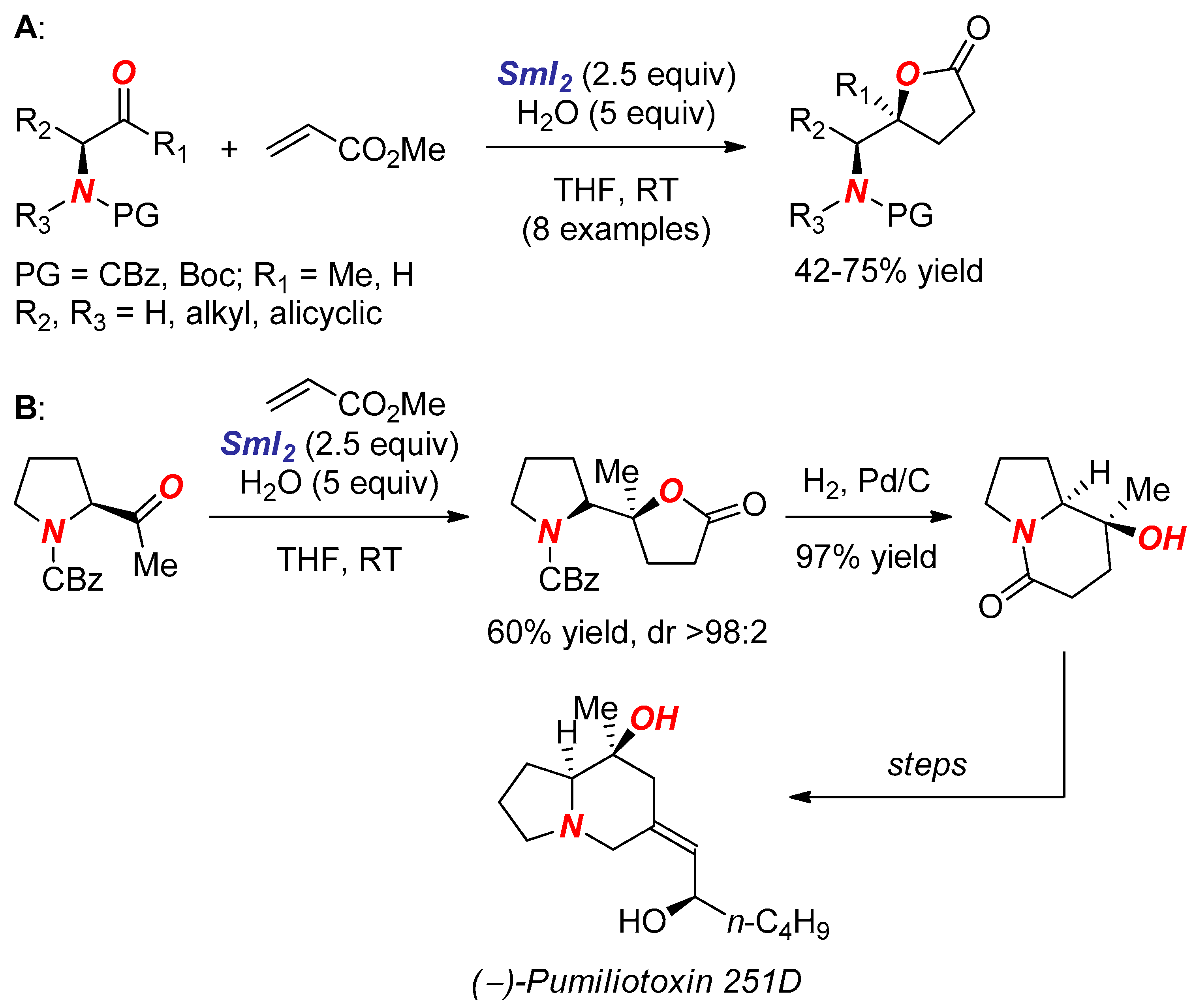
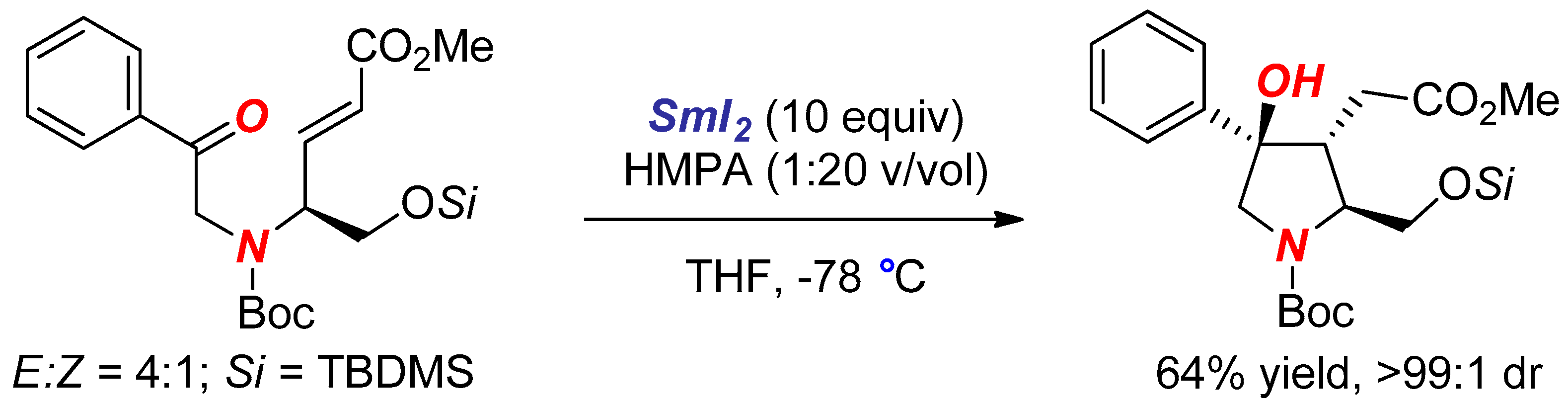
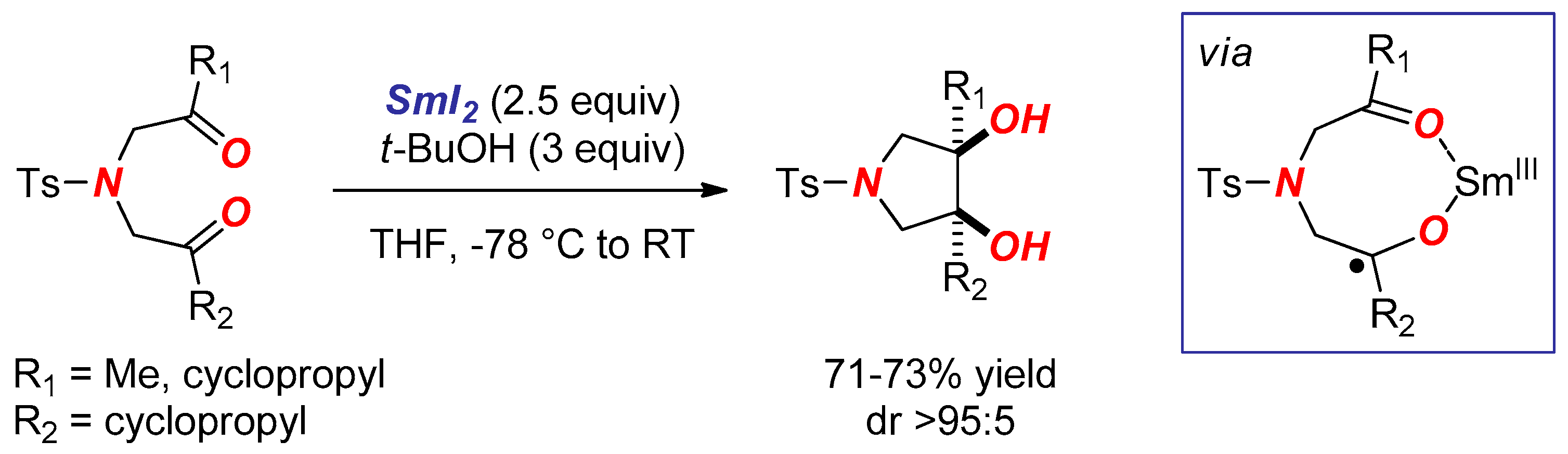
2. Synthesis of Nitrogen Heterocycles via Aminoketyl Radicals
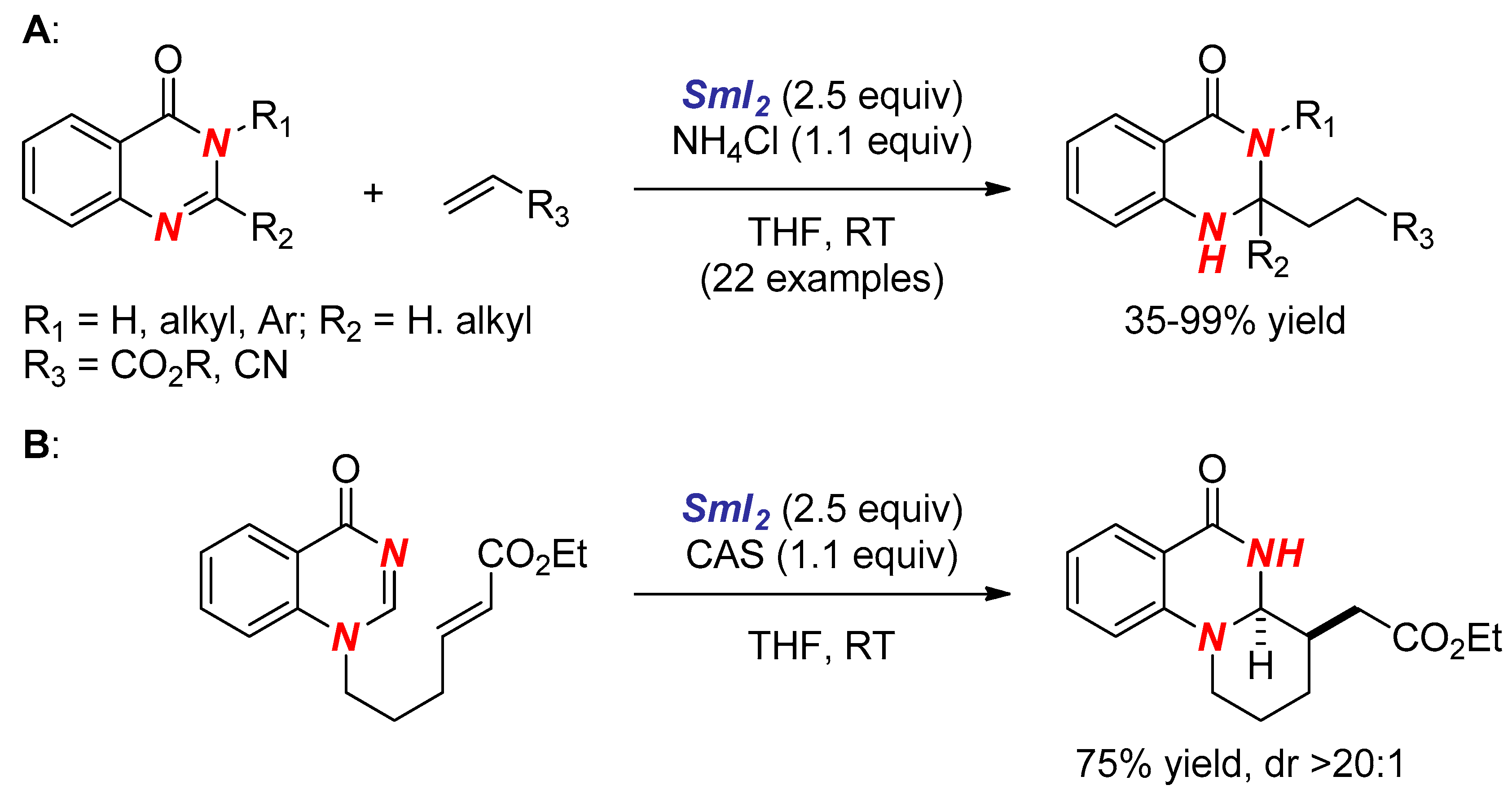
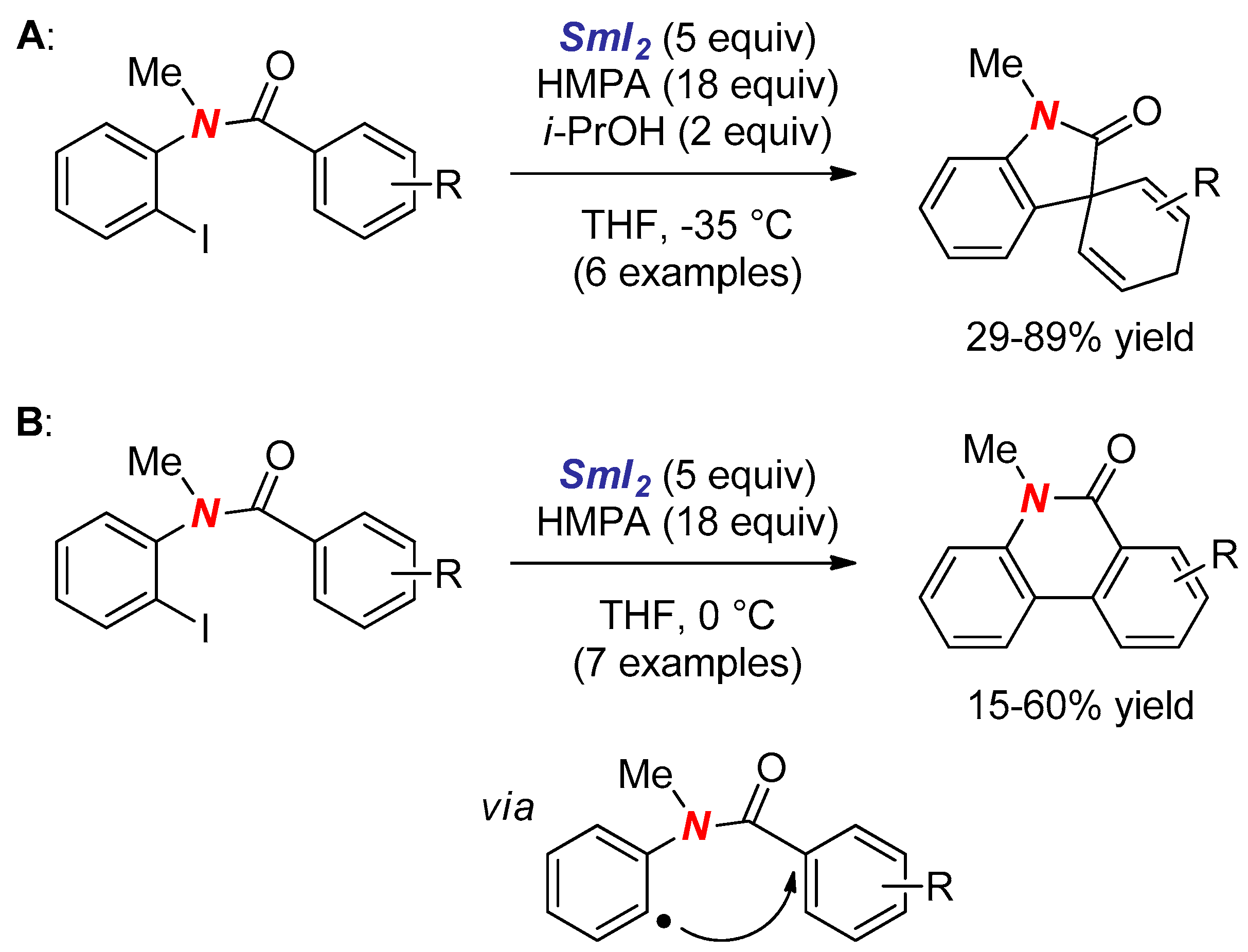
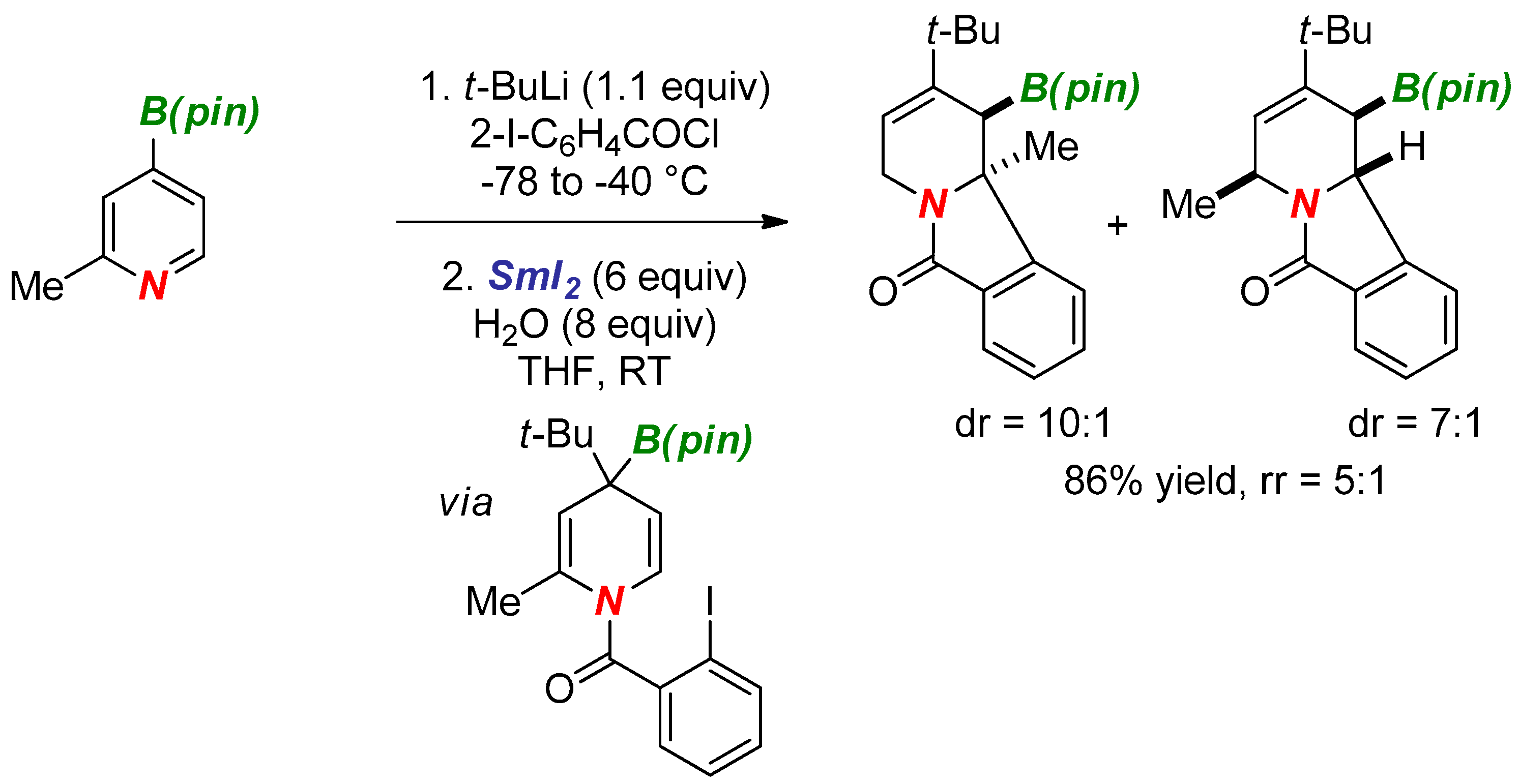
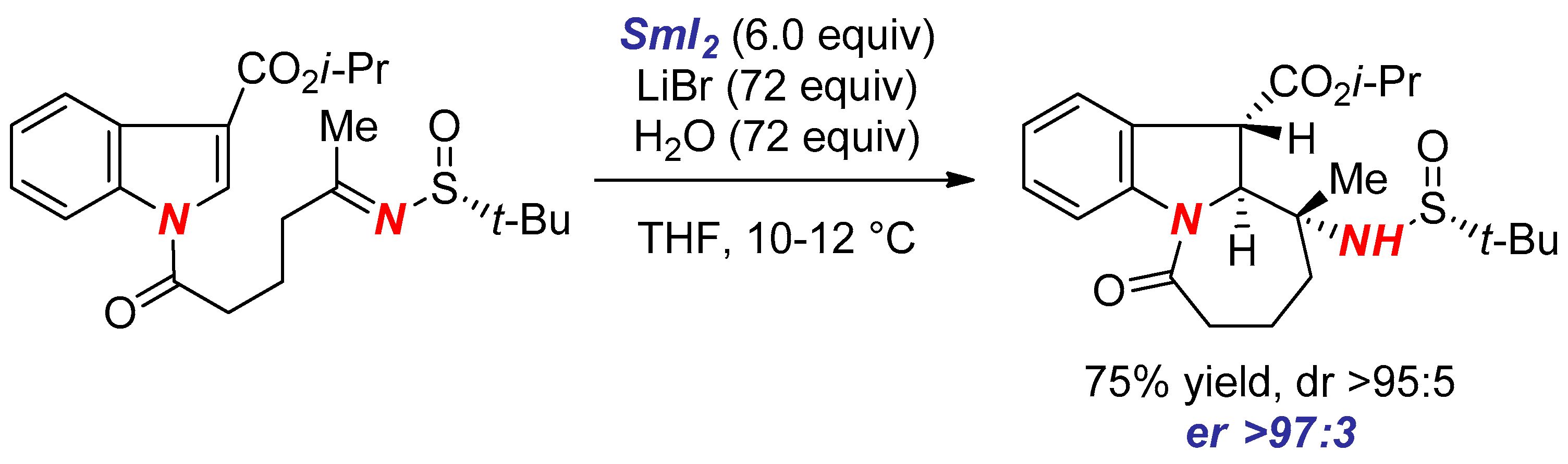
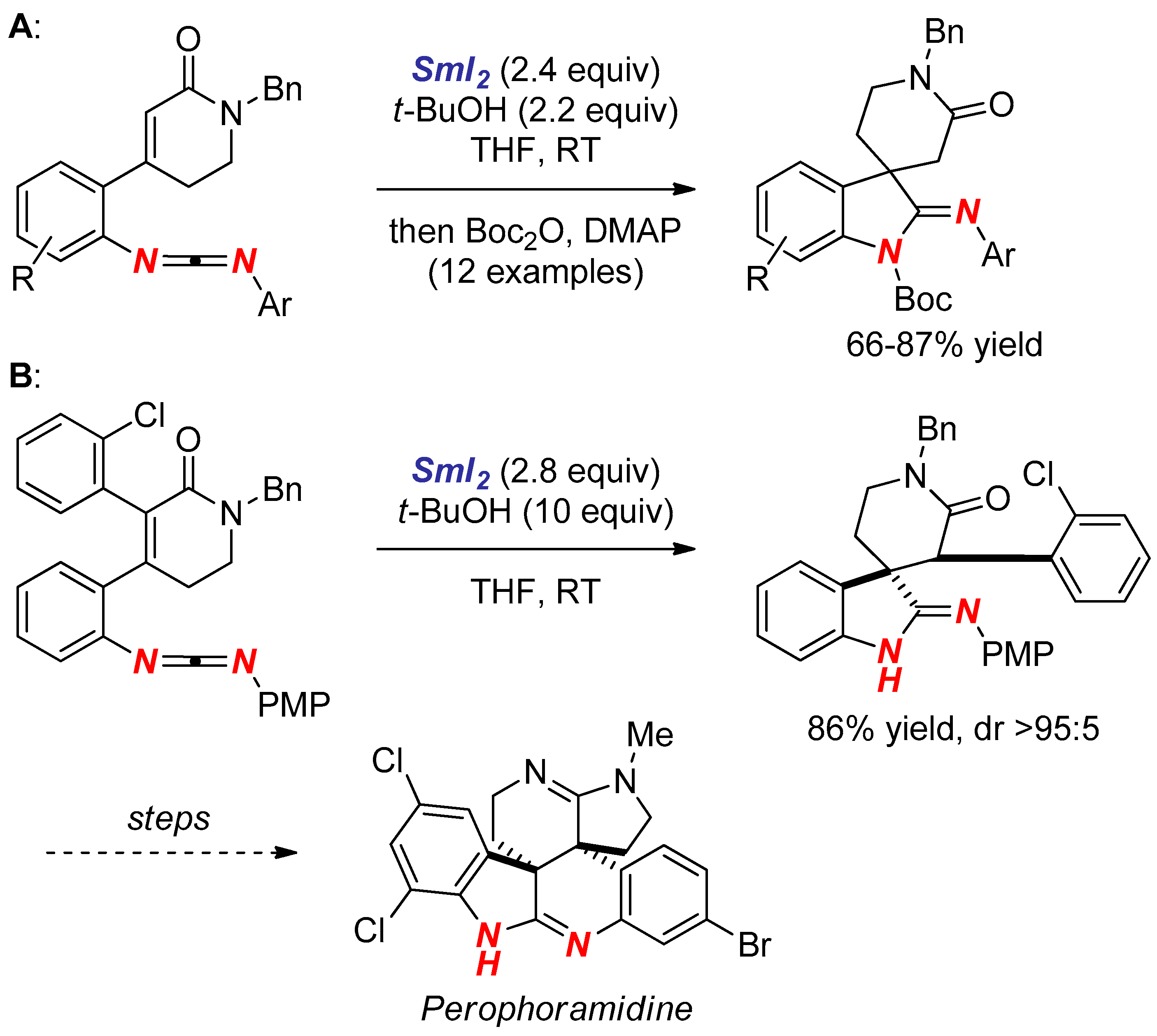
3. Synthesis of Nitrogen Heterocycles via Aminyl Radicals
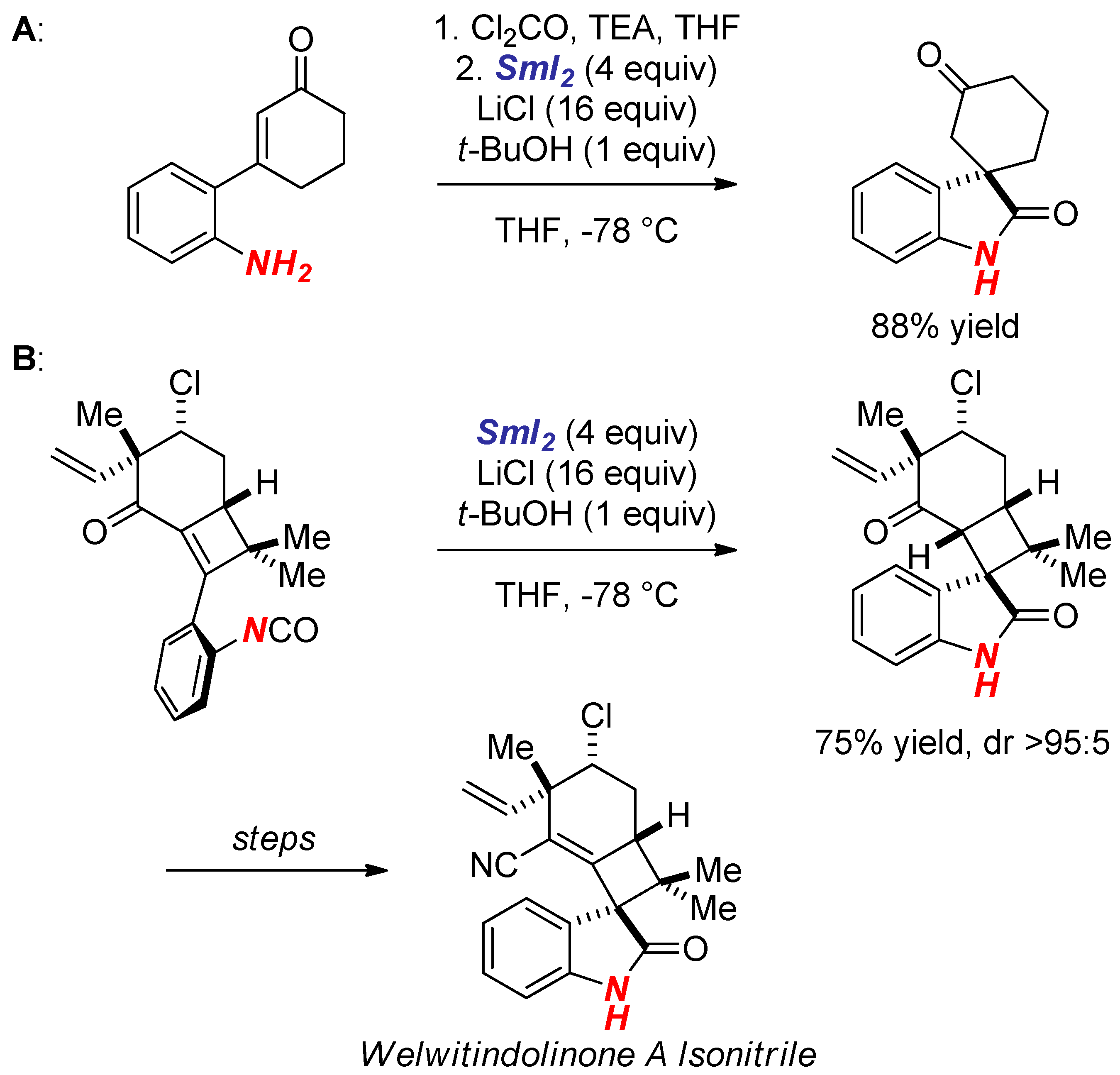
4. Synthesis of Nitrogen Heterocycles via Fragmentation/Cyclization Pathways
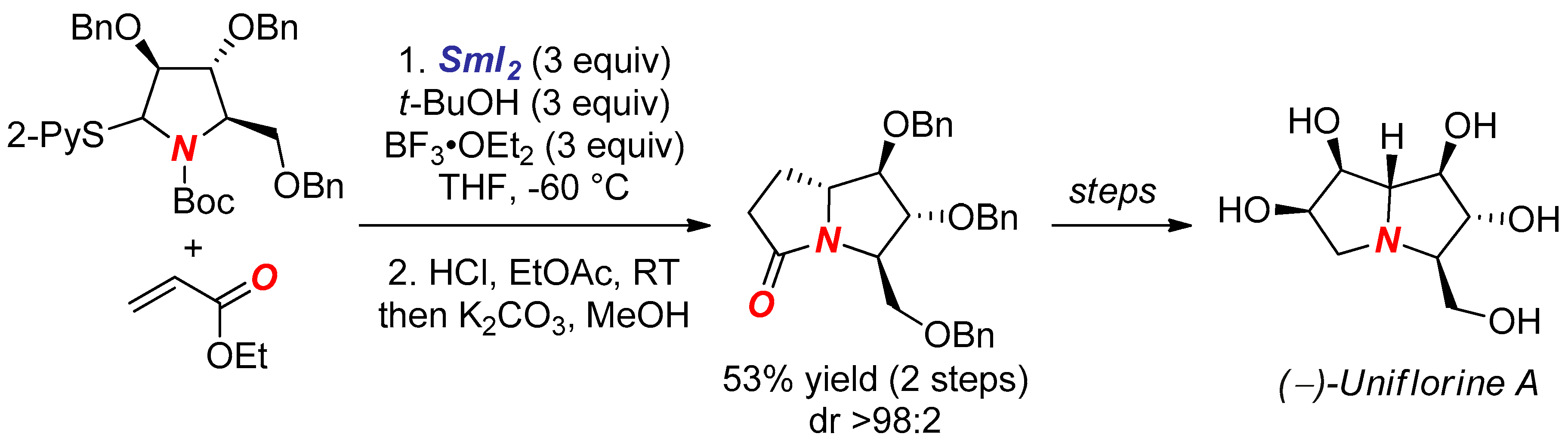
5. Synthesis of Nitrogen Heterocycles via Tethered Approach
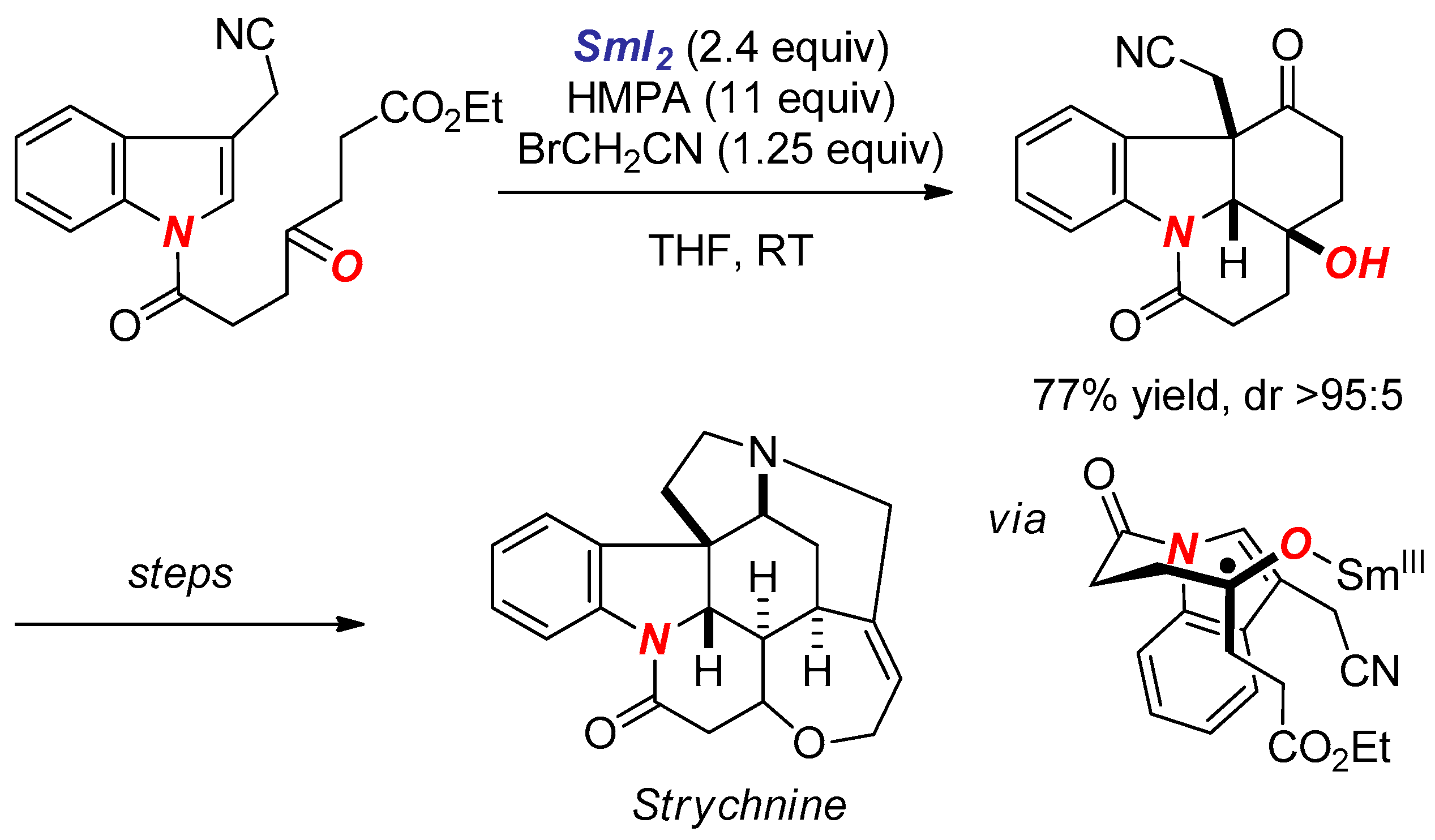
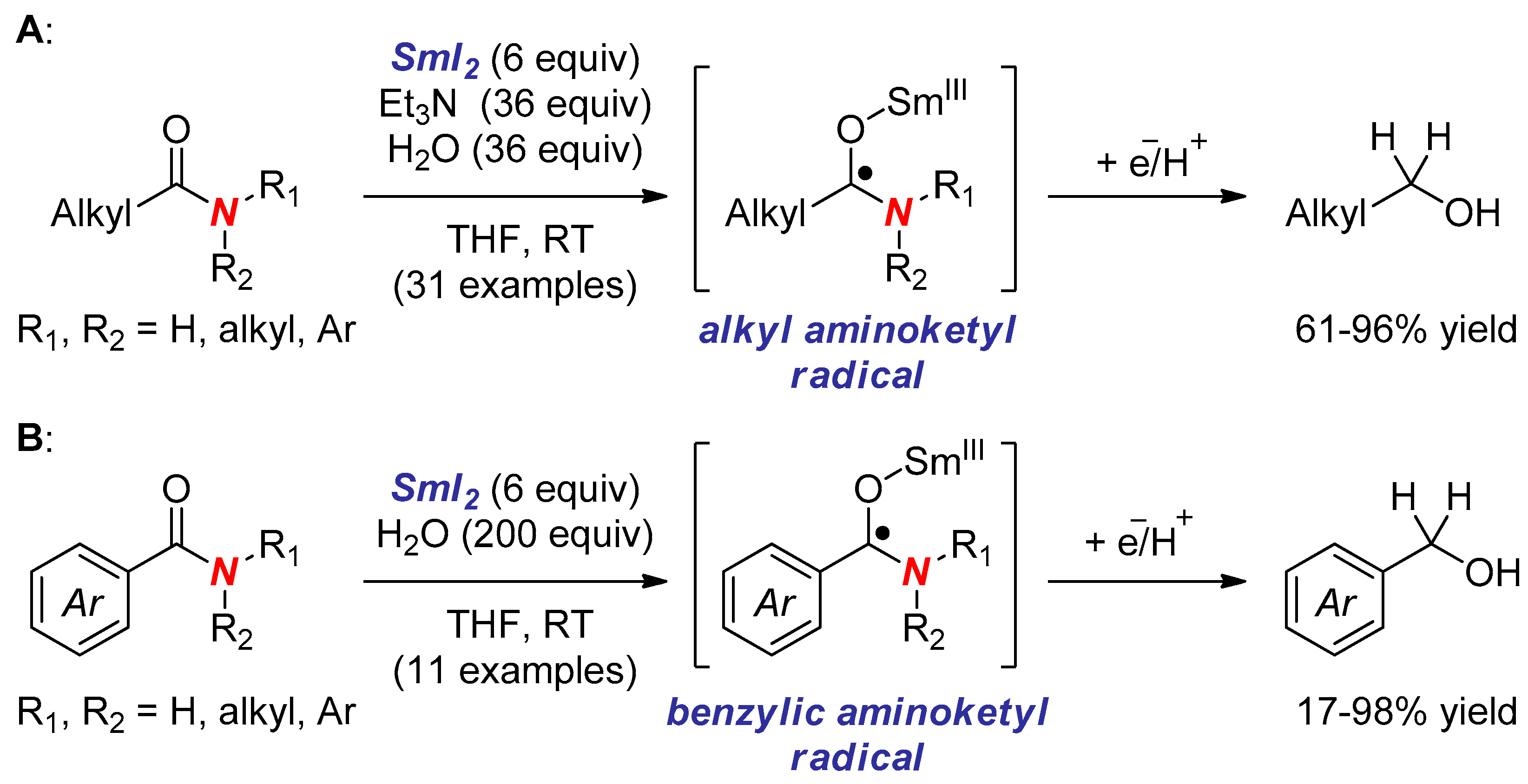
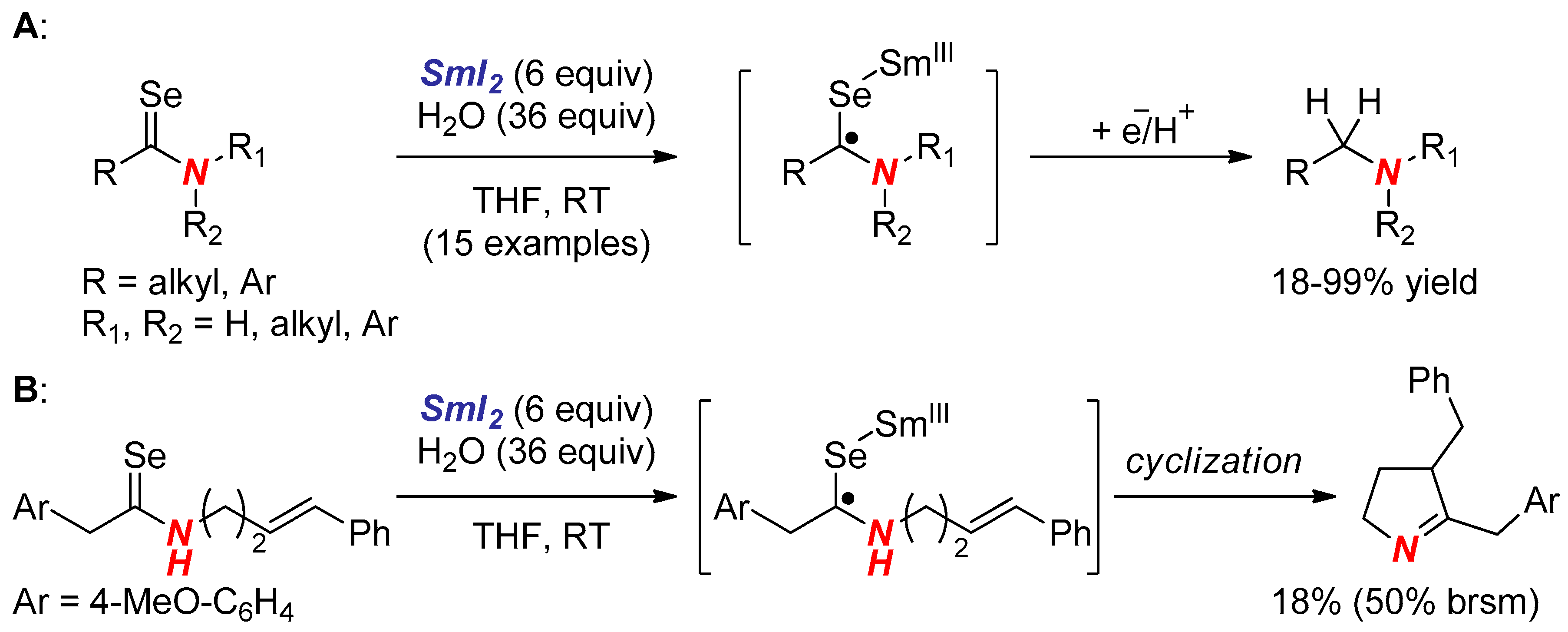
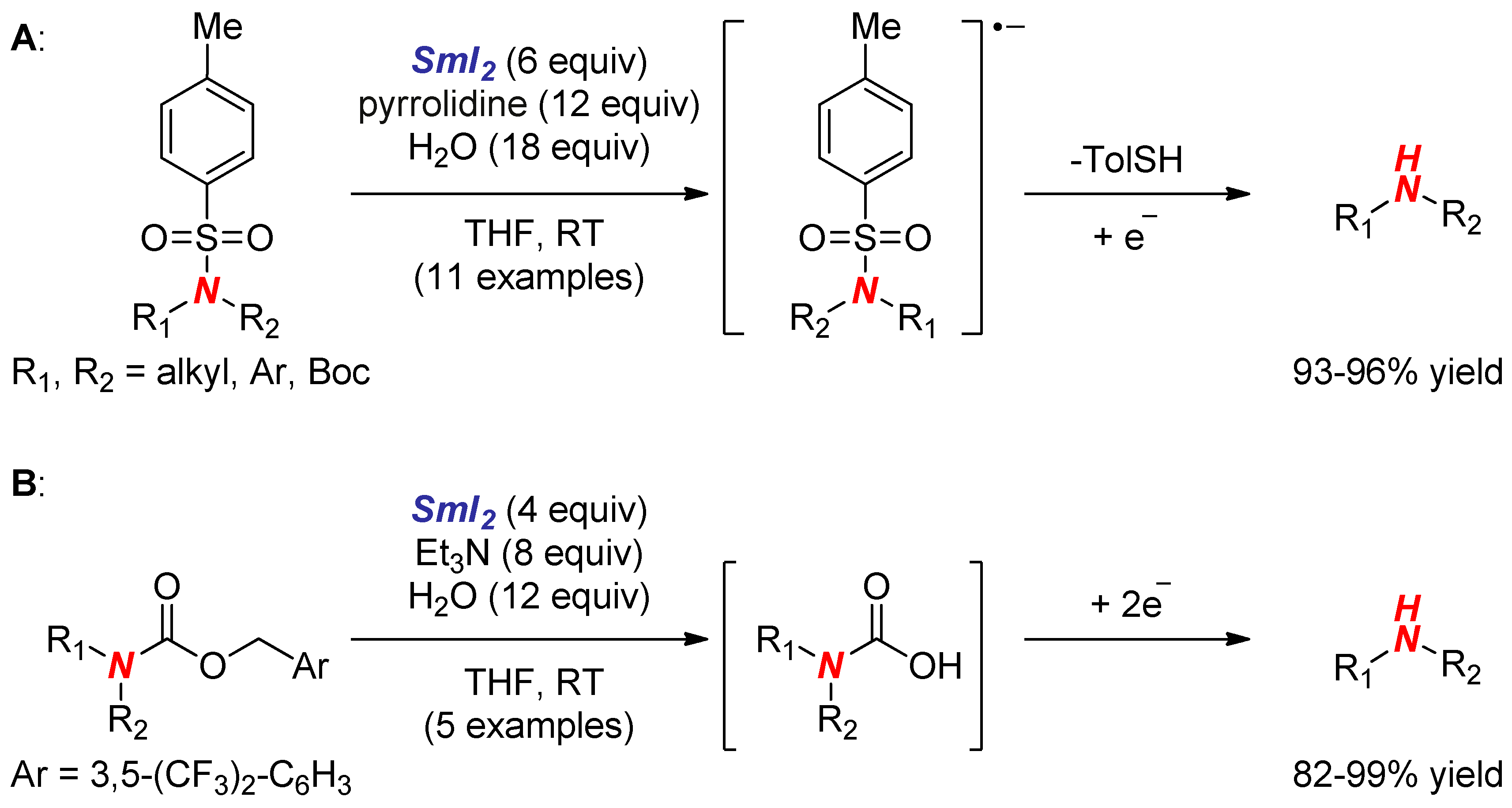
6. Reactions Involving Aminoketyl and Related Radicals
7. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Szostak, M.; Fazakerley, N.J.; Parmar, D.; Procter, D.J. Cross-Coupling Reactions Using Samarium(II) Iodide. Chem. Rev. 2014, 114, 5959–6039. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Procter, D.J. Beyond Samarium Diiodide: Vistas in Reductive Chemistry Mediated by Lanthanides(II). Angew. Chem. Int. Ed. 2012, 51, 9238–9256. [Google Scholar] [CrossRef] [PubMed]
- Steel, P.G. Recent developments in lanthanide mediated organic synthesis. J. Chem. Soc. Perkin Trans. 2001, 1, 2727–2751. [Google Scholar] [CrossRef]
- Molander, G.A.; Harris, C.R. Sequencing Reactions with Samarium(II) Iodide. Chem. Rev. 1996, 96, 307–338. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.P.; Fevig, T.L.; Jasperse, C.P.; Totleben, J. New mechanistic insights into reductions of halides and radicals with samarium(II) iodide. Synlett 1992, 1992, 943–961. [Google Scholar] [CrossRef]
- Edmonds, D.J.; Johnston, D.; Procter, D.J. Samarium(II)-Iodide-Mediated Cyclizations in Natural Product Synthesis. Chem. Rev. 2004, 104, 3371–3404. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Ellery, S.P.; Chen, J.S. Samarium Diiodide Mediated Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2009, 48, 7140–7165. [Google Scholar] [CrossRef] [PubMed]
- Austad, B.C.; Calkins, T.L.; Chase, C.E.; Fang, F.G.; Horstmann, T.E.; Hu, Y.; Lewis, B.M.; Niu, X.; Noland, T.A.; Orr, J.D.; et al. Commercial Manufacture of Halaven®: Chemoselective Transformations En Route to Structurally Complex Macrocyclic Ketones. Synlett 2013, 24, 333–337. [Google Scholar] [CrossRef]
- Szostak, M.; Spain, M.; Procter, D.J. Determination of the Effective Redox Potentials of SmI2, SmBr2, SmCl2, and their Complexes with Water by Reduction of Aromatic Hydrocarbons. Reduction of Anthracene and Stilbene by Samarium(II) Iodide-Water Complex. J. Org. Chem. 2014, 79, 2522–2537. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Spain, M.; Procter, D.J. Recent advances in the chemoselective reduction of functional groups mediated by samarium(II) iodide: A single electron transfer approach. Chem. Soc. Rev. 2013, 42, 9155–9183. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, A.; Hilmersson, G. Samarium(II) Iodide Mediated Reductions—Influence of Various Additives. Eur. J. Inorg. Chem. 2004, 2004, 3393–3403. [Google Scholar] [CrossRef]
- Szostak, M.; Spain, M.; Parmar, D.; Procter, D.J. Selective reductive transformations using samarium diiodide-water. Chem. Commun. 2012, 48, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Krief, A.; Laval, A.M. Coupling of Organic Halides with Carbonyl Compounds Promoted by SmI2, the Kagan Reagent. Chem. Rev. 1999, 99, 745–778. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Spain, M.; Procter, D.J. Ketyl-Type Radicals from Cyclic and Acyclic Esters are Stabilized by SmI2(H2O)n: The Role of SmI2(H2O)n in Post-Electron Transfer Steps. J. Am. Chem. Soc. 2014, 136, 8459–8466. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, H.; Yamaguchi, K.; Imamoto, T. Evaluation of the relative Lewis acidities of lanthanoid(III) compounds by tandem mass spectrometry. Chem. Commun. 1999, 17, 1703–1704. [Google Scholar] [CrossRef]
- Szostak, M.; Spain, M.; Procter, D.J. Preparation of Samarium(II) Iodide: Quantitative Evaluation of the Effect of Water, Oxygen, and Peroxide Content, Preparative Methods, and the Activation of Samarium Metal. J. Org. Chem. 2012, 77, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Helm, M.D.; Da Silva, M.; Sucunza, D.; Findley, T.J.K.; Procter, D.J. A dialdehyde cyclization cascade: An approach to pleuromutilin. Angew. Chem. Int. Ed. 2009, 48, 9315–9317. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Yeoman, J.T.S.; Reisman, S.E. A Concise Total Synthesis of (−)-Maoecrystal Z. J. Am. Chem. Soc. 2011, 133, 14964–14967. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Price, K.; Spain, M.; Matsubara, H.; Bradley, P.A.; Procter, D.J. Reductive Cyclization Cascades of Lactones Using SmI2−H2O. J. Am. Chem. Soc. 2011, 133, 2418–2420. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Matsubara, H.; Price, K.; Spain, M.; Procter, D.J. Lactone Radical Cyclizations and Cyclization Cascades Mediated by SmI2–H2O. J. Am. Chem. Soc. 2012, 134, 12751–12757. [Google Scholar] [CrossRef] [PubMed]
- Just-Baringo, X.; Procter, D.J. Sm(II)-Mediated Electron Transfer to Carboxylic Acid Derivatives: Development of Complexity-Generating Cascade. Acc. Chem. Res. 2015, 48, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, J.T.S.; Mak, V.W.; Reisman, S.E. A Unified Strategy to ent-Kauranoid Natural Products: Total Syntheses of (−)-Trichorabdal A and (−)-Longikaurin E. J. Am. Chem. Soc. 2013, 135, 11764–11767. [Google Scholar] [CrossRef] [PubMed]
- Kern, N.; Plesniak, M.P.; McDouall, J.W.W.; Procter, D.J. Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals. Nat. Chem. 2017, in press. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Majumdar, K.C.; Chattopadhyay, S.K. Heterocycles in Natural Product Synthesis, 1st ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Vitaku, E.; Smith, D.T.; Njardson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Shi, S.; Szostak, M. Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. [Google Scholar] [CrossRef]
- Liu, C.; Szostak, M. Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N−C Amide Bond Activation. Chem. Eur. J. 2017, 23, 7157–7173. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Szostak, M. Aminoketyl Radicals in Organic Synthesis: Stereoselective Cyclization of Five- and Six-Membered Cyclic Imides to 2-Azabicycles Using SmI2–H2O. Org. Lett. 2015, 17, 5144–5147. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Lalancette, R.; Szostak, M. Cyclization of Imides to 2-Azabicycles via Aminoketyl Radicals by Using Samarium(II) Iodide-Water: Reaction Development, Synthetic Scope, and Mechanistic Studies. Synthesis 2016, 48, 1825–1854. [Google Scholar] [CrossRef]
- Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Highly Chemoselective Synthesis of Indolizidine Lactams by SmI2-Induced Umpolung of the Amide Bond via Aminoketyl Radicals: Efficient Entry to Alkaloid Scaffolds. Chem. Eur. J. 2016, 22, 11949–11953. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Spain, M.; Choquette, K.A.; Flowers, R.A., II; Procter, D.J. Substrate-Directable Electron Transfer Reactions. Dramatic Rate Enhancement in the Chemoselective Reduction of Cyclic Esters Using SmI2–H2O: Mechanism, Scope, and Synthetic Utility. J. Am. Chem. Soc. 2013, 135, 15702–15705. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Szostak, R.; Szostak, M. Proton-coupled electron transfer in the reduction of carbonyls using SmI2–H2O: Implications for the reductive coupling of acyl-type ketyl radicals with SmI2–H2O. Org. Biomol. Chem. 2016, 14, 9151–9157. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Sautier, B.; Spain, M.; Behlendorf, M.; Procter, D.J. Selective Reduction of Barbituric Acids Using SmI2/H2O: Synthesis, Reactivity, and Structural Analysis of Tetrahedral Adduct. Angew. Chem. Int. Ed. 2013, 52, 12559–12563. [Google Scholar] [CrossRef] [PubMed]
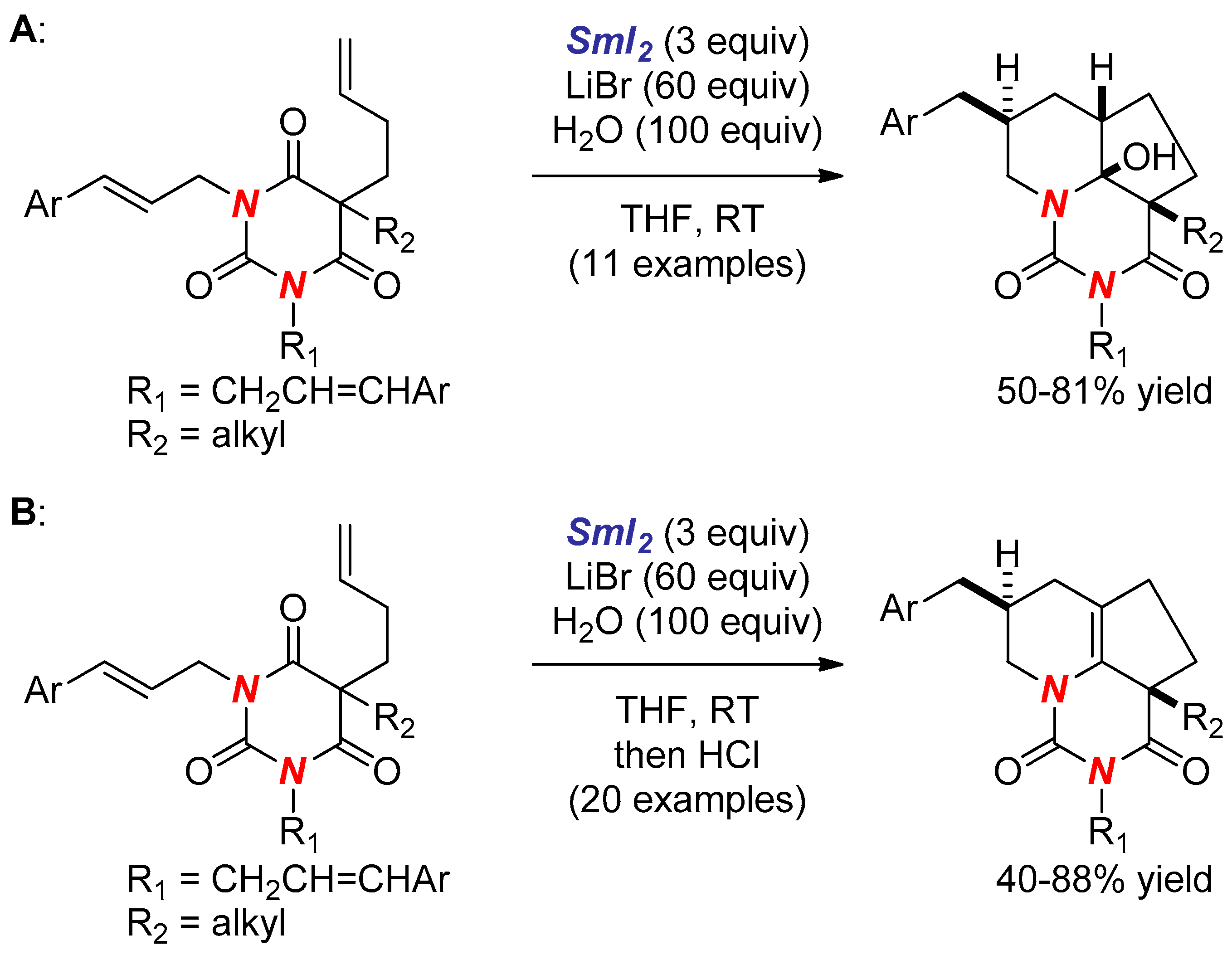
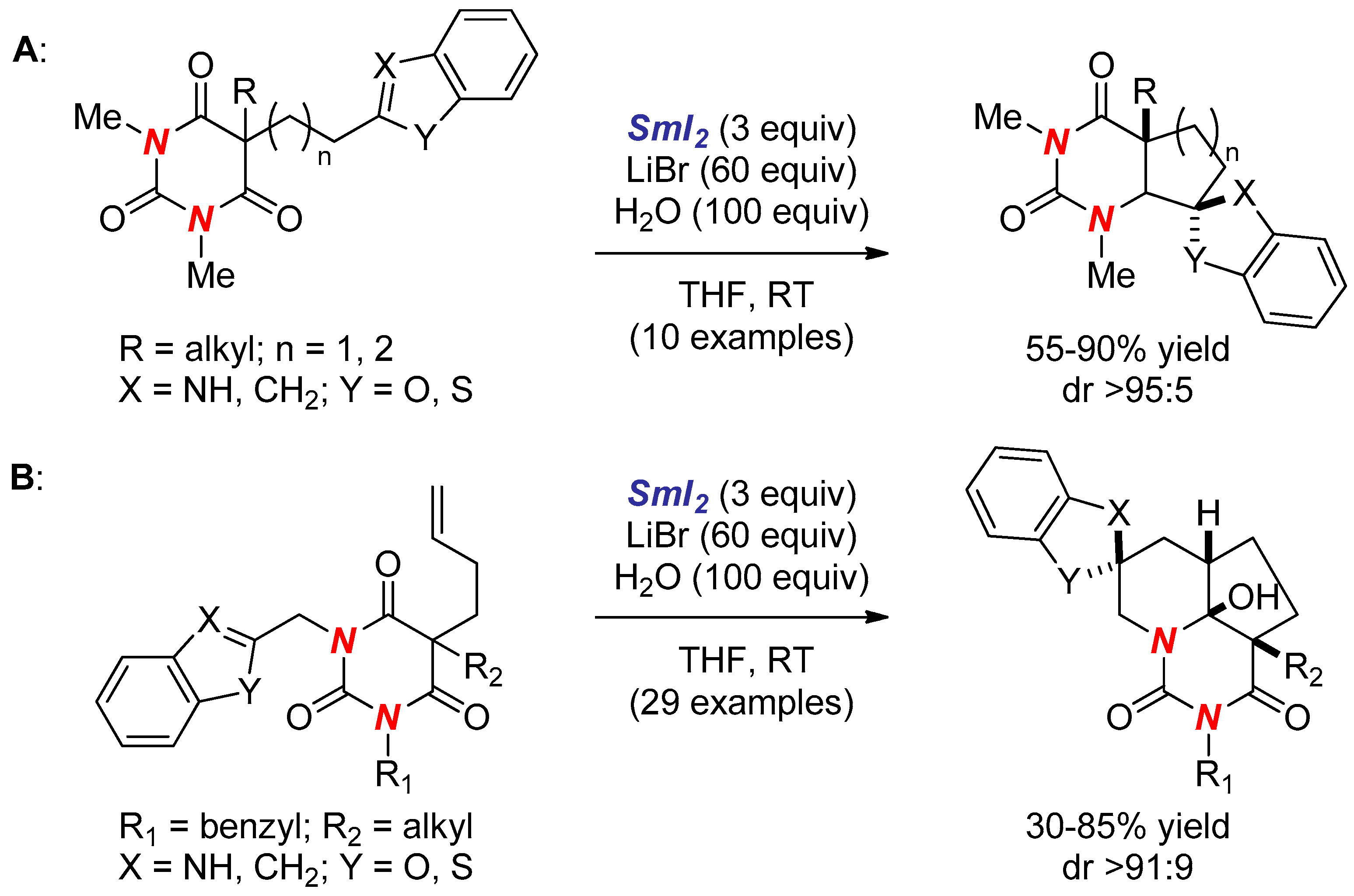
- Huang, H.M.; Procter, D.J. Radical–Radical Cyclization Cascades of Barbiturates Triggered by Electron-Transfer Reduction of Amide-Type Carbonyls. J. Am. Chem. Soc. 2016, 138, 7770–7775. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Procter, D.J. Dearomatizing Radical Cyclizations and Cyclization Cascades Triggered by Electron-Transfer Reduction of Amide-Type Carbonyls. J. Am. Chem. Soc. 2017, 139, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Procter, D.J. Selective construction of quaternary stereocentres in radical cyclisation cascades triggered by electron-transfer reduction of amide-type carbonyls. Org. Biomol. Chem. 2017, 15, 4159–4164. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Procter, D.J. Radical Heterocyclization and Heterocyclization Cascades Triggered by Electron Transfer to Amide-Type Carbonyl Compounds. Angew. Chem. Int. Ed. 2017. [Google Scholar] [CrossRef]
- Vacas, T.; Álvarez, E.; Chiara, J.L. Phthalimides as Exceptionally Efficient Single Electron Transfer Acceptors in Reductive Coupling Reactions Promoted by Samarium Diiodide. Org. Lett. 2007, 9, 5445–5448. [Google Scholar] [CrossRef] [PubMed]
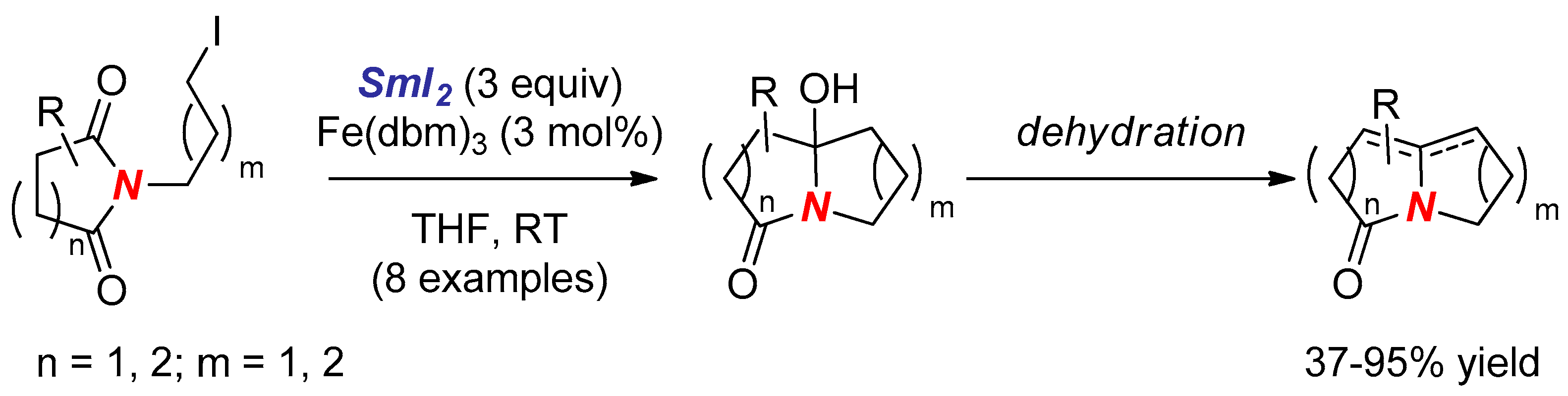
- Ha, D.C.; Yun, C.S.; Yu, E. Reductive cyclization of N-iodoalkyl cyclic imides to nitrogen-fused polycyclic amides induced by samarium diiodide. Tetrahedron Lett. 1996, 37, 2577–2580. [Google Scholar] [CrossRef]
- Ha, D.C.; Yun, C.S.; Lee, Y. Samarium Diiodide-Promoted Cyclization of N-(ω-Iodoalkyl)imides to Polyhydroxylated Indolizidinones and Pyrrolizidinones: Synthesis of (+)-Lentiginosine. J. Org. Chem. 2000, 65, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Burchak, O.N.; Py, S. Reductive cross-coupling reactions (RCCR) between CN and CO for β-amino alcohol synthesis. Tetrahedron 2009, 65, 7333–7356. [Google Scholar] [CrossRef]
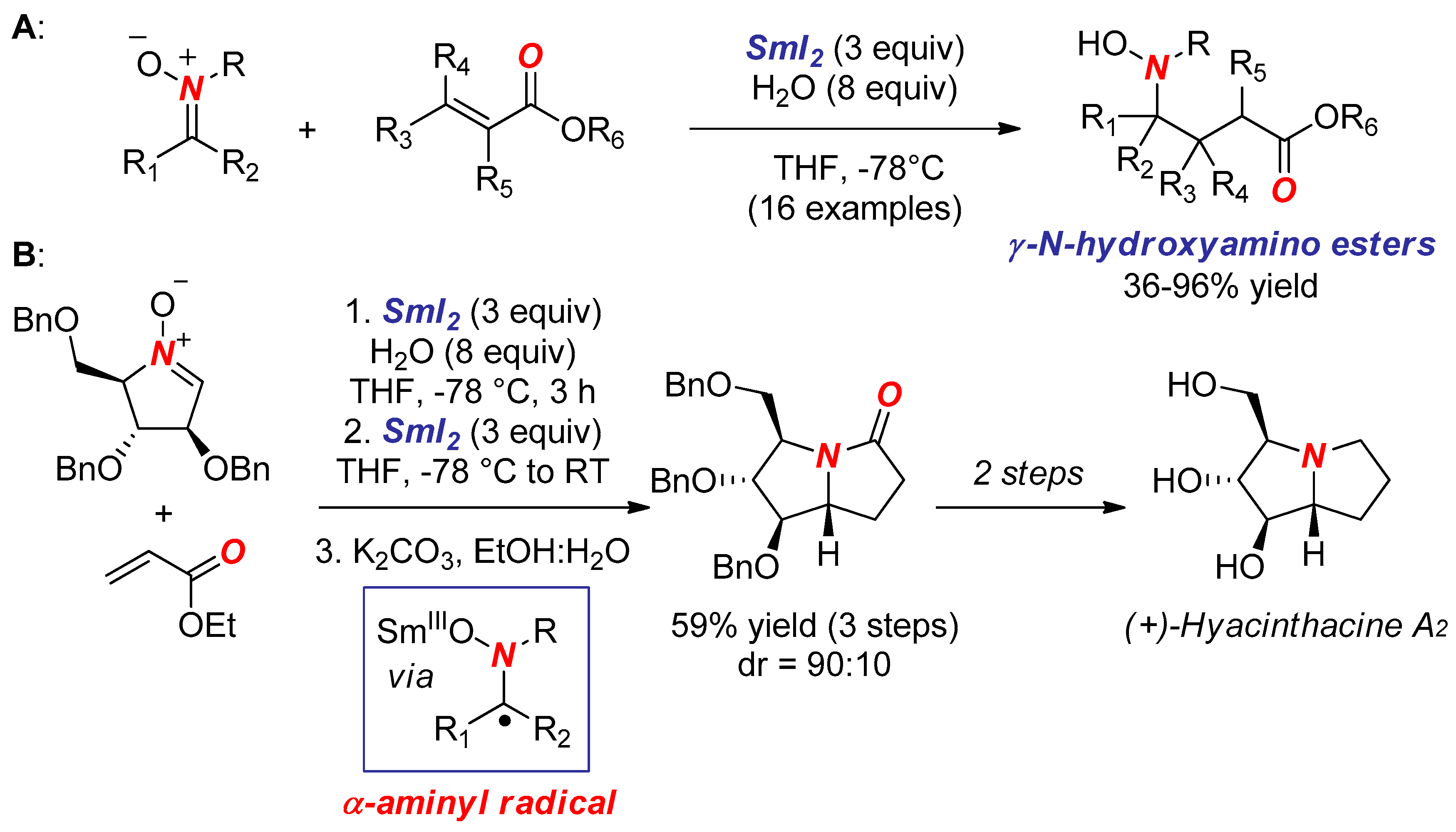
- Masson, G.; Py, S.; Vallée, Y. Samarium Diiodide-Induced Reductive Cross-Coupling of Nitrones with Aldehydes and Ketones. Angew. Chem. Int. Ed. 2002, 41, 1772–1775. [Google Scholar] [CrossRef]
- Masson, G.; Cividino, P.; Py, S.; Vallée, Y. SmI2-Induced Umpolung of the C=N Bond: First Reductive Conjugate Addition of Nitrones to α,β-Unsaturated Esters. Angew. Chem. Int. Ed. 2003, 42, 2265–2268. [Google Scholar] [CrossRef] [PubMed]
- Masson, G.; Zeghida, W.; Cividino, P.; Py, S.; Vallée, Y. A Concise Formal Synthesis of (S)-Vigabatrin Based on Nitrone Umpolung. Synlett 2003, 1527–1529. [Google Scholar] [CrossRef]
- Riber, D.; Skrydstrup, T. SmI2-Promoted Radical Addition of Nitrones to α,β-Unsaturated Amides and Esters: Synthesis of γ-Amino Acids via a Nitrogen Equivalent to the Ketyl Radical. Org. Lett. 2003, 5, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, S.A.; Albu, S.; Hazell, R.G.; Skrydstrup, T. Radical addition of nitrones to acrylates mediated by SmI2: Asymmetric synthesis of γ-amino acids employing carbohydrate-based chiral auxiliaries. Chem. Commun. 2004, 1962–1963. [Google Scholar] [CrossRef] [PubMed]
- Cividino, P.; Py, S.; Delair, P.; Greene, A.E. 1-(2,4,6-Triisopropylphenyl)ethylamine: A New Chiral Auxiliary for the Asymmetric Synthesis of γ-Amino Acid Derivatives. J. Org. Chem. 2007, 72, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.P.; Huang, P.Q.; Py, S. SmI2-Mediated Coupling of Nitrones and tert-Butanesulfinyl Imines with Allenoates: Synthesis of β-Methylenyl-γ-lactams and Tetramic Acids. Org. Lett. 2012, 14, 2034–2037. [Google Scholar] [CrossRef] [PubMed]
- Desvergnes, S.; Desvergnes, V.; Martin, O.R.; Itoh, K.; Liu, H.W.; Py, S. Stereoselective synthesis of β-1-c-substituted 1,4-dideoxy-1,4-imino-d-galactitols and evaluation as UDP-galactopyranose mutase inhibitors. Bioorg. Med. Chem. 2007, 15, 6443–6449. [Google Scholar] [CrossRef] [PubMed]
- Desvergnes, S.; Py, S.; Vallée, Y. Total Synthesis of (+)-Hyacinthacine A2 Based on SmI2-Induced Nitrone Umpolung. J. Org. Chem. 2005, 70, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
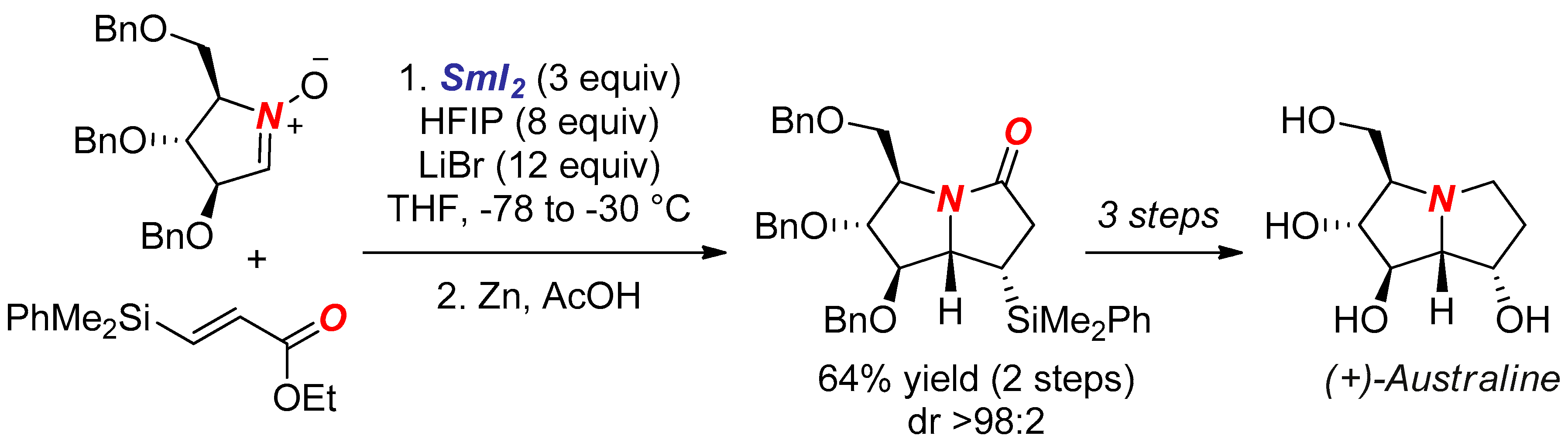
- Gilles, P.; Py, S. SmI2-Mediated Cross-Coupling of Nitrones with β-Silyl Acrylates: Synthesis of (+)-Australine. Org. Lett. 2012, 14, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Ebran, J.P.; Hazell, R.G.; Skrydstrup, T. Samarium diiodide-induced intramolecular pinacol coupling of dinitrones: Synthesis of cyclic cis-vicinal diamines. Chem. Commun. 2005, 45, 5402–5404. [Google Scholar] [CrossRef] [PubMed]
- Volz, N.; Clayden, J. The Urea Renaissance. Angew. Chem. Int. Ed. 2011, 50, 12148–12155. [Google Scholar] [CrossRef] [PubMed]
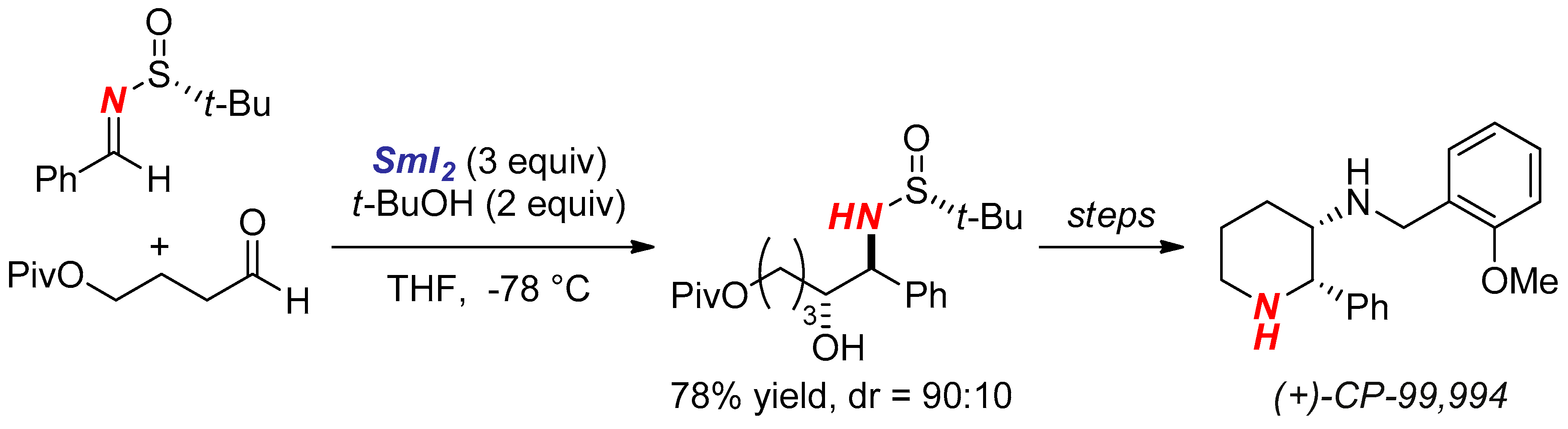
- Robak, M.A.T.; Herbage, M.A.; Ellman, J.A. Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.W.; Dong, Y.Z.; Fang, K.; Izumi, K.; Xu, M.H.; Lin, G.Q. A Highly Efficient and Direct Approach for Synthesis of Enantiopure β-Amino Alcohols by Reductive Cross-Coupling of Chiral N-tert-Butanesulfinyl Imines with Aldehydes. J. Am. Chem. Soc. 2005, 127, 11956–11957. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.W.; Izumi, K.; Xu, M.H.; Lin, G.Q. Highly Diastereoselective and Enantioselective Synthesis of Enantiopure C2-Symmetrical Vicinal Diamines by Reductive Homocoupling of Chiral N-tert-Butanesulfinyl Imines. Org. Lett. 2004, 6, 4747–4750. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.J. SmI2-Promoted Intramolecular Asymmetric Pinacol-Type Ketone−tert-Butanesulfinyl Imine Reductive Coupling: Stereoselectivity and Mechanism. Org. Lett. 2009, 11, 3410–3413. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Fang, K.; Wang, B.; Xu, M.H.; Lin, G.Q. Concise Asymmetric Synthesis of (+)-CP-99,994 and (+)-L-733,060 via Efficient Construction of Homochiral syn-1,2-Diamines and syn-1,2-Amino Alcohols. J. Org. Chem. 2008, 73, 3307–3310. [Google Scholar] [CrossRef] [PubMed]
- Maryanoff, B.E.; Zhang, H.C.; Cohen, J.H.; Turchi, I.J.; Maryanoff, C. Cyclizations of N-Acyliminium Ions. Chem. Rev. 2004, 104, 1431–1628. [Google Scholar] [CrossRef] [PubMed]
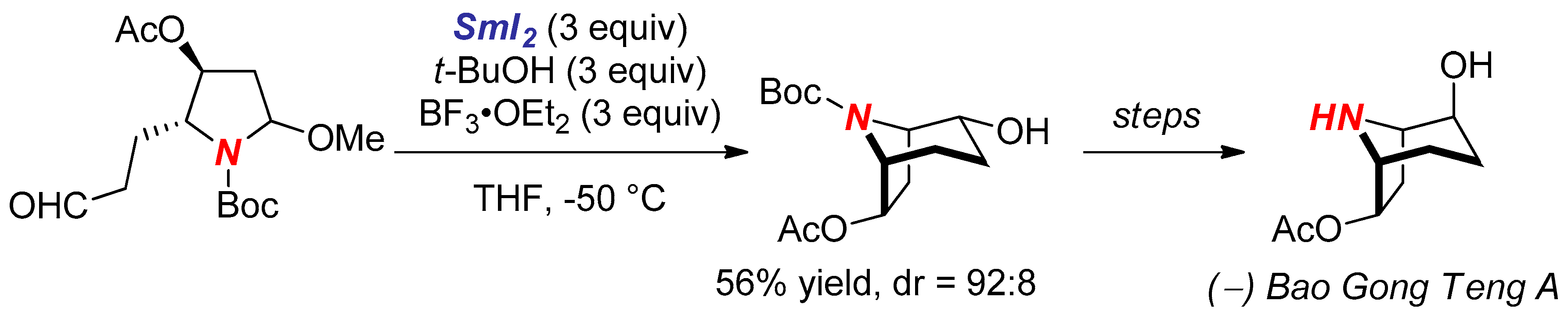
- Lin, G.J.; Zheng, X.; Huang, P.Q. A new method for the construction of the hydroxylated tropane skeleton: Enantioselective synthesis of (−)-Bao Gong Teng A. Chem Commun. 2011, 47, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.K.; Qiu, S.; Xiang, Y.G.; Ruan, Y.P.; Zheng, X.; Huang, P.Q. SmI2-Mediated Radical Cross-Couplings of α-Hydroxylated Aza-hemiacetals and N,S-Acetals with α,β-Unsaturated Compounds: Asymmetric Synthesis of (+)-Hyacinthacine A2, (−)-Uniflorine A, and (+)-7-epi-Casuarine. J. Org. Chem. 2011, 76, 4952–4963. [Google Scholar] [CrossRef] [PubMed]
- Schiedler, D.A.; Lu, Y.; Beaudry, C.M. Reductive Synthesis of Aminal Radicals for Carbon–Carbon Bond Formation. Org. Lett. 2014, 16, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Schiedler, D.A.; Vellucci, J.K.; Lu, Y.; Beaudry, C.M. The development of carbon–carbon bond forming reactions of aminal radicals. Tetrahedron 2015, 71, 1448–1465. [Google Scholar] [CrossRef]
- Schiedler, D.A.; Vellucci, J.K.; Beaudry, C.M. Formation of Carbon–Carbon Bonds Using Aminal Radicals. Org. Lett. 2012, 14, 6092–6095. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Ishikawa, F. Reductive deamination of α-amino carbonyl compounds by means of samarium iodide. Chem. Commun. 1999, 12, 1065–1066. [Google Scholar] [CrossRef]
- Honda, T.; Takahashi, R.; Namiki, H. Syntheses of (+)-Cytisine, (−)-Kuraramine, (−)-Isokuraramine, and (−)-Jussiaeiine A. J. Org. Chem. 2005, 70, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Honda, T. Development of Samarium Diiodide-Promoted Reductive Carbon-Nitrogen Bond Cleavage Reaction of α-Amino Carbonyl Compounds: Application to the Synthesis of Biologically Active Alkaloids. Heterocycles 2011, 83, 1–46. [Google Scholar] [CrossRef]
- Pinho, V.D.; Procter, D.J.; Burtoloso, A.C.B. SmI2-Mediated Couplings of α-Amino Acid Derivatives. Formal Synthesis of (−)-Pumiliotoxin 251D and (±)-Epiquinamide. Org. Lett. 2013, 15, 2434–2437. [Google Scholar] [CrossRef] [PubMed]
- Bernardim, B.; Pinho, V.D.; Burtoloso, A.C.D. α,β-Unsaturated Diazoketones as Platforms in the Asymmetric Synthesis of Hydroxylated Alkaloids. Total Synthesis of 1-Deoxy-8,8a-diepicastanospermine and 1,6-Dideoxyepicastanospermine and Formal Synthesis of Pumiliotoxin 251D. J. Org. Chem. 2012, 77, 9926–9931. [Google Scholar] [CrossRef] [PubMed]
- Burtoloso, A.C.D.; Dias, R.M.P.; Bernardim, B. α,β-Unsaturated Diazoketones as Useful Platforms in the Synthesis of Nitrogen Heterocycles. Acc. Chem. Res. 2015, 48, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Iwasaki, H.; Eguchi, T.; Tanaka, T. The first samarium(II)-mediated aryl radical cyclisation onto an aromatic ring. Chem. Commun. 2004, 2228–2229. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Coffin, A.; Nguyen, Q.N.; Tantillo, D.J.; Ready, J.M. Synthesis and Utility of Dihydropyridine Boronic Esters. Angew. Chem. Int. Ed. 2016, 55, 2205–2209. [Google Scholar] [CrossRef] [PubMed]
- Kamabe, M.; Miyazaki, T.; Hashimoto, K.; Shirahama, H. Formal Synthesis of FPA, a Kainoid Amino Acid, via Ketyl Radical Cyclization. Heterocycles 2002, 56, 105–111. [Google Scholar] [CrossRef]
- Foster, S.L.; Handa, S.; Krafft, M.; Rowling, D. Samarium(II) iodide-mediated intramolecular pinacol coupling reactions with cyclopropyl ketones. Chem. Commun. 2007, 45, 4791–4793. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, H.G.; Siegel, S.; Bats, J.W. Radical Additions to (η6-Arene)(tricarbonyl)-chromium Complexes: Diastereoselective Synthesis of Hydrophenalene and Hydrobenzindene Derivatives by Samarium(II) Iodide Induced Cyclization. Angew. Chem. Int. Ed. 1995, 34, 2383–2385. [Google Scholar] [CrossRef]
- Beemelmanns, C.; Reissig, H.U. Samarium diiodide induced ketyl-(het)arene cyclisations towards novel N-heterocycles. Chem. Soc. Rev. 2011, 40, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Beemelmanns, C.; Reissig, H.U. A Short Formal Total Synthesis of Strychnine with a Samarium Diiodide Induced Cascade Reaction as the Key Step. Angew. Chem. Int. Ed. 2010, 49, 8021–8025. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Procter, D.J. Concise Syntheses of Strychnine and Englerin A: The Power of Reductive Cyclizations Triggered by Samarium Iodide. Angew. Chem. Int. Ed. 2011, 50, 7737–7739. [Google Scholar] [CrossRef] [PubMed]
- Beemelmanns, C.; Reissig, H.U. New samarium diiodide-induced cyclizations. Pure Appl. Chem. 2011, 83, 507–518. [Google Scholar] [CrossRef]
- Rao, N.C.; Lentz, D.; Reissig, H.U. Synthesis of Polycyclic Tertiary Carbinamines by Samarium Diiodide Mediated Cyclizations of Indolyl Sulfinyl Imines. Angew. Chem. Int. Ed. 2015, 54, 2750–2753. [Google Scholar] [CrossRef] [PubMed]
- Ready, J.M.; Reisman, S.E.; Hirata, M.; Weiss, M.M.; Tamaki, K.; Ovaska, T.V.; Wood, J.L. A Mild and Efficient Synthesis of Oxindoles: Progress towards the Synthesis of Welwitindolinone A Isonitrile. Angew. Chem. Int. Ed. 2004, 43, 1270–1272. [Google Scholar] [CrossRef]
- Reisman, S.E.; Ready, J.M.; Weiss, M.M.; Hasuoka, A.; Hirata, M.; Tamaki, K.; Ovaska, T.V.; Smith, C.J.; Wood, J.L. Evolution of a Synthetic Strategy: Total Synthesis of (±)-Welwitindolinone A Isonitrile. J. Am. Chem. Soc. 2008, 130, 2087–2100. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Tsukano, C.; Takemoto, Y. Synthesis of 2-Iminoindolines via Samarium Diiodide Mediated Reductive Cyclization of Carbodiimides. Chem. Lett. 2012, 41, 44–46. [Google Scholar] [CrossRef]
- Ishida, T.; Takemoto, Y. Synthetic study of perophoramidine: Construction of pentacyclic core structure via SmI2-mediated reductive cyclization. Tetrahedron 2013, 69, 4517–4523. [Google Scholar] [CrossRef]
- Bai, W.J.; Jackson, S.K.; Pettus, T.R.R. Mild Construction of 3-Methyl Tetramic Acids Enabling a Formal Synthesis of Palau’imide. Org. Lett. 2012, 14, 3862–3865. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Spain, M.; Eberhart, A.J.; Procter, D.J. Highly Chemoselective Reduction of Amides (Primary, Secondary, Tertiary) to Alcohols using SmI2/Amine/H2O under Mild Conditions. J. Am. Chem. Soc. 2014, 136, 2268–2271. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, A.; Hilmersson, G. Instantaneous SmI2–H2O-mediated reduction of dialkyl ketones induced by amines in THF. Tetrahedron Lett. 2002, 43, 7197–7200. [Google Scholar] [CrossRef]
- Dahlén, A.; Hilmersson, G. Mechanistic Study of the SmI2/H2O/Amine-Mediated Reduction of Alkyl Halides: Amine Base Strength (pKBH+) Dependent Rate. J. Am. Chem. Soc. 2005, 127, 8340–8347. [Google Scholar] [CrossRef] [PubMed]
- Huq, S.; Shi, S.; Diao, R.; Szostak, M. Mechanistic Study of SmI2/H2O and SmI2/Amine/H2O-Promoted Chemoselective Reduction of Aromatic Amides (Primary, Secondary, Tertiary) to Alcohols via Aminoketyl Radicals. J. Org. Chem. 2017, 82, 6528–6540. [Google Scholar] [CrossRef] [PubMed]
- Thurow, S.; Lenardao, E.J.; Just-Baringo, X.; Procter, D.J. Reduction of Selenoamides to Amines Using SmI2–H2O. Org. Lett. 2017, 19, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Ankner, T.; Hilmersson, G. Instantaneous Deprotection of Tosylamides and Esters with SmI2/Amine/Water. Org. Lett. 2009, 11, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Ankner, T.; Stålsmeden, A.S.; Hilmersson, G. Selective cleavage of 3,5-bis-(trifluoromethyl)benzylcarbamate by SmI2–Et3N–H2O. Chem. Commun. 2013, 49, 6867–6869. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, A.; Hilmersson, G. Instantaneous SmI2/H2O/Amine-Mediated Reductions in THF. Chem. Eur. J. 2003, 9, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Ankner, T.; Hilmersson, G. SmI2/H2O/amine promoted reductive cleavage of benzyl-heteroatom bonds: optimization and mechanism. Tetrahedron 2009, 65, 10856–10862. [Google Scholar] [CrossRef]

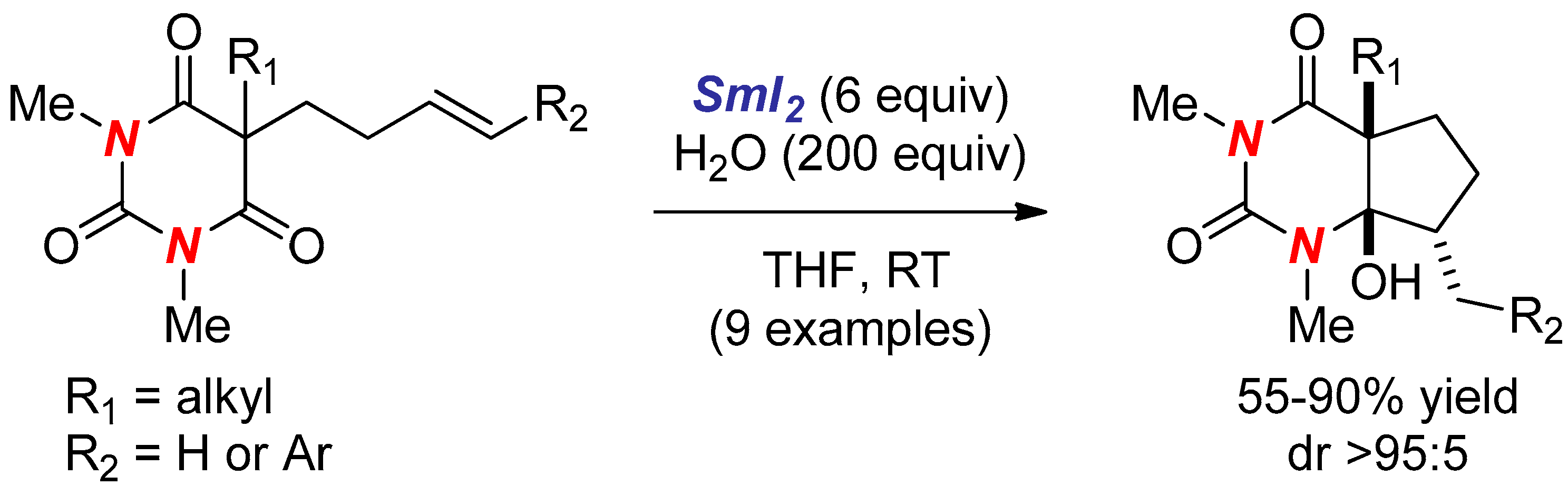

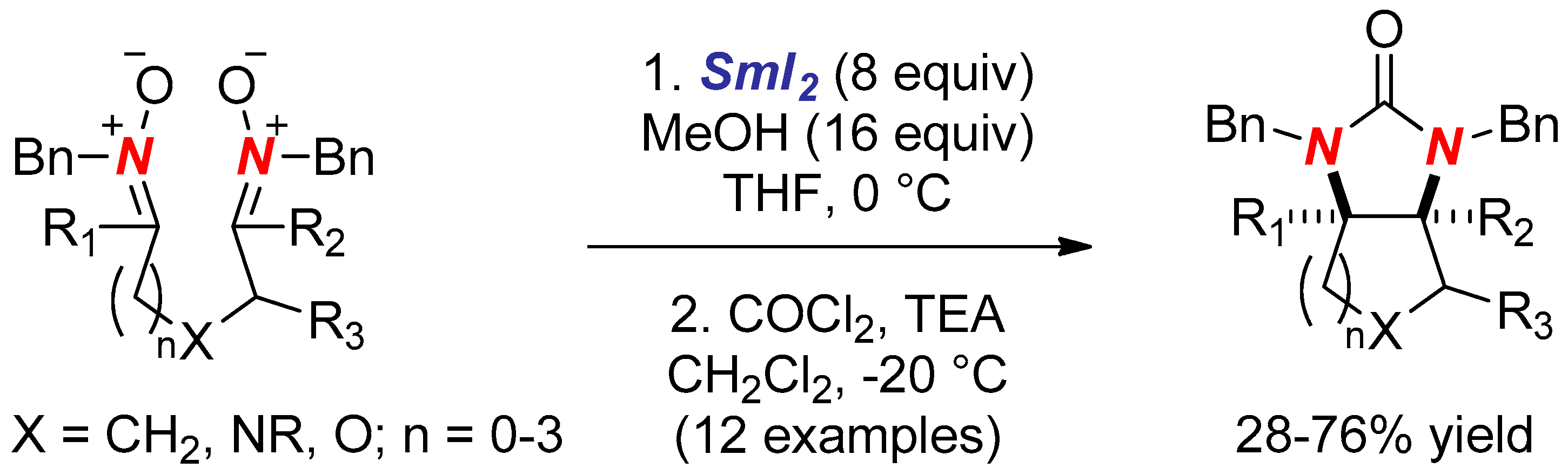
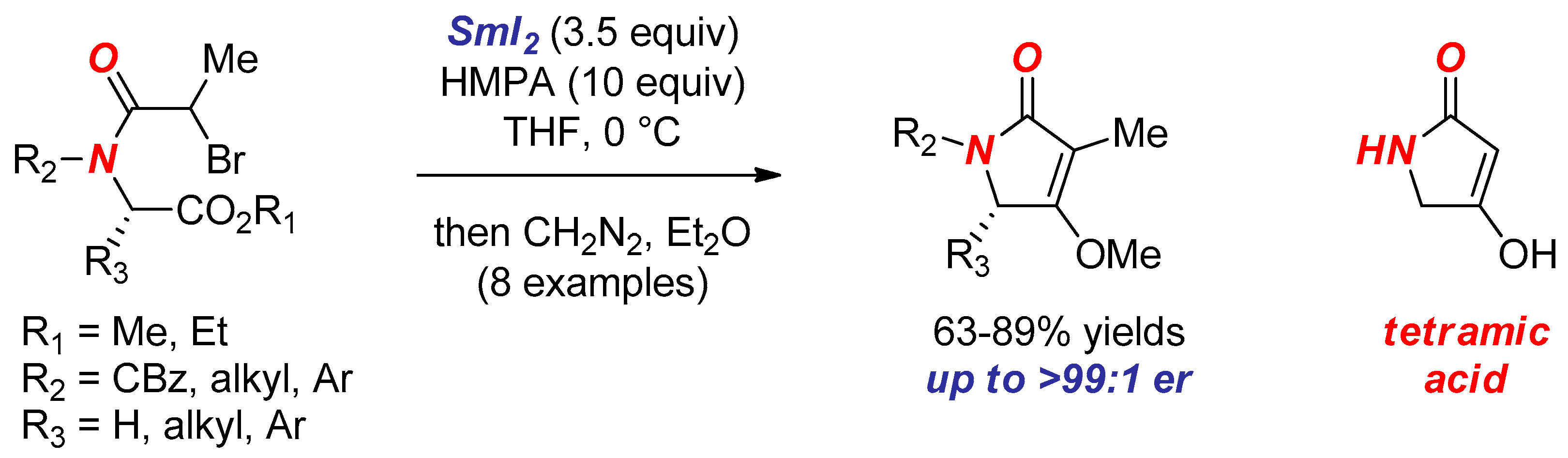
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Szostak, M. Synthesis of Nitrogen Heterocycles Using Samarium(II) Iodide. Molecules 2017, 22, 2018. https://doi.org/10.3390/molecules22112018
Shi S, Szostak M. Synthesis of Nitrogen Heterocycles Using Samarium(II) Iodide. Molecules. 2017; 22(11):2018. https://doi.org/10.3390/molecules22112018
Chicago/Turabian StyleShi, Shicheng, and Michal Szostak. 2017. "Synthesis of Nitrogen Heterocycles Using Samarium(II) Iodide" Molecules 22, no. 11: 2018. https://doi.org/10.3390/molecules22112018
APA StyleShi, S., & Szostak, M. (2017). Synthesis of Nitrogen Heterocycles Using Samarium(II) Iodide. Molecules, 22(11), 2018. https://doi.org/10.3390/molecules22112018